Introduction
Type 2 diabetes (T2D) is a chronic disease that
affects glucose metabolism. T2D has a number of associated serious
complications, including heart disease, retinopathy and renal
failure (1,2). The prevalence of diabetes mellitus,
particularly T2D, is increasing with contributing factors, such as
body weight and obesity (3). The
T2D population is predicted to be double the size as it is
currently by 2030 (4). T2D is
affected by genetic (5) and
environmental factors, including an unhealthy lifestyle (6), diet (7) and obesity (8).
Although there are hundreds of genetic loci
associated with T2D (9), >90%
of T2D trait variations remain to be explained. Epigenetic
modification, including DNA methylation, plays an important role in
the pathogenesis of T2D (10). DNA
methylation and histone modification have become alternative
approaches that have aided the understanding of β-cell dysfunction
in the pathogenesis (11) and the
high growth rate of T2D (12).
Aberrant DNA methylation of genes, such as PGC-1α (13), PDX1 (14), MCP-1 (15) and leptin (16), have been shown to contribute to the
risk of T2D. In addition, a number of environmental risk factors,
including malnutrition and a lack of physical exercise, interfere
with DNA methylation modification and increase the risk of T2D
(17).
The BCL11A gene encodes a CH2H2 type
zinc-finger protein that is necessary for lymphopoiesis and the
negative regulation of p53 activity (18), functioning as a transcriptional
repressor (19). Elevated levels
of insulin and leptin and decreased levels of adiponectin in the
serum are known to be associated with T2D risk, and they may also
downregulate p53 expression,thus, induce a cancer risk (20). Expression of human fetal hemoglobin
is controlled by BCL11A (21). BCL11A deficiency is
associated with decreased fetal hemoglobin (22), which is significantly associated
with a decreased risk of T2D (23). BCL11A gene variants affect
the insulin response to glucose (24) and glucagon secretion (25), thus, have been shown to increase
the risk of T2D in Europeans, North African Arabs (26) and African-Americans (27). The aim of the present study was to
investigate the contribution of BCL11A DNA methylation to
the risk of T2D.
Materials and methods
Sample collection
A total of 48 T2D cases and 48 age- and
gender-matched controls were selected from patients in the
Affiliated Hospital of Ningbo University and Ningbo No. 2 Hospital
(Ningbo, China). Patients were included in the study if they met
the following criteria. Firstly, all the subjects were recruited
without hypertension, coronary heart disease or other serious
diseases. Secondly, the subjects were of Han Chinese origin and had
lived in Ningbo city for at least three generations. Thirdly,
standard clinical criteria (World Health Organization, 2007; 28)
were applied with regard to T2D diagnosis, while the selection for
healthy controls was based on the standard that the fasting blood
glucose level was <6.1 mmol/l. Blood samples were collected from
all the participants and were stored at −80°C in 3.2% citrate
sodium-treated tubes. All the involved individuals provided
informed consent, which was approved by the Ethical Committees of
the Affiliated Hospital of Ningbo University and Ningbo No. 2
Hospital.
Phenotype and biochemical analyses
Phenotype analysis included total cholesterol (TC),
triglyceride (TG), alanine aminotransferase (ALT), uric acid (UA)
and glucose levels. Plasma levels of TG and TC were measured using
an enzymatic end point assay (29). Concentrations of ALT and blood
glucose were measured using the International Federation of
Clinical Chemistry reference measurement systems (30) and the glucose oxidase and
peroxidase assay (31),
respectively. UA levels were measured with a CX77 Analyzer (Beckman
Coulter, Inc., Fullerton, CA, USA). Genomic DNA was isolated from
peripheral blood samples using a nucleic acid extraction analyzer
(Lab-Aid 820; Xiamen City, China), and the concentration was
measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Wilmington, DE, USA). BCL11A methylation was
conducted with pyrosequencing technology combined with sodium
bisulfite DNA conversion chemistry (EpiTech Bisulfite kits; Qiagen,
Venlo, Netherlands) and polymerase chain reaction (PCR)
amplification (Pyromark PCR kit; Qiagen). PyroMark Assay Design
software automatically selected the appropriate CpG sites with high
scores in a 70-nt fragment to design the PCR and sequencing
primers, which included the forward
(5′-GTTTAGGTTAGAGGTGGGTGTTT-3′), reverse
(5′-biotin-TATACCAATCTTCTCCTTACTACCT-3′) and sequencing primers
(5′-GAAGGGTAGGAGTTA-3′). The biotin in reverse primer was used to
identify the sequences.
Statistical analysis
SPSS software (version 16.0; SPSS, Inc., Chicago,
IL, USA) was used for all the statistical tests, including the
t-test for two independent samples, two-way analysis of variance
(ANOVA) and Pearson’s regression analysis. Using the two
independent samples t-test, BCL11A methylation and other
phenotypes were compared between the T2D cases and controls. The
interaction between gender and T2D status was assessed by applying
two-way ANOVA, while the correlation analyses between BCL11A
methylation and other phenotypes (including TG, TC, UA and ALT)
were performed with Pearson’s regression analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between mean BCL11A
methylation and T2D
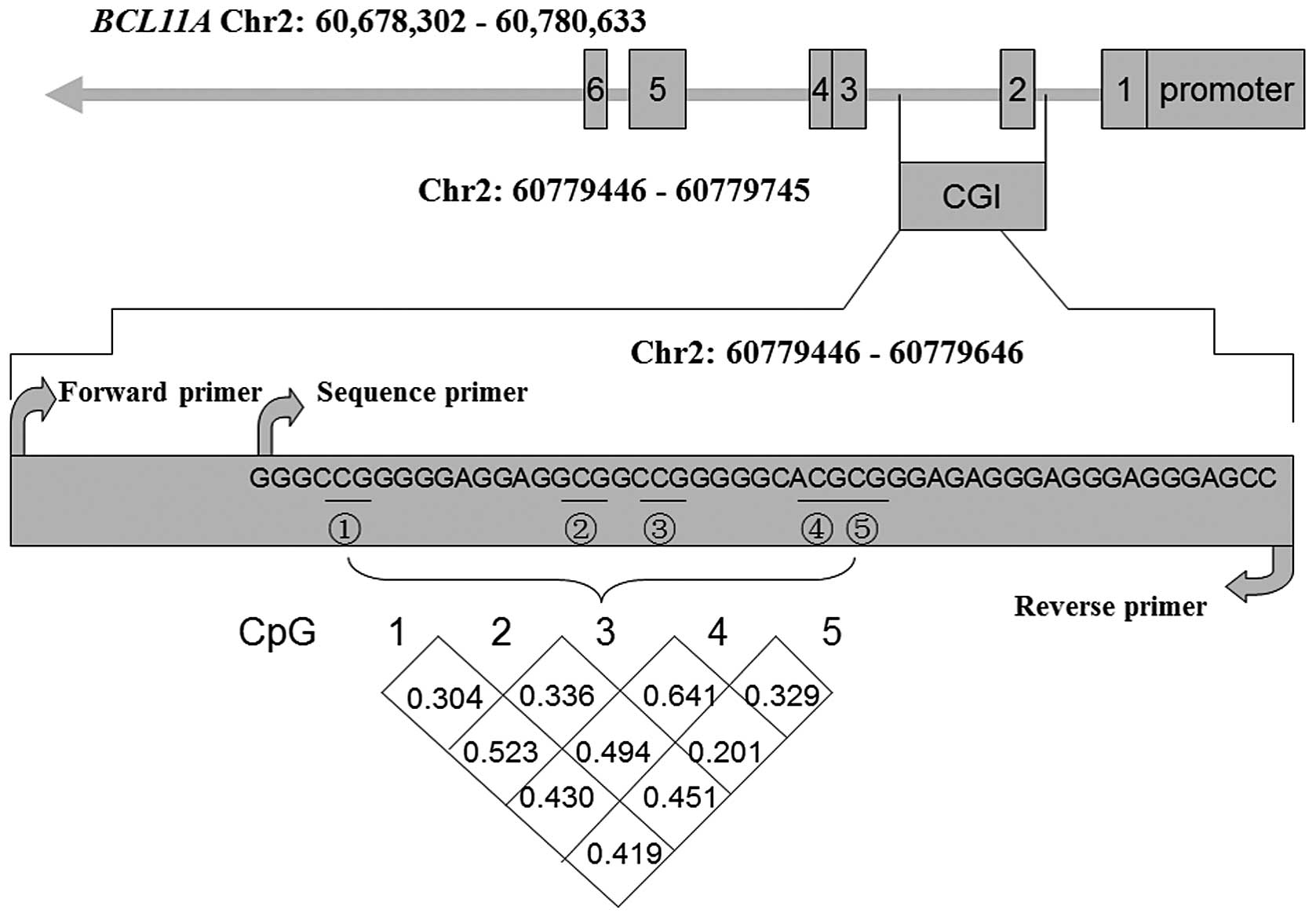
As shown in Fig. 1,
the intragenic CpG island (CGI) was close to the promoter. A total
of five CGI sites that exhibited a strong correlation were used to
evaluate the DNA methylation of the BCL11A gene (r>0.3;
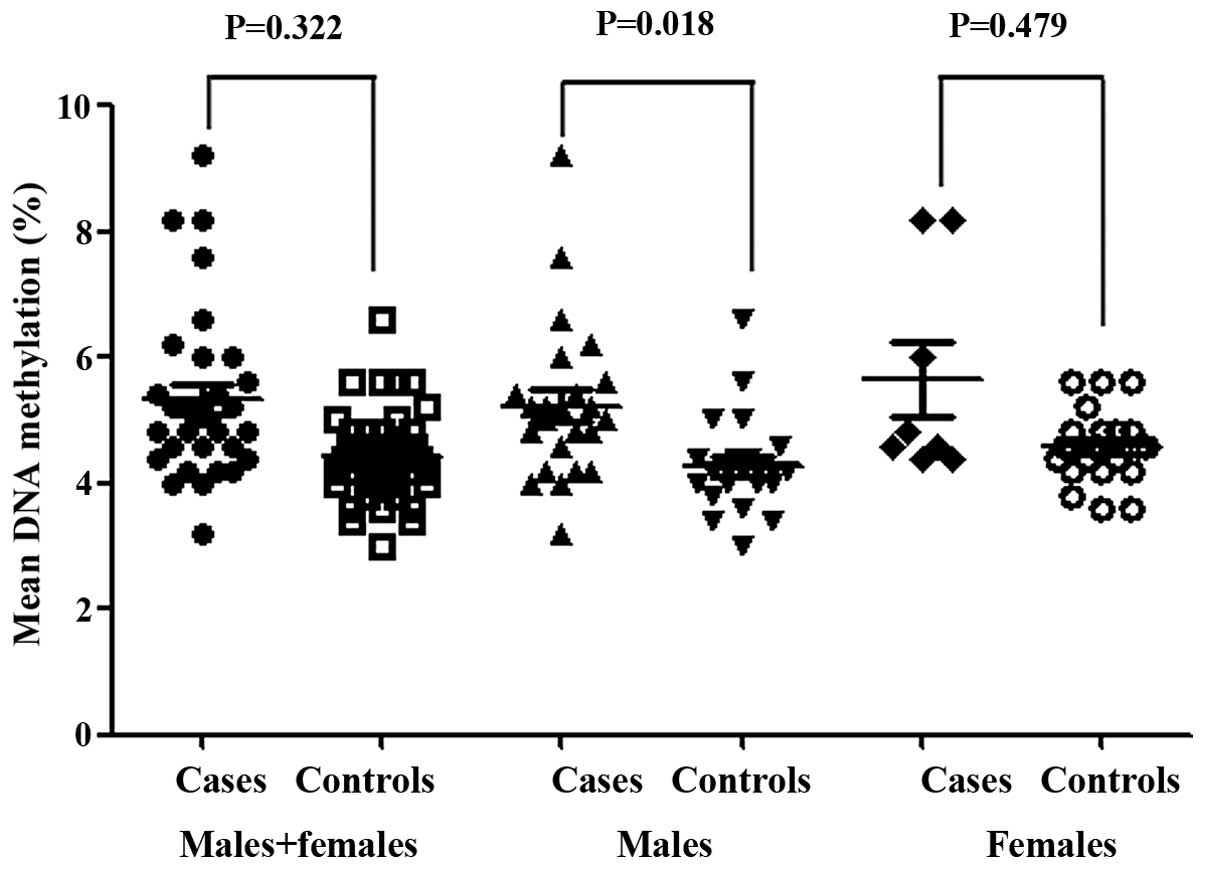
Fig. 1). Although there was no
statistically significant gender difference with regard to mean DNA
methylation (Table I; P=0.102), a
significant difference in the mean DNA methylation of the
BCL11A gene was observed in males (Fig. 2; P=0.018).
 | Table ICharacteristics of the subjects. |
Table I
Characteristics of the subjects.
|
Characteristics | Males (n=47) | Females
(n=47)b | P1 | Diabetic
(n=48) | Non-diabetic
(n=46) | P2 | P3 |
|---|
| Age (years) | 59.0±8.7 | 59.2±6.2 | 0.902 | 59.2±7.5 | 58.9±7.5 | 0.839 | 0.948 |
| TC (mmol/l) | 4.96±0.95 | 5.42±0.93 | 0.021 | 5.34±0.83 | 5.04±1.08 | 0.133 | 0.037 |
| TG (mmol/l) | 1.60±1.29 | 1.62±1.46 | 0.965 | 1.90±1.69 | 1.32±0.84 | 0.038 | 0.546 |
| ALT (mmol/l) | 26.64±19.22 | 17.38±7.42 | 0.019 | 25.10±18.49 | 16.74±8.23 | 0.006 | 0.236 |
| UA (μmol/l) | 323.24±78.08 | 263.56±72.24 | <0.001 | 289.30±70.48 | 297.67±90.57 | 0.065 | 0.058 |
| DNA methylation
(%)a |
| CpG1 | 5.89±1.784 | 5.447±2.224 | 0.334 | 5.98±2.46 | 5.35±1.37 | 0.076 | 0.094 |
| CpG2 | 2.319±1.520 | 2.213±1.587 | 0.705 | 2.56±1.60 | 1.96±1.44 | 0.036 | 0.012 |
| CpG3 | 7.234±1.902 | 6.298±2.255 | 0.107 | 6.96±2.63 | 6.57±1.44 | 0.427 | 0.019 |
| CpG4 | 6.809±1.262 | 6.021±2.101 | 0.011 | 6.17±2.26 | 6.67±0.99 | 0.318 | 0.002 |
| CpG5 | 1.575±1.315 | 1.511±1.040 | 0.935 | 1.46±1.20 | 1.63±1.16 | 0.592 | 0.264 |
| Mean | 4.766±1.139 | 4.298±1.373 | 0.102 | 4.63±1.66 | 4.43±0.67 | 0.322 | 0.003 |
Association between mean BCL11A
methylation and clinical phenotypes
As shown in Table
I, among the five tested phenotypes, the results demonstrated
that TC (P=0.021), ALT (P=0.019) and UA (P<0.001) levels were
significantly different between males and females, and that levels
of TG (P=0.038) and ALT (P=0.006) were significantly different
between the T2D cases and controls. The results also revealed a
significant interaction between gender and T2D status for the
association study of mean BCL11A methylation (P=0.003). In
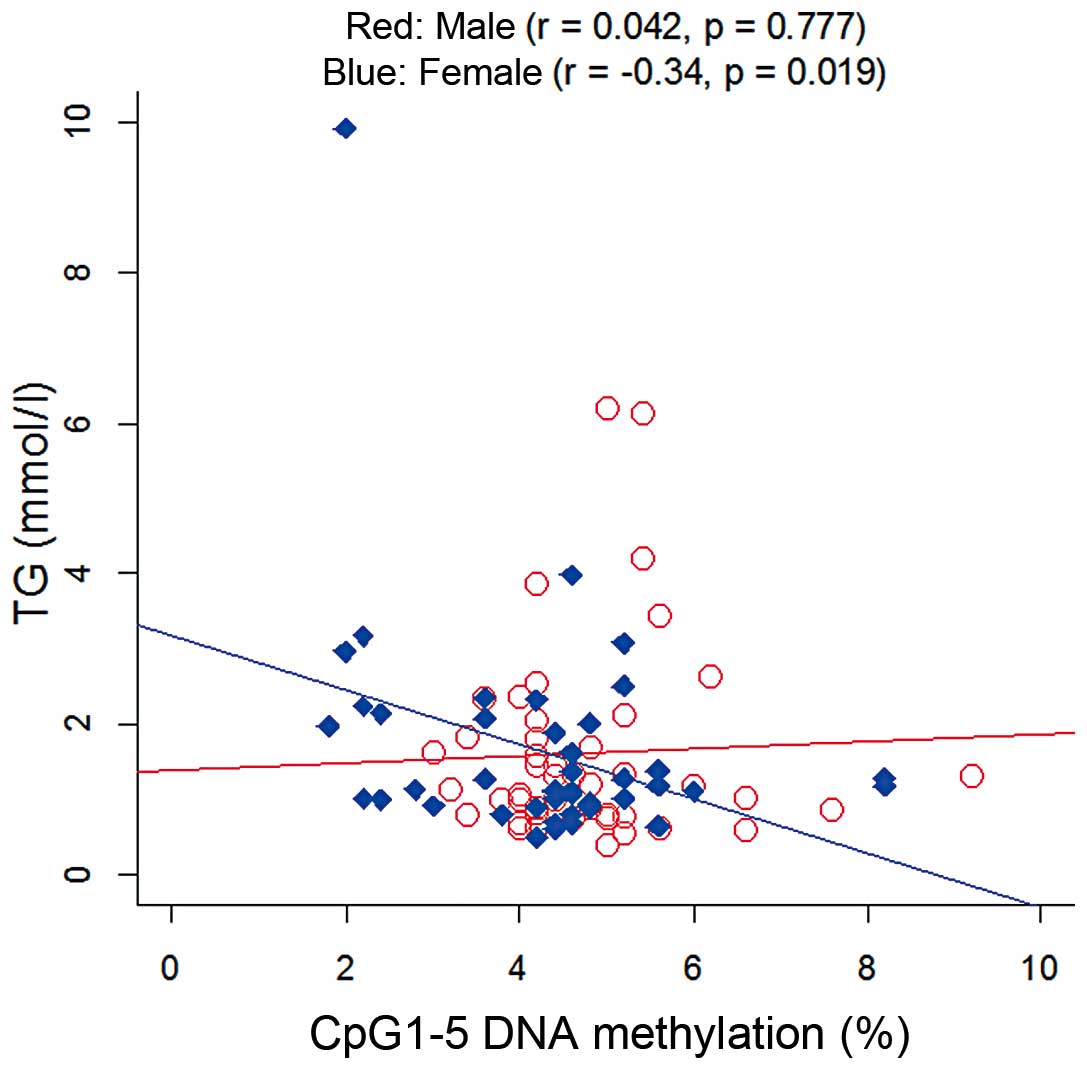
addition, a female-specific correlation between the TG level and
mean DNA methylation was observed (Fig. 3; P=0.019). However, no correlations
were observed between the other phenotypes (age, TC, ALT and UA)
and mean DNA methylation (P>0.05).
Discussion
In recent years, an increasing number of studies
have investigated DNA methylation in a variety of diseases,
including coronary heart disease (32), lung cancer (33) and T2D (34). The present study investigated the
association between BCL11A DNA methylation and the risk of
T2D in 48 T2D cases and 48 age- and gender-matched controls. The
results revealed that BCL11A DNA methylation was
specifically associated with the risk of T2D in males
(P=0.018).
A previous study demonstrated gender differences in
T2D (35). Compared with males, a
higher prevalence for cardiovascular disease was shown in diabetic
females (36). Female T2D patients
have compact clots and compromised fibrinolysis, thus, are much
more likely to suffer from atherothrombotic disease (37) compared with male T2D patients. In
addition, serum ferritin levels have been shown to be significantly
associated with fasting glucose levels in female T2D patients
(38). Gender differences have
also been observed in the association between other diseases and
the methylation of genes, including PLA2G7 (32), MIR375 (34) and MTHFR (39). The present study demonstrated a
male specific association between BCL11A DNA methylation and
the risk of T2D, but a female-specific correlation between TG
levels and DNA methylation.
High TG/high-density lipoprotein cholesterol levels
are associated with the risk of microvascular complications in T2D
(40). Continuous insulin infusion
can correct hypertriglyceridemia in T2D patients and markedly
reduce the risk of metabolic complications (41). The development of T2D may be
associated with DNA methylation in the BCL11A gene via
affecting TG levels.
CGIs in the promoter regions of diabetic candidate
genes, such as MIR375 (34), are associated with the risk of T2D.
Although DNA methylation of gene promoters has a significant impact
on gene expression, a correlation exists between intragenic DNA
methylation and gene expression (42). The present study demonstrated that
intragenic DNA methylation in the BCL11A gene was associated
with T2D. However, there were limitations to the study. For
example, the sample size of the study was relatively small, which
should be expanded for future study. In addition, DNA methylation
is tissue specific and the observations in the peripheral blood may
not reflect the other tissues of interest.
In conclusion, the present study revealed a
male-specific significant association between BCL11A DNA
methylation and the risk of T2D and a female-specific association
between TG levels and and BCL11A DNA methylation. These
observations may improve the understanding of the molecular
mechanisms underlying T2D pathogenesis.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (nos. 31100919 and 81371469),
the Natural Science Foundation of Zhejiang Province (no.
LR13H020003) and the K.C. Wong Magna Fund in Ningbo University,
Ningbo Social Development Research Projects (no. 2012C50032).
Abbreviations:
|
T2D
|
type 2 diabetes
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
ALT
|
alanine aminotransferase
|
|
UA
|
uric acid
|
|
CGI
|
CpG island
|
References
|
1
|
Afkarian M, Sachs MC, Kestenbaum B, et al:
Kidney disease and increased mortality risk in type 2 diabetes. J
Am Soc Nephrol. 24:302–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papa G, Degano C, Iurato MP, Licciardello
C, Maiorana R and Finocchiaro C: Macrovascular complication
phenotypes in type 2 diabetic patients. Cardiovasc Diabetol.
12:202013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ginter E and Simko V: Global prevalence
and future of diabetes mellitus. Adv Exp Med Biol. 771:35–41.
2012.PubMed/NCBI
|
|
4
|
Biasini E, Unterberger U, Solomon IH, et
al: A mutant prion protein sensitizes neurons to glutamate-induced
excitotoxicity. J Neurosci. 33:2408–2418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franks PW: Gene x environment interactions
in type 2 diabetes. Curr Diab Rep. 11:552–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wittmeier KD, Wicklow BA, Sellers EA,
Griffith AT, Dean HJ and McGavock JM: Success with lifestyle
monotherapy in youth with new-onset type 2 diabetes. Paediatr Child
Health. 17:129–132. 2012.PubMed/NCBI
|
|
7
|
Tobias DK, Hu FB, Chavarro J, Rosner B,
Mozaffarian D and Zhang C: Healthful dietary patterns and type 2
diabetes mellitus risk among women with a history of gestational
diabetes mellitus. Arch Intern Med. 172:1566–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tentolouris N, Andrianakos A, Karanikolas
G, et al: Type 2 diabetes mellitus is associated with obesity,
smoking and low socioeconomic status in large and representative
samples of rural, urban, and suburban adult Greek populations.
Hormones (Athens). 11:458–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drong AW, Lindgren CM and McCarthy MI: The
genetic and epigenetic basis of type 2 diabetes and obesity. Clin
Pharmacol Ther. 92:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simmons RA: Programming of DNA methylation
in type 2 diabetes. Diabetologia. 56:947–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilbert ER and Liu D: Epigenetics: the
missing link to understanding β-cell dysfunction in the
pathogenesis of type 2 diabetes. Epigenetics. 7:841–852. 2012.
|
|
12
|
Kirchner H, Osler ME, Krook A and Zierath
JR: Epigenetic flexibility in metabolic regulation: disease cause
and prevention? Trends Cell Biol. 23:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Y, Gu P, Liu K and Huang P: Maternal
protein restriction in rats leads to reduced PGC-1α expression via
altered DNA methylation in skeletal muscle. Mol Med Rep. 7:306–312.
2013.PubMed/NCBI
|
|
14
|
Yang BT, Dayeh TA, Volkov PA, et al:
Increased DNA methylation and decreased expression of PDX-1 in
pancreatic islets from patients with type 2 diabetes. Mol
Endocrinol. 26:1203–1212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZH, Chen LL, Deng XL, et al:
Methylation status of CpG sites in the MCP-1 promoter is correlated
to serum MCP-1 in type 2 diabetes. J Endocrinol Invest. 35:585–589.
2012.PubMed/NCBI
|
|
16
|
Yang M, Sun JZ, Sun YL, You W, Dai J and
Li GS: Association between leptin gene promoter methylation and
type 2 diabetes mellitus. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
29:474–477. 2012.(In Chinese).
|
|
17
|
Burdge GC, Hoile SP and Lillycrop KA:
Epigenetics: are there implications for personalised nutrition?
Curr Opin Clin Nutr Metab Care. 15:442–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Wang J, Khaled W, et al: Bcl11a is
essential for lymphoid development and negatively regulates p53. J
Exp Med. 209:2467–2483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uda M, Galanello R, Sanna S, et al:
Genome-wide association study shows BCL11A associated with
persistent fetal hemoglobin and amelioration of the phenotype of
beta-thalassemia. Proc Natl Acad Sci USA. 105:1620–1625. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii N, Wei M, Kakehashi A, et al:
Enhanced urinary bladder, liver and colon carcinogenesis in zucker
diabetic fatty rats in a multiorgan carcinogenesis bioassay:
Evidence for mechanisms involving activation of PI3K signaling and
impairment of p53 on urinary bladder carcinogenesis. J Toxicol
Pathol. 24:25–36. 2011. View Article : Google Scholar
|
|
21
|
Labie D: BCL11A controls the expression of
the human fetal hemoglobin. Med Sci (Paris). 28:923–925. 2012.(In
French).
|
|
22
|
Xu J, Bauer DE, Kerenyi MA, et al:
Corepressor-dependent silencing of fetal hemoglobin expression by
BCL11A. Proc Natl Acad Sci USA. 110:6518–6523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pardini VC, Victória IM, Pieroni FB, et
al: Fetal hemoglobin levels are related to metabolic control in
diabetic subjects. Braz J Med Biol Res. 32:695–701. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simonis-Bik AM, Nijpels G, van Haeften TW,
et al: Gene variants in the novel type 2 diabetes loci
CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different
aspects of pancreatic beta-cell function. Diabetes. 59:293–301.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jonsson A, Ladenvall C, Ahluwalia TS, et
al: Effects of common genetic variants associated with type 2
diabetes and glycemic traits on α- and β-cell function and insulin
action in humans. Diabetes. 62:2978–2983. 2013.
|
|
26
|
Cauchi S, Ezzidi I, El Achhab Y, et al:
European genetic variants associated with type 2 diabetes in North
African Arabs. Diabetes Metab. 38:316–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langberg KA, Ma L, Sharma NK, et al:
American Diabetes Association GENNID Study Group: Single nucleotide
polymorphisms in JAZF1 and BCL11A gene are nominally associated
with type 2 diabetes in African-American families from the GENNID
study. J Hum Genet. 57:57–61. 2012. View Article : Google Scholar
|
|
28
|
Definition and diagnosis of diabetes
mellitus and intermediate hyperglycemia: report of a WHO/IDF
consultation. World Health Organization; Geneva, Switzerland: pp.
212006
|
|
29
|
Lolekha PH, Srisawasdi P, Jearanaikoon P,
Wetprasit N, Sriwanthana B and Kroll MH: Performance of four
sources of cholesterol oxidase for serum cholesterol determination
by the enzymatic endpoint method. Clin Chim Acta. 339:135–145.
2004.PubMed/NCBI
|
|
30
|
Schumann G, Klauke R, Canalias F, et al:
IFCC primary reference procedures for the measurement of catalytic
activity concentrations of enzymes at 37°C. Part 9: reference
procedure for the measurement of catalytic concentration of
alkaline phosphatase International Federation of Clinical Chemistry
and Laboratory Medicine (IFCC) Scientific Division, Committee on
Reference Systems of Enzymes (C-RSE) (1)). Clin Chem Lab Med.
49:1439–1446. 2011.
|
|
31
|
Guo JA, Mo PS and Li GX: Immobilization of
glucose oxidase and peroxidase and their application in
flow-injection analysis for glucose in serum. Appl Biochem
Biotechnol. 23:15–24. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang D, Zheng D, Wang L, et al: Elevated
PLA2G7 gene promoter methylation as a gender-specific marker of
aging increases the risk of coronary heart disease in females. PLoS
One. 8:e597522013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harada H, Miyamoto K, Yamashita Y, et al:
Methylation of breast cancer susceptibility gene 1 (BRCA1) predicts
recurrence in patients with curatively resected stage I non-small
cell lung cancer. Cancer. 119:792–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng J, Wang L, Xu L, et al:
Gender-dependent miR-375 promoter methylation and the risk of type
2 diabetes. Exp Ther Med. 5:1687–1692. 2013.PubMed/NCBI
|
|
35
|
Legato MJ, Gelzer A, Goland R, et al;
Writing Group for The Partnership for Gender-Specific Medicine.
Gender-specific care of the patient with diabetes: review and
recommendations. Gend Med. 3:131–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huxley R, Barzi F and Woodward M: Excess
risk of fatal coronary heart disease associated with diabetes in
men and women: meta-analysis of 37 prospective cohort studies. BMJ.
332:73–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alzahrani SH, Hess K, Price JF, et al:
Gender-specific alterations in fibrin structure function in type 2
diabetes: associations with cardiometabolic and vascular markers. J
Clin Endocrinol Metab. 97:E2282–E2287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dekker LH, Nicolaou M, van der ADL, et al:
Sex differences in the association between serum ferritin and
fasting glucose in type 2 diabetes among South Asian Surinamese,
African Surinamese, and ethnic Dutch: the population-based SUNSET
study. Diabetes Care. 36:965–971. 2013. View Article : Google Scholar
|
|
39
|
Burghardt KJ, Pilsner JR, Bly MJ and
Ellingrod VL: DNA methylation in schizophrenia subjects: gender and
MTHFR 677C/T genotype differences. Epigenomics. 4:261–268. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zoppini G, Negri C, Stoico V, Casati S,
Pichiri I and Bonora E: Triglyceride-high-density lipoprotein
cholesterol is associated with microvascular complications in type
2 diabetes mellitus. Metabolism. 61:22–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henderson SR, Maitland R, Mustafa OG,
Miell J, Crook MA and Kottegoda SR: Severe hypertriglyceridaemia in
type 2 diabetes mellitus: beneficial effect of continuous insulin
infusion. QJM. 106:355–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kulis M, Queiros AC, Beekman R and
Martin-Subero JI: Intragenic DNA methylation in transcriptional
regulation, normal differentiation and cancer. Biochim Biophys
Acta. 1829:1161–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|