Introduction
As a major global chronic health problem, type 2
diabetes affects 336 million people worldwide (1). Type 2 diabetes develops when
pancreatic β cells fail to secrete enough insulin in the face of
insulin resistance or increased insulin demand, most likely due to
β-cell exhaustion (2). Therefore,
it is critical to reveal the mechanism of β-cell compensation and
prevent the progression of β-cell decompensation for the long-term
management of the disease.
Cells continually adjust their gene expression
profiles in order to adapt to the availability of nutrients such as
glucose (3,4). The majority of nonproliferating,
differentiated cells depend on the efficiency of ATP production
through oxidative phosphorylation to maintain their integrity.
These cells metabolize glucose to pyruvate through glycolysis
following which they oxidize the majority of the pyruvate to
CO2 through the tricarboxylic acid (TCA) cycle in the
mitochondria (5). In lipogenic
tissues or rapidly proliferating cells, citrate, generated by the
TCA cycle from glucose, is preferentially exported from the
mitochondria to the cytosol and cleaved by ATP citrate lyase to
produce cytosolic acetyl coenzyme A (acetyl-CoA) (6–8),
which is a vital building block for the de novo biosynthesis
of fatty acids and cholesterol. Previous studies have reported that
islets from mice with a specific inactivation of the ATP-binding
cassette transporter 1 (Abca1), a cellular cholesterol transporter,
in their β cells, demonstrated altered cholesterol homeostasis and
impaired insulin secretion (9–11).
This suggests that abnormality of the cholesterol metabolism may
contribute to the impaired β-cell function in diabetes. However, as
glucose is a major regulator of pancreatic β-cell function and the
main source of precursors (including acetyl-CoA) for cholesterol
synthesis, whether high levels of glucose regulate the expression
levels of genes responsible for de novo cholesterol
biosynthesis in islets has not yet been validated.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum and other
culture reagents were obtained from Gibco Life Technologies (Grand
Island, NY, USA). The cell culture plates were purchased from Nalge
Nunc International (Roskilde, Denmark). Collagenase type XI was
purchased from Sigma (St. Louis, MO, USA). The
anti-3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr) antibody was
purchased from Abcam (Cambridge, MA, USA). Anti-rabbit IgG
conjugated with horseradish peroxidase was obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
Rat infusions
The animal treatment was reviewed and approved by
the Animal Care Committee of Shanghai Jiao Tong University School
of Medicine (Shanghai, China). Male Sprague Dawley rats (Shanghai
Laboratory Animal Center, Shanghai, China), weighing 250–300 g,
were housed under a controlled temperature (21°C) and a 12 h
light-dark cycle with unrestricted access to water and a standard
laboratory diet. The animals were randomly divided into two groups,
4 rats in each group, receiving either saline or glucose, with
heparin (40 U/ml). The infusion technique was similar to that
described by Bonner-Weir et al (12). Under general anesthesia, indwelling
catheters were inserted into the right jugular vein. The catheters
were tunneled subcutaneously and exteriorized at the base of the
neck. Following a recovery period of three to five days, the rats
were infused (via the jugular vein catheter at 2 ml/h) with either
0.45% saline or 50% glucose in 0.45% saline, for 12, 24, 48 and 72
h. Animals were allowed access to food and water ad libitum
during the infusion period. Upon completion of the infusion, the
animals were sacrificed and their islets were isolated. The
isolated islets were stored at −80°C with TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) until RNA isolation.
Islet isolation and treatment
Islets of Langerhans were isolated from male Sprague
Dawley rats by an in situ infusion of the pancreas with
collagenase, and separated by density gradient centrifugation
(13). The concentration of
collagenase type XI was 0.5 mg/ml. Isolated rat islets were
transferred to 6-well plates (300 islets per well) and cultured for
6, 12, 24, 48 or 72 h in RPMI-1640 medium containing 3.3, 8.3, 11.1
or 16.7 mmol/l glucose, supplemented with 0.25% bovine serum
albumin (BSA) at 37°C and with 5% CO2.
RNA sample preparation and array
hybridization
Total RNA was extracted from isolated islets using
TRIzol according to the manufacturer’s instructions. Sample
labeling and array hybridization were performed according to the
instructions for the Agilent One-Color Microarray-Based Gene
Expression Analysis protocol (Agilent Technologies, Inc., Santa
Clara, CA, USA). The total RNA from each sample was linearly
amplified and labeled with Cy3-CTP (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The labeled cRNAs were purified using an
RNeasy Mini kit (Qiagen, Hilden, Germany). The concentration and
specific activity (pmol Cy3/μg cRNA) of the labeled cRNAs were
measured by NanoDrop (ND-1000; Thermo Scientific, Wilmington, DE,
USA). A total of 1 μg of each labeled cRNA was fragmented by adding
11 μl of 10X blocking agent and 2.2 μl of 25X fragmentation buffer
(both from Agilent Technologies, Inc.,). The mixture was heated at
60°C for 30 min and 55 μl 2X gene expression (GE) hybridization
buffer (Agilent Technologies, Inc.,) was added to dilute the
labeled cRNA. A total of 100 μl of the hybridization solution was
dispensed into a gasket slide and assembled to the gene expression
microarray slide. The slides were incubated for 17 h at 65°C in an
Agilent Hybridization Oven (Agilent Technologies, Inc.). The
hybridized arrays were washed and scanned using the Agilent DNA
Microarray Scanner system (part no. G2565BA; Agilent Technologies,
Inc.).
Microarray data analysis
Agilent Feature Extraction software (version
11.0.1.1; Agilent Technologies, Inc.) was used to analyze the
acquired array images. Quantile normalization and subsequent data
processing were performed using the GeneSpring GX software package
(version 11.5.1; Agilent Technologies, Inc.). Differentially
expressed genes were identified through volcano plot filtering.
Gene ontology (GO) and pathway analyses were performed using the
standard enrichment computation method.
Quantitative polymerase chain reaction
(qPCR)
The total RNA isolated from the islets was
reverse-transcribed using a Promega Reverse Transcription kit
(Promega Corporation, Madison, WI, USA). In order to quantify the
transcript abundance of the genes of interest, qPCR was performed
using SYBR Green Premix Ex Taq (Takara Bio, Inc., Shiga, Japan)
with a 7300 Real-Time PCR machine (Applied Biosystems, Foster City,
CA, USA). The relative expression levels were normalized to 18S
mRNA levels in each sample. The sequences of the specific primers
that were used were as follows: 3-hydroxy-3-methylglutaryl-CoA
synthase 1 (Hmgcs1), 5′-GTCCCTCCACAAATGACCAC-3′ (forward), 3′-ATG
ACAGCCGACTCAGGTTC-5′ (reverse); Hmgcr, 5′-TGCTGCTTTGGCTGTATGTC-3′
(forward), 3′-TGAGCG TGAACAAGAACCAG-5′ (reverse); mevalonate kinase
(Mvk), 5′-TGGAGCAACTGGAGAAGCTG-3′ (forward),
3′-ATGTCCAGGCTTGGGAGTGT-5′ (reverse); phosphomevalonate kinase
(Pmvk), 5′-TCAGCTGTAGGCCTGGTG AA-3′ (forward),
3′-TGCTCCTTGAGTGGACCAGA-5′ (reverse); mevalonate (diphospho)
decarboxylase (Mvd), 5′-GAAAACTTCGCTGGCTACGG-3′ (forward) and
3′-CAACCCCTTTCTCCAGATGC-5′ (reverse); isopentenyl diphosphate
δ-isomerase 1 (Idi1), 5′-CACTGG CAGGAGTGATTGGA-3′ (forward),
3′-TTGCTGGCATTG ATTTCAGG-5′ (reverse); farnesyl diphosphate
farnesyl transferase 1 (Fdft1), 5′-ACATGGCCATCAGTGTGGAG-3′
(forward), 3′-AATTCTGCCATCCCACATCC-5′ (reverse); squalene epoxidase
(Sqle), 5′-TGGTGGAGGAATGACAGT CG-3′ (forward),
3′-AAGCAAGCTTTTCGGAGCTG-5′ (reverse); 7-dehydrocholesterol
reductase (Dhcr7), 5′-CGC TTCCAAAGCCAAGAATC-3′ (forward), 3′-ACACAA
TGAACGGTGCGAAG-5′ (reverse); sterol regulatory element binding
protein 1 (Srebp1), 5′-CATCCGCTT CTTACAGCACA-3′ (forward),
3′-TCATGCCCTCCA TAGACACA-5′ (reverse); 18S, 5′-CACGGGTGACGGGGA
ATCAG-3′ (forward), 3′-CGGGTCGGGAGTGGGTAA TTTG-5′ (reverse).
Western blot analysis
The cultured islets in the 6-well plates were washed
twice with ice-cold phosphate-buffered saline (PBS) and immediately
placed into a lysis buffer containing 25 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4),
1% Nonidet P-40, 100 mmol/l NaCl, 2% glycerol, 5 mmol/l NaF, 1
mmol/l ethylenediamine tetraacetic acid (EDTA), 1 mmol/l
Na3VO4, 1 mmol/l sodium pyrophosphate
(NaPPi), 1 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml
aprotinin, 5 μg/ml leupeptin and 5 μg/ml pepstatin. Lysates were
centrifuged at 14,000 × g for 10 min at 4°C. The protein
concentration of the extracts was determined according to the
Bradford method, using BSA as the standard. Samples were separated
by sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) on 8% polyacrylamide gels and transferred to
polyvinylidene fluoride (PVDF)-Plus membranes (Bio-Rad, Hercules,
CA, USA). The transferred membranes were incubated with anti-Hmgcr
antibody at 1:1,000 dilution overnight at 4°C. Primary antibodies
were detected with donkey anti-rabbit IgG conjugated with
horseradish peroxidase at 1:2,000 for 1 h at room temperature. The
blotted membrane was developed with enhanced chemiluminescence
(ECL) Advance (Cell Signaling Technology, Inc., Boston, MA, USA)
and imaged with a LAS-4000 Super CCD Remote Control Science Imaging
system (Fujifilm, Tokyo, Japan).
Statistical analysis
Data are presented as mean ± standard error of the
mean. Comparisons were performed using analysis of variance (ANOVA)
for multiple groups or the Student’s t-test for two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Genes involved in cholesterol
biosynthesis are upregulated in rat islets exposed to high levels
of glucose
To identify the specific targets responsible for the
adaptation to high levels of glucose, the differentially expressed
genes from primary rat islets treated with 3.3 and 16.7 mM glucose
for 24 h were detected by a whole-genome DNA microarray. Among a
total of 44,000 probe sets, 894 genes were upregulated by high
levels of glucose, revealing changes of ≥2-fold. GO analysis was
used to identify the underlying biological themes in the genes in
response to treatment with high levels of glucose. As shown in
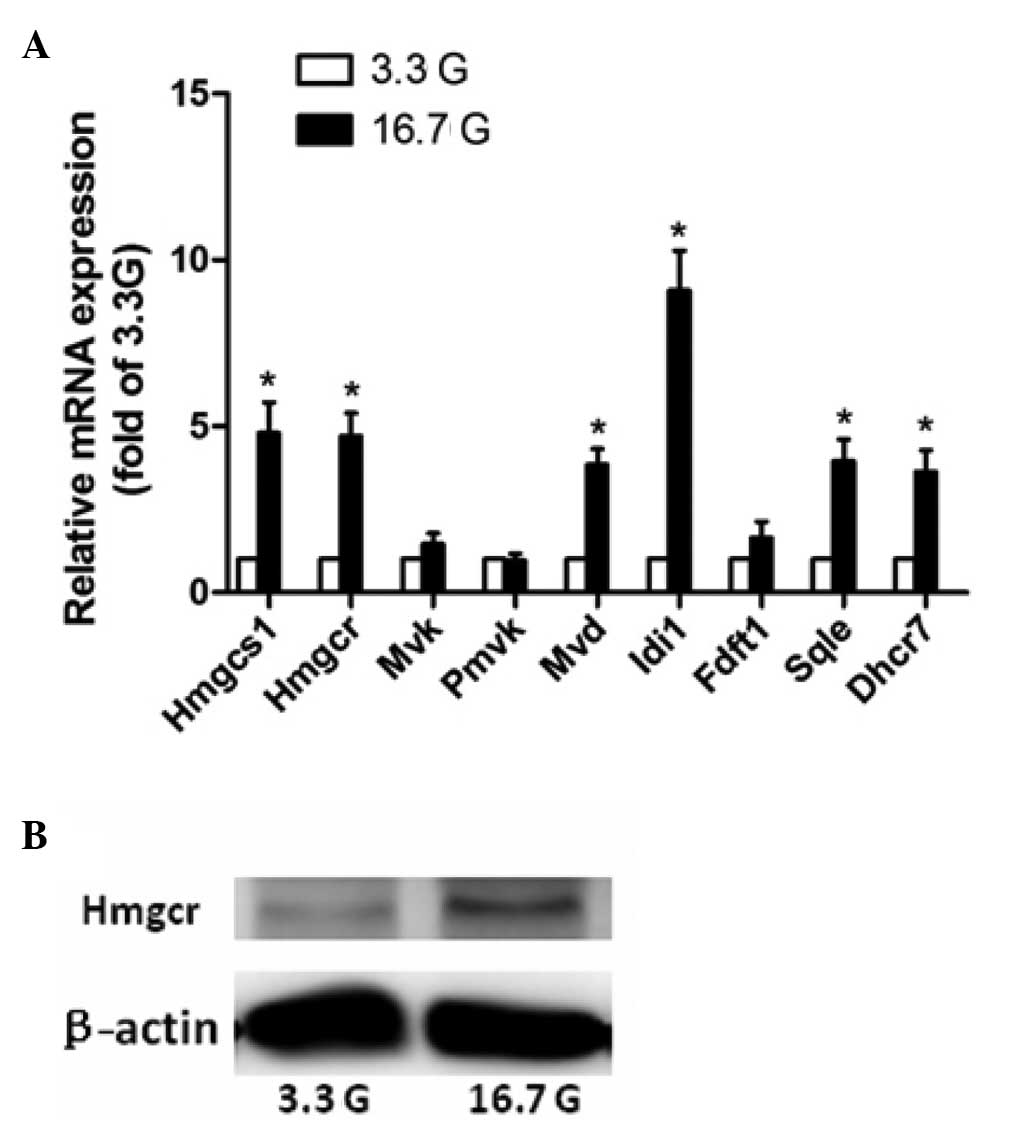
Table I, seven genes, termed in
the gene ontology as ‘cholesterol biosynthetic process’ (GO:
0006695), were significantly upregulated in group treated with high
levels of glucose. The qPCR results for Hmgcs1, Hmgcr, Mvd, Idi1,
Sqle and Dhcr7 confirmed the genomic analyses, whereas those for
Fdft1 did not (Fig. 1). Hmgcr, the
rate-limiting enzyme in cholesterol biosynthesis, catalyzes the
synthesis of mevalonate from hydroxymethyl glutaric acid acyl
coenzymeA. Western blot analysis revealed increased protein levels
of Hmgcr (Fig. 1). When combined,
these data demonstrate that high levels of glucose markedly
increased the expression levels of various genes involved in
cholesterol biosynthesis in rat islets.
 | Table IIncreased expression folds of
cholesterol biosynthesis-associated genes in isolated rat islets
incubated with 16.7 compared with 3.3 mmol/l glucose as
demonstrated by microarray analysis. |
Table I
Increased expression folds of
cholesterol biosynthesis-associated genes in isolated rat islets
incubated with 16.7 compared with 3.3 mmol/l glucose as
demonstrated by microarray analysis.
| Gene symbol | Gene name | Gene function | mRNA fold change |
|---|
| Hmgcs1 |
3-Hydroxy-3-methylglutaryl- CoA synthase
1 | Catalyzes the
conversion of (S)-3-hydroxy-3-methylglutaryl-CoA and CoA to
acetyl-CoA, acetoacetyl-CoA and H2O | 2.494 |
| Hmgcr |
3-Hydroxy-3-methylglutaryl- CoA
reductase | Enzyme involved in
mevalonate synthesis | 2.675 |
| Mvd | Mevalonate
(diphospho) decarboxylase | Enzyme that catalyzes
the conversion of mevalonate pyrophosphate to isopentenyl
pyrophosphate | 2.044 |
| Idi1 |
Isopentenyl-diphosphate δ-isomerase 1 | Catalyzes the
interconversion of isopentenyl diphosphate to dimethylallyl
diphosphate | 5.411 |
| Fdft1 | Farnesyl-diphosphate
farnesyl transferase 1 | Catalyzes the
conversion of trans-farnesyl diphosphate to squalene | 2.279 |
| Sqle | Squalene
epoxidase | Enzyme that catalyzes
sterol biosynthesis | 2.591 |
| Dhcr7 | 7-Dehydrocholesterol
reductase | Catalyzes the
reduction of 7-dehydrocholesterol and is involved in cholesterol
biosynthesis | 2.139 |
| Srebp1 | Sterol regulatory
element binding protein 1 | Transcription factor:
binds to the sterol regulatory element 1 | 2.289 |
Cholesterol biosynthetic gene expression
levels are increased in the islets from rats infused with high
levels of glucose
To further investigate the effect of high levels of
glucose on cholesterol biosynthetic gene expression levels in
vivo, the present study used a continuous glucose-infusion
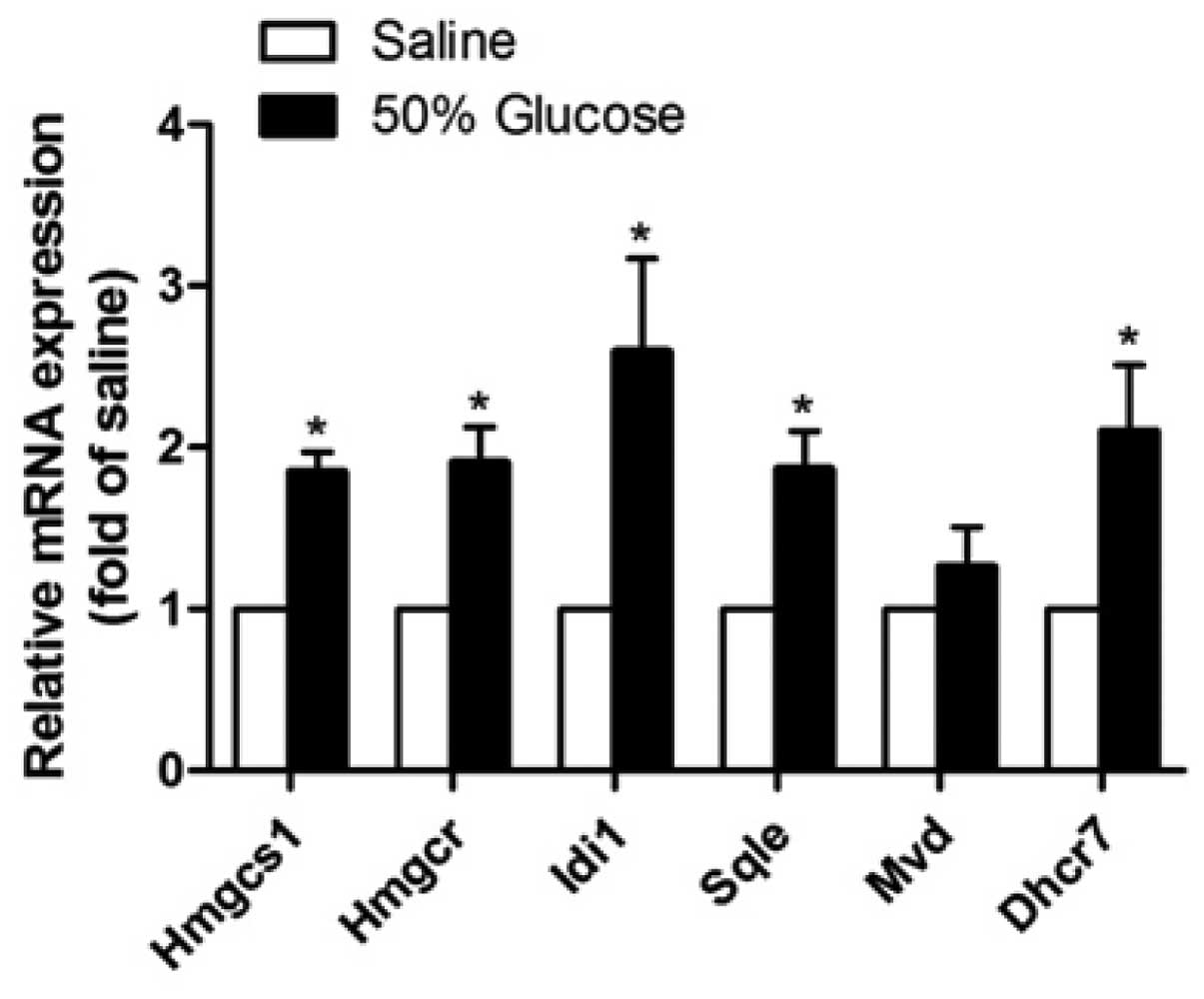
model as previously described (12). Infusion with 50% glucose (2 ml/h)
for 24 h causes a significant increase in the plasma glucose level
in rats (14,15). Consistent with the in vitro
results of the current study, Hmgcs1, Hmgcr, Idi1, Sqle and Dhcr7
mRNA expression levels were significantly higher in islets isolated
from rats infused with high levels of glucose for 24 h compared
with those in the saline-infused control rats (Fig. 2). Furthermore, the expression level
of the Idi1 gene revealed the highest increase (259±58%, P<0.05)
among all genes tested, in accordance with in vitro
results.
Glucose increases the expression levels
of cholesterol biosynthetic genes in islets in a dose-dependent
manner
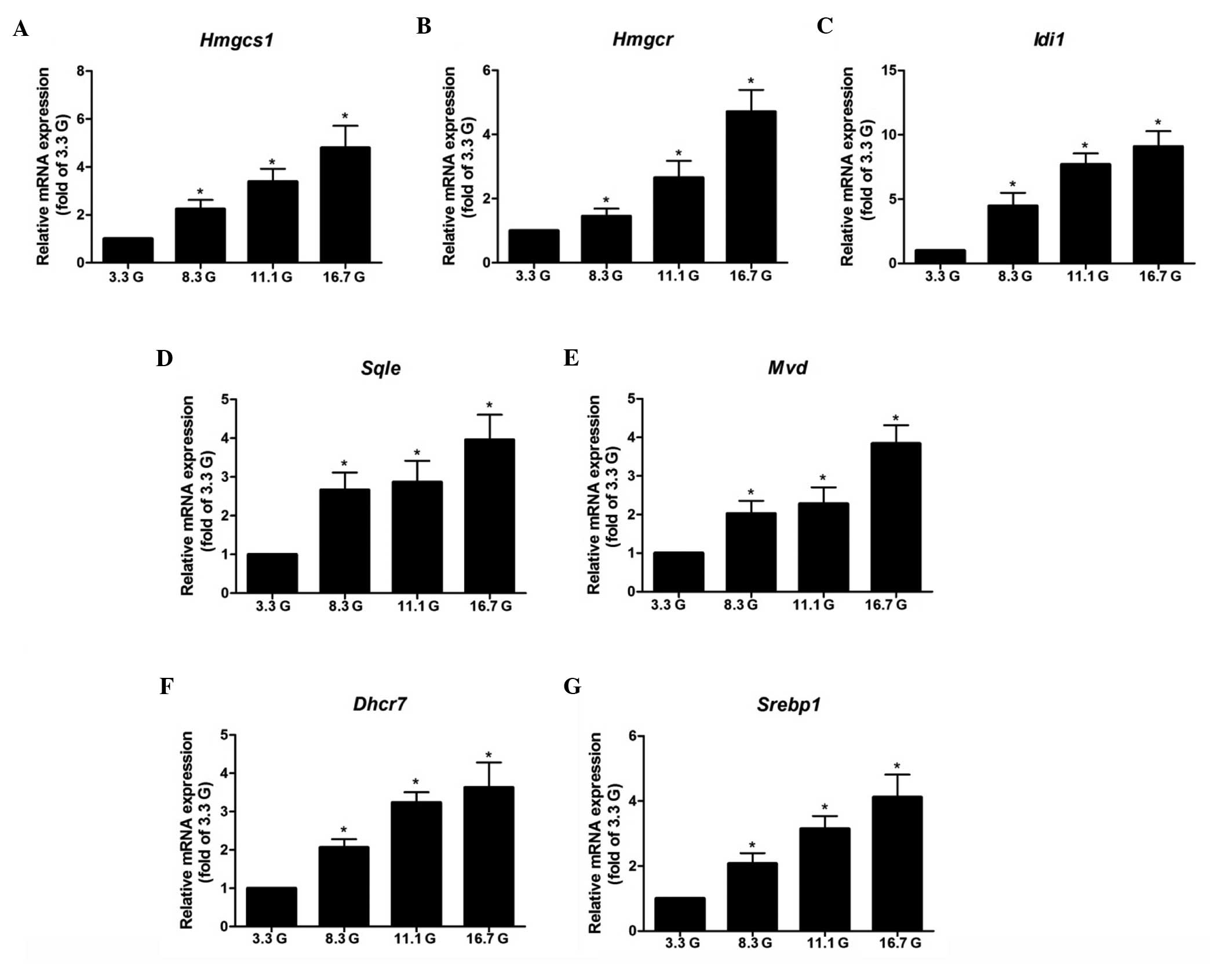
Isolated rat islets were incubated in 3.3, 8.3, 11.1
and 16.7 mmol/l glucose for 24 h. The qPCR results demonstrated
that glucose increased the mRNA expression levels of Hmgcs1, Hmgcr,
Mvd, Idi1, Sqle, Dhcr7 and Srebp1 in a dose-dependent manner, with
notable effects observed at the concentration of 8.3 mmol/l
(Fig. 3). The expression level of
the of Srebp1 gene, which functions as a transcription factor for
the gene expression of cholesterol biosynthetic enzymes, was also
assessed. The qPCR results revealed that glucose markedly enhanced
the expression level of Srebp1 in the same pattern as its target
genes (Fig. 3).
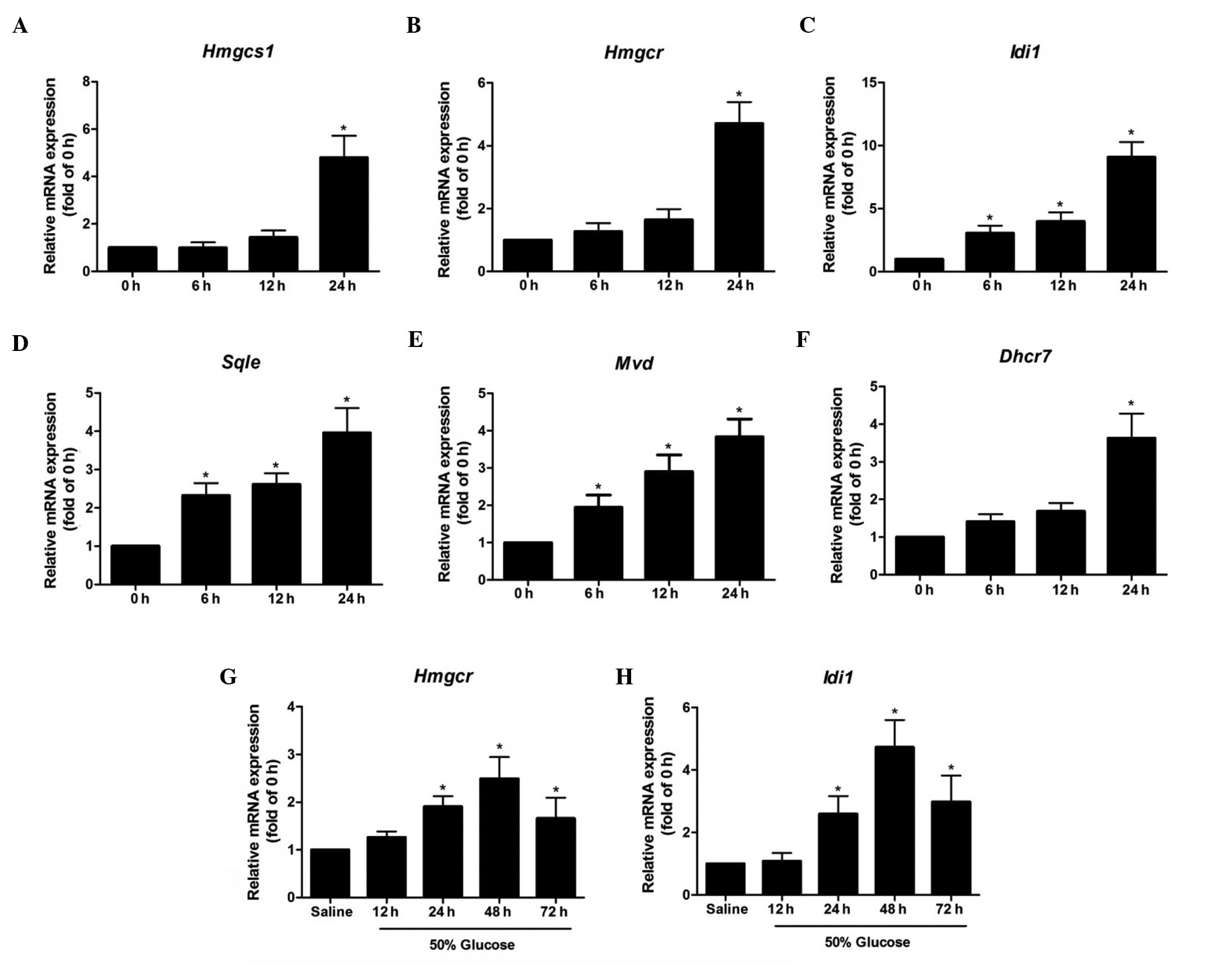
Time course for the expression of
cholesterol biosynthetic genes in islets exposed to high levels of
glucose in vitro or in vivo
The time-dependent effects of high levels of glucose
on the expression levels of cholesterol biosynthetic genes in
isolated rat islets were detected (Fig. 4). In islets exposed to 16.7 mmol/l
glucose for 6 and 12 h, the mRNA levels of Hmgcs1, Hmgcr and Dhcr7
remained comparable with those at 0 h, and increased significantly
at 24 h by 380±92, 371±68, and 263±65%, respectively (P<0.05).
However, the gene expression levels of Sqle and Mvd were increased
~2-fold by high levels of glucose at 6 h, and increased markedly
with the lengthening of the exposure time. The Idi1 gene exhibited
the most notable change in expression level among the genes tested,
increasing by 206±59% at 6 h and 808±119% at 24 h (P<0.05). In
islets isolated from 50% glucose-infused rats for 12 h, the mRNA
level of Hmgcr remained unchanged when compared with that of the
saline control group; however, it significantly increased with the
lengthening of the infusion time to 24 h, and reached a peak level
after 48 h (Fig. 4). The
expression level pattern of the Idi1 gene was similar to that of
Hmgcr in islets from glucose-infused rats (Fig. 4).
Discussion
As a main source for precursors (including
acetyl-CoA), glucose has notable long-term actions on the de
novo biosynthesis of lipids (16). Numerous studies investigating the
link between chronic high levels of glucose and pancreatic β-cell
lipid biosynthesis have focused on fatty acids (FAs). Chronic high
levels of glucose markedly enhance the gene expression levels of
acetyl-CoA carboxylase and fatty acid synthase and induce de
novo fatty acid synthesis in pancreatic β cells (17–19).
In the present study, the expression levels of genes involved in
de novo cholesterol biosynthesis (Hmgcs1, Hmgcr, Mvd, Idi1,
Sqle and Dhcr7) were significantly increased in islets treated with
high levels of glucose. These results were further confirmed in
islets isolated from rats subjected to 12, 24, 48 and 72 h
continuous glucose infusion. This suggests that high levels of
glucose may enhance cholesterol biosynthesis in the islets through
increasing the gene expression levels of associated enzymes.
Cholesterol is synthesized via a cascade of
enzymatic reactions known as the mevalonate pathway, which involves
>20 enzymes from several subcellular compartments (20). This series of reactions is
primarily regulated by a rate-limiting step involving the
conversion of hydroxylmethylglutaryl-coenzyme A (HMG-CoA) into
mevalonate. The rate-limiting reduction of HMG-CoA to mevalonate is
an important regulatory step in cholesterol synthesis (21). The results of the present study
revealed that high levels of glucose enhanced the gene expression
levels of Hmgcr in rat islets in a dose-dependent manner when the
cells were exposed to them for 24 h, and exposure to high levels of
glucose also increased the protein levels of the gene. The qPCR
results from glucose-infused rat islets confirmed this effect of
high levels of glucose in vivo. Hmgcr acts early in the
cholesterol synthesis pathway, with >20 subsequent enzymes
required to produce cholesterol. The process of their regulation
remains largely unstudied. The current study has provided the first
published evidence, to the best of our knowledge, that high levels
of glucose significantly increase the gene expression levels of
Mvd, Idi1, Sqle and Dhcr7 in rat islets; these genes encode enzymes
that serve as flux-controlling points in the cholesterol synthesis
process beyond Hmgcr.
It has long been known that chronic high levels of
glucose stimulate β-cell proliferation (12,22–26);
however, the mechanism underlying the role of glucose in these
events remains to be fully determined. Cell proliferation requires
nutrients, energy and biosynthetic activity to duplicate all the
macromolecular components during each passage through the cell
cycle (27). The cholesterol
content and rate of cholesterol biosynthesis are elevated in
proliferating normal tissues and a reduction in cholesterol
biosynthesis inhibits cell growth (28). This suggests the presence of a link
between cell proliferation and the cholesterol biosynthetic
pathway. The mevalonate pathway is also a crucial biochemical
process for the generation of other key metabolic end products.
There is an expanding list of intermediates that are known to be
involved in cholesterol synthesis, and which have been credited
with regulatory functions in the control of cell growth (28). Farnesyl diphosphate and other
phosphorylated products of the mevalonate pathway are essential to
the post-translational processing and physiological function of
small G proteins, nuclear lamins and growth factor receptors
(29–30), which are crucially involved in the
regulation of proliferation. In addition, the expression levels of
the steroidogenic acute regulatory protein (StAR) and
steroid-5-α-reductase (Srd5a1), involved in the synthesis of
steroid hormones from cholesterol, were observed to be increased by
treatment with high levels of glucose in the DNA microarray data in
the present study (data not shown). The regulatory mechanism of
these genes by glucose and the possible role of these intermediates
in glucose-stimulated β-cell proliferation requires further
investigation.
In conclusion, based on the microarray analysis and
qPCR validation, the present study identified a set of genes
encoding cholesterol biosynthetic enzymes that were induced by high
levels of glucose in rat islets. The current results provide
evidence that enhanced cholesterol biosynthesis may play a role in
the adaptive changes of β cells to high levels of glucose in
vivo and in vitro.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30600294, 81170720 and
81270910), Natural Science Foundation of Shanghai (11ZR1429500),
Science and Technology Funds from Pudong New Area (PKJ2012-Y07),
and the Academic Leaders Training Program of Pudong Health Bureau
of Shanghai (PWRd2011-01).
Abbreviations:
|
TCA
|
tricarboxylic acid
|
|
BSA
|
bovine serum albumin
|
|
Hmgcs1
|
3-hydroxy-3-methylglutaryl-CoA
synthase 1
|
|
Hmgcr
|
3-hydroxy-3-methylglutaryl-CoA
reductase
|
|
Mvk
|
mevalonate kinase
|
|
Pmvk
|
phosphomevalonate kinase
|
|
Mvd
|
mevalonate (diphospho)
decarboxylase
|
|
Idi1
|
isopentenyl-diphosphate δ-isomerase
1
|
|
Fdft1
|
farnesyl diphosphate farnesyl
transferase 1
|
|
Dhcr7
|
7-dehydrocholesterol reductase
|
|
Sqle
|
squalene epoxidase
|
|
Srebp1
|
sterol regulatory element binding
protein 1
|
|
HMG-CoA
|
hydroxymethylglutaryl-coenzyme A
|
References
|
1
|
Ashcroft FM and Rorsman P: Diabetes
mellitus and the β cell: the last ten years. Cell. 148:1160–1171.
2012.
|
|
2
|
Shi Y, Taylor SI, Tan SL and Sonenberg N:
When translation meets metabolism: multiple links to diabetes.
Endocr Rev. 24:91–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganapathy V, Thangaraju M and Prasad PD:
Nutrient transporters in cancer: relevance to Warburg hypothesis
and beyond. Pharmacol Ther. 121:29–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiaschi T, Marini A, Giannoni E, Taddei
ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P and
Chiarugi P: Reciprocal metabolic reprogramming through lactate
shuttle coordinately influences tumor-stroma interplay. Cancer Res.
72:5130–5140. 2012. View Article : Google Scholar
|
|
5
|
Ward PS and Thompson CB: Metabolic
reprogramming: a cancer hallmark even Warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wellen KE, Hatzivassiliou G, Sachdeva UM,
Bui TV, Cross JR and Thompson CB: ATP-citrate lyase links cellular
metabolism to histone acetylation. Science. 324:1076–1080. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chypre M, Zaidi N and Smans K: ATP-citrate
lyase: a mini-review. Biochem Biophys Res Commun. 422:1–4. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beigneux AP, Kosinski C, Gavino B, Horton
JD, Skarnes WC and Young SG: ATP-citrate lyase deficiency in the
mouse. J Biol Chem. 279:9557–9564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brunham LR, Kruit JK, Pape TD, Timmins JM,
Reuwer AQ, Vasanji Z, et al: β-cell ABCA1 influences insulin
secretion, glucose homeostasis and response to thiazolidinedione
treatment. Nat Med. 13:340–347. 2007.
|
|
10
|
Kruit JK, Wijesekara N, Fox JE, Dai XQ,
Brunham LR, Searle GJ, et al: Islet cholesterol accumulation due to
loss of ABCA1 leads to impaired exocytosis of insulin granules.
Diabetes. 60:3186–3196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wijesekara N, Zhang LH, Kang MH, Abraham
T, Bhattacharjee A, Warnock GL, Verchere CB and Hayden MR: miR-33a
modulates ABCA1 expression, cholesterol accumulation, and insulin
secretion in pancreatic islets. Diabetes. 61:653–658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonner-Weir S, Deery D, Leahy JL and Weir
GC: Compensatory growth of pancreatic β-cells in adult rats after
short-term glucose infusion. Diabetes. 38:49–53. 1989.
|
|
13
|
Kinasiewicz A, Juszczak M, Pachecka J and
Fiedor P: Pancreatic islets isolation using different protocols
with in situ flushing and intraductal collagenase injection.
Physiol Res. 53:327–333. 2004.PubMed/NCBI
|
|
14
|
Zhang H, Li W, Wang Q, Wang X, Li F, Zhang
C, et al: Glucose-mediated repression of menin promotes pancreatic
β-cell proliferation. Endocrinology. 153:602–611. 2012.PubMed/NCBI
|
|
15
|
Ammon HP, Bacher M, Brändle WF, Waheed A,
Roenfeldt M, el-Sayed ME, Ahmed AA and Wahl MA: Effect of
forty-eight-hour glucose infusion into rats on islet ion fluxes,
ATP/ADP ratio and redox ratios of pyridine nucleotides. J
Endocrinol. 156:583–590. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dashty M: A quick look at biochemistry:
carbohydrate metabolism. Clin Biochem. 46:1339–1352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roche E, Farfari S, Witters LA,
Assimacopoulos-Jeannet F, Thumelin S, Brun T, Corkey BE, Saha AK
and Prentki M: Long-term exposure of β-INS cells to high glucose
concentrations increases anaplerosis, lipogenesis, and lipogenic
gene expression. Diabetes. 47:1086–1094. 1998.
|
|
18
|
Berne C: The metabolism of lipids in mouse
pancreatic islets: the biosynthesis of triacylglycerols and
phospholipids. Biochem J. 152:667–673. 1975.PubMed/NCBI
|
|
19
|
Diraison F, Ravier MA, Richards SK, Smith
RM, Shimano H and Rutter GA: SREBP1 is required for the induction
by glucose of pancreatic β-cell genes involved in glucose sensing.
J Lipid Res. 49:814–822. 2008.PubMed/NCBI
|
|
20
|
Sharpe LJ and Brown AJ: Controlling
cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA
reductase (HMGCR). J Biol Chem. 288:18707–18715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Medina MW and Krauss RM: The role of HMGCR
alternative splicing in statin efficacy. Trends Cardiovasc Med.
19:173–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Swenne I: Effects of aging on the
regenerative capacity of the pancreatic B-cell of the rat.
Diabetes. 32:14–19. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swenne I: Glucose-stimulated DNA
replication of the pancreatic islets during the development of the
rat fetus. Effects of nutrients, growth hormone, and
triiodothyronine. Diabetes. 34:803–807. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chick WL: β-cell replication in rat
pancreatic monolayer cultures. Effects of glucose, tolbutamide,
glucocorticoid, growth hormone and glucagons. Diabetes. 22:687–693.
1973.
|
|
25
|
Paris M, Bernard-Kargar C, Berthault MF,
Bouwens L and Ktorza A: Specific and combined effects of insulin
and glucose on functional pancreatic β-cell mass in vivo in
adult rats. Endocrinology. 144:2717–2727. 2003.
|
|
26
|
Heit JJ, Karnik SK and Kim SK: Intrinsic
regulators of pancreatic β-cell proliferation. Annu Rev Cell Dev
Biol. 22:311–338. 2006.
|
|
27
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao KN: The significance of the
cholesterol biosynthetic pathway in cell growth and carcinogenesis
(review). Anticancer Res. 15:309–314. 1995.PubMed/NCBI
|
|
29
|
Thurnher M, Gruenbacher G and Nussbaumer
O: Regulation of mevalonate metabolism in cancer and immune cells.
Biochim Biophys Acta. 1831:1009–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mo H and Elson CE: Studies of the
isoprenoid-mediated inhibition of mevalonate synthesis applied to
cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood).
229:567–585. 2004.PubMed/NCBI
|