Introduction
Pancreatic fistula and anastomotic leakage are major
complications following pancreatic head resection or distal
pancreatectomy (1,2), which are often associated with
subsequent dangerous infectious complications, including
peritonitis and sepsis (3).
Therefore, these complications represent the leading cause of
mortality following pancreatic resection. A primary aim during
pancreatic surgery is to reduce the incidence rate of fistulas and
thus the mortality rate. Although the mortality rate has been
reduced in centers of excellence to <5% (4), the morbidity rate remains high
(2). The outcome of pancreatic
surgery, including the leakage rate, is dependent on the experience
of the center and the individual surgeon, as well as the surgical
technique. However, there are also patient-specific and
tissue-determined risk factors for pancreatic leakage or fistula,
which include the soft tissue texture of the pancreas (3) and a small pancreatic duct of <3 mm
(5–7). While these risk factors are widely
accepted, to date, no histological or molecular factors have been
identified. Postoperative pancreatic fistulas (POPFs) are a
consequence of inadequate regeneration of the pancreatic tissue
following resection and insufficient wound healing of the
pancreatic remnant. Wound healing is a complex process involving a
coordinated interplay of cells, extracellular matrix and numerous
regulatory mediators. It also includes an organized stimulation of
angiogenesis, fibroblast proliferation with stimulation of
extracellular matrix and the growth of epithelial tissue. The
process can be divided into three well-defined phases:
Inflammatory, proliferative and tissue remodeling phases (8). In each phase there are regulated and
regulatory mediators. Impaired wound healing has been hypothesized
to be caused by the dysfunctional coordination of wound healing
mediators, which may result in the overexpression or suppression of
certain factors (9). Impaired
healing of chronic wounds is known to be mediated by the
dysregulation of numerous factors (10,11),
including pro- and anti-inflammatory cytokines,
angiogenesis-associated proteins, proteins associated with diabetic
conditions and matrix metalloproteinases (MMPs) that remodel
damaged tissue.
The aim of the present study was to investigate
whether the tissues of patients with POPF may be predicted and
differentiated from those without complications using the molecular
composition of the pancreatic resection margins.
Materials and methods
Patients
Between August 2008 and November 2009, 435
pancreatic head resections and distal pancreatectomies were
performed at the Department of General Surgery of the University of
Heidelberg (Heidelberg, Germany). In total, 12% of these resections
developed a pancreatic fistula. In this study, 31 patients were
selected randomly. Indications for their pancreatic resection were
malignant pathologies (n=25) or chronic pancreatitis (n=6). The
tissue with the resection margins was preserved in each case.
Informed consent was obtained preoperatively from all the patients
(males, 19; females, 12; mean age, 63.8 years; age range, 48–79
years) and approval was obtained from the designated Ethics
Commission of the University of Heidelberg. Pancreaticoenteric
anastomosis was performed using the same method for all the
patients, as previously described (12). Experienced surgeons performed all
the resections. Patients without a fistula (n=16), as well as those
with POPF (n=15), were selected according to the criteria of the
International Study Group on Pancreatic Fistula definition
(1,2). POPF is the failure of healing/sealing
a pancreatic-enteric anastomosis or a parenchymal leak not directly
associated with an anastomosis, and was defined as a drain output
of any measurable volume on or following day three postoperatively,
with an amylase content greater than three times the serum amylase
activity. According to the clinical impact on the patient’s
hospital course, three different grades of POPF may be
differentiated (grades A, B and C) (4,13).
Tissue
Tissue samples were immediately shock frozen in
liquid nitrogen and fixed in formalin for paraffin embedding. For
multiplex protein analysis and immunohistochemistry (IHC), all the
cryopreserved samples were sectioned into aliquots and stored at
−80°C until required for further analysis.
Histological analysis
Paraffin-embedded pancreatic tissue sections were
cut into 3–4 μm slices using a microtome (Leica, Wetzlar, Germany),
deparaffinized, rehydrated in graded alcohols, stained with
hematoxylin and eosin, then dehydrated and examined using an
Axioplan 2 Imaging, Zeiss light microscope (Carl Zeiss, Goettingen,
Germany). An experienced pathologist investigated the samples for
their histological grade of fibrosis, lipomatous atrophy,
inflammatory activity, inflammatory infiltration and necrosis using
a semi-quantitative scoring system developed in consultation with a
specialized pathologist (Table
I).
 | Table IHistological grading scores. |
Table I
Histological grading scores.
| Grading | Fibrosis | Lipomatous
atrophy | Inflammatory
infiltration | Inflammatory
activity | Microscopic
necrosis |
|---|
| 0 | No | No | No | No | No |
| 1 | Periductal | Little | Little | little | Single cells |
| 2 | Periductal, intra-
and interlobular | Moderate | Moderate | Moderate | Grouped necrosis |
| 3 | Extensive | Severe | Severe | Severe | Broad |
Multiplex protein analysis
Tissue sample concentrations of the selected wound
healing mediators were determined using multiplex protein arrays
(Biorad, Biorad Laboratories GmbH, Munich, Germany), enabling
quantification of all the parameters in one sample. The factors
were assessed in four group panels: (i) The cytokine panel, using
the Bio-Plex Pro Human Cytokine 17-plex panel (Bio-Rad
Laboratories, Inc., Munich, Germany) composed of interleukin
(IL)-1β, -2, -4, -5, -6, -8, -10, -12, -13 and -17, granulocyte
colony-stimulating factor (G-CSF), granulocyte-macrophage
colony-stimulating factor, interferon-γ, monocyte chemotactic
protein-1, macrophage inflammatory protein-1β and tumor necrosis
factor (TNF)-α; (ii) the angiogenesis panel, using the Bio-Plex Pro
Human Angiogenesis 9-plex panel (Bio-Rad Laboratories, Inc.)
composed of vascular endothelial growth factor (VEGF),
angiopoetin-2, follistatin, G-CSF, hepatocyte growth factor, IL-8,
platelet-derived growth factor-BB, platelet endothelial cell
adhesion molecule-1 and leptin; (iii) the diabetes panel, using the
Bio-Plex Pro Human Diabetes 9-customized plex (Bio-Rad
Laboratories, Inc.) consisting of c-peptide, gastric inhibitory
polypeptide (GIP), ghrelin, glucagon-like peptide-1 (GLP-1),
insulin, leptin, plasminogen activator inhibitor-1, TNF-α and IL-6;
and (iv) the MMP panel (MMP-kit; R&D Systems, Abingdon, UK),
including MMP-1, -2, -3, -7, -8, -9, -12 and -13.
In order to quantify the 38 parameters, 100 mg
frozen tissue were cut with a cryotome in thin serial section
slices (Leica CM3050 S Cryostat; Leica Biosystems, Nussloch,
Germany) and subsequently ground with a mortar in liquid nitrogen.
The powder was transferred into prechilled 15-ml Falcon tubes and
500 μl lysis buffer (Bio-Rad Laboratories, Inc.) was added. The
suspension was subjected to a 30 sec sonification step on ice
(amplification 80%; 0.99 kJ; Sonoplus; Bandelin, Berlin, Germany)
and subsequently centrifuged at 16,000 × g for 10 min. Supernatants
were collected and the total protein concentration was determined
using the bicinchoninic acid assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). For multiplexing, the samples were adjusted
with a Sample Diluent Buffer (Bio-Rad Laboratories, Inc.) to a
final protein concentration of 2 mg/ml.
Multiplexing was performed in accordance with the
manufacturer’s instructions. Briefly, beads coated with anti-human
antibodies against the examined biomarker antigen were mixed with
200 μl each diluted patient sample (50 μl supernatant and 150 μl
dilution buffer) and then incubated for 30 min. Following a wash
cycle, a biotinylated detection antibody specific to another
epitope of the examined biomarker-antigen was added and the samples
were incubated for an additional 30 min. A second wash cycle was
then performed, after which streptavidin-phycoerythrin was added to
the beads and a third wash cycle was conducted, followed by
incubation for 10 min. Following the removal of excess conjugate,
the bead mixture was analyzed using a BioPlex 200 System (Bio-Rad
Laboratories, Inc.).
Raw data were initially measured as the relative
fluorescence intensity and then converted to a fluorescence ratio
using predyed internal standard beads (Bio-Rad Laboratories, Inc.).
A series of calibrators were analyzed with the patient samples to
convert the fluorescence ratio to international units per
milliliter. All the samples were measured in triplicate.
Overlapping analytes from different panels were analyzed and a
combined evaluation was performed. Standard curves and
concentrations were calculated using Bioplex Manager 6.1 software
(Bio-Rad Laboratories, Inc.).
IHC
For IHC detection, polyclonal antibodies against
IL-6 (1:500), IL-8 (1:25) and VEGF (1:100; Abcam, Cambridge, UK)
and monoclonal antibodies against MMP-1 (1:100; Merck Calbiochem,
Darmstadt, Germany) and MMP-2 (1:100; Dianova, Hamburg, Germany)
were used. Frozen sections were cut into 10-μm thick slices using a
Leica cryotome at −20°C. The IHC staining protocol was performed as
previously described (14). Tissue
sections were scored semi-quantitatively and the IHC scores were
assigned a numerical value in consultation with the pathologist.
The staining distribution and intensity were graded according to
the following criteria. Distribution of the staining: 0, no
staining; 1, focal staining; 2, moderate staining; and 3, diffuse
staining. Intensity of the staining: 0, none; 1, weak intensity; 2,
moderate intensity; and 3, strong intensity. All the slides were
examined by an experienced pathologist.
Statistical analysis
Continuous variables are expressed as the median and
range. The non-parametric Mann-Whitney U test was used to compare
the continuous variables between the two study groups. Categorical
variables are presented as absolute and relative frequencies, and
comparisons of the categorical variables between the two study
groups were performed using Fisher’s exact test. The exact
Cochran-Armitage trend test was used to compare three or four
categories. P<0.05 was considered to indicate a statistically
significant difference, and all the tests used were two-sided.
Statistical analysis was performed using the
GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA,
USA) and SAS software (Release 9.1; SAS Institute, Inc., Cary, NC,
USA).
Results
Histology
A histological comparison of the tissue sections
derived from the groups with and without a fistula revealed
differences in the grade of fibrosis, lipomatous atrophy,
inflammatory activity, inflammatory invasion and necrosis.
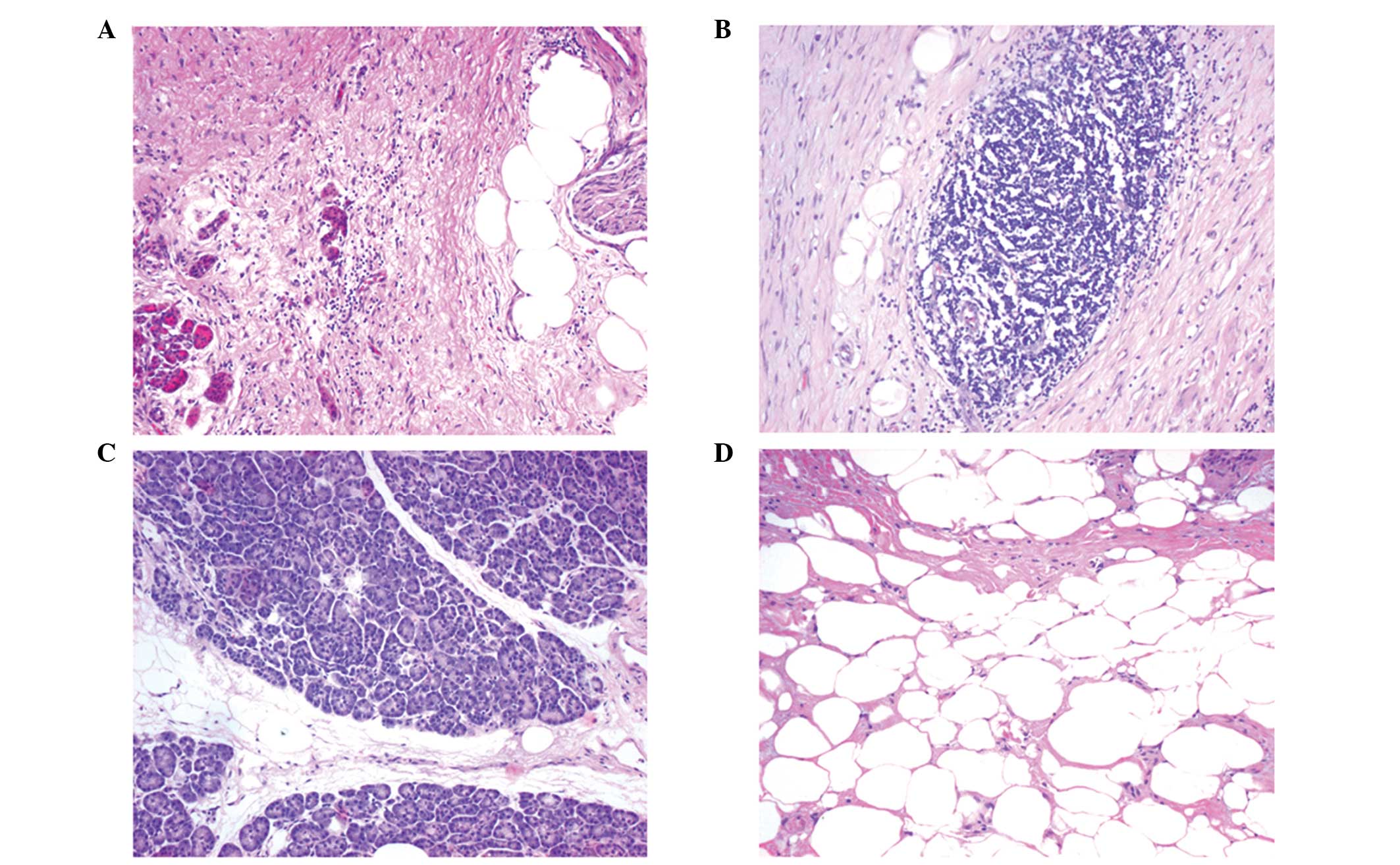
Representative histological sections for lipomatous atrophy,
chronic inflammatory infiltration and their grading are presented
in Fig. 1.
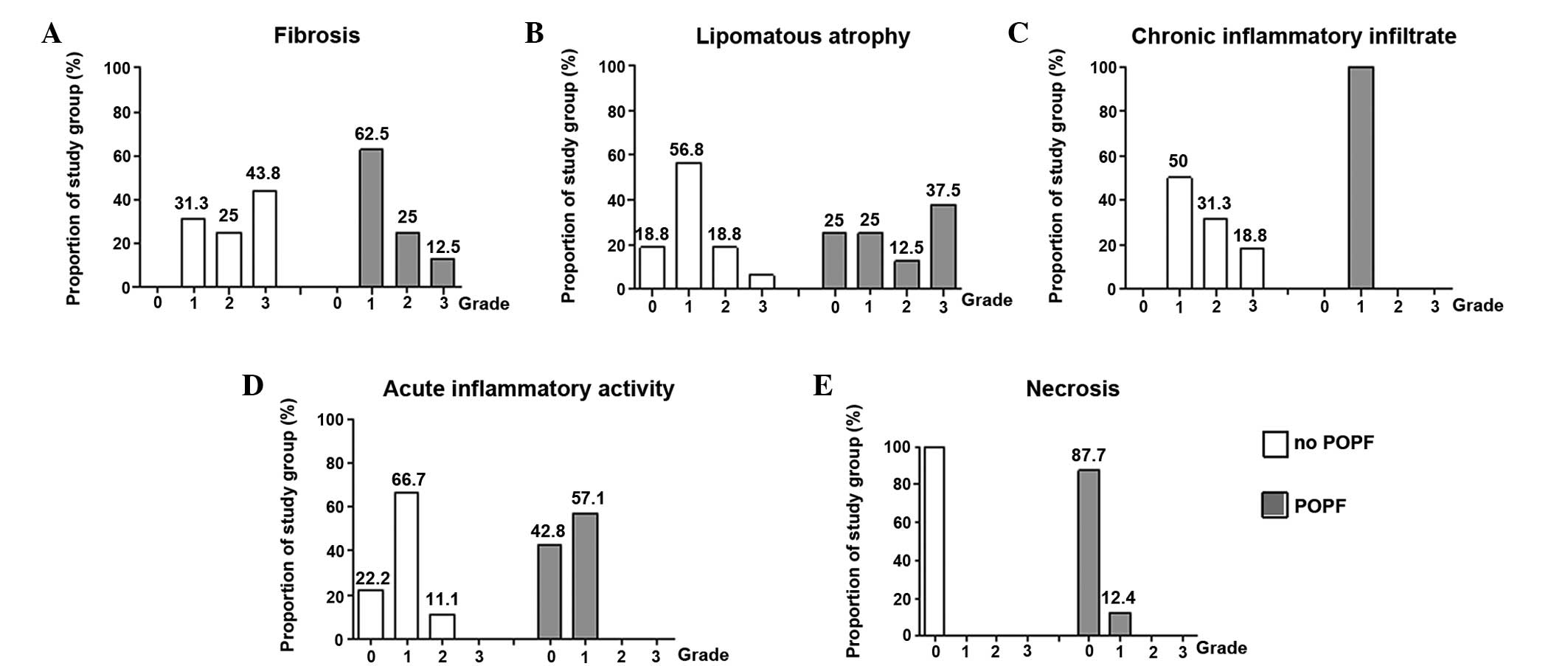
Fibrosis
Fibrosis was observed in all the samples to a
certain extent. The majority (62.5%) of the patients with a fistula
had weak periductal fibrosis (grade 1) compared with 31.3% in the
group without a fistula (Fig. 2A).
However, extensive fibrosis was more frequently observed in the
samples from the group without a fistula (43.8%) compared with the
group with a fistula (12.5%).
Lipomatous atrophy
Lipomatous atrophy was observed in the two groups
with and without fistulas; however, the distribution and extent of
lipomatous atrophy differed between the groups. In the group
without a fistula, the majority of the samples were classified as
little (56.3%) and rarely with severe lipomatous atrophy (6.3%),
whereas in the fistula group, the majority of the samples had
severe (37.5%) or little lipomatous atrophy (25%). These results
revealed more extensive lipomatous atrophy in the resection margins
from patients developing a pancreatic fistula (Fig. 2B).
Chronic inflammatory infiltration
In order to determine the grade of chronic
infiltration, the overall infiltration of inflammatory cells
(leukocytes, macrophages and plasma cells) was investigated. All
the resection edge tissues of the fistula group exhibited little
(grade 1) chronic inflammatory infiltration when compared with the
tissues without a fistula, which exhibited little (50% grade 1),
moderate (31.3% grade 2) or even severe chronic inflammatory
infiltration (18.8% grade 3). These observations indicated an
overall increased chronic inflammatory infiltration rate in the
samples without a fistula (Fig.
2C).
Acute inflammatory activity
To classify the degree of inflammatory activity, the
quantity of neutrophil granulocytes was evaluated. In the fistula
group, no neutrophil granulocytes were observed in 42.8% of the
individuals, while 57.1% of the group exhibited little acute
inflammatory activity. Furthermore, none of the patients with a
fistula exhibited moderate or severe inflammatory activity (grade 2
or 3). However, patients without a fistula exhibited no (22.2%),
little (66.7% grade 1) or even severe acute inflammatory
infiltration (11.1% grade 2). Similar to chronic inflammatory
infiltration, slightly increased acute inflammatory activity was
observed in the group without fistulas (Fig. 2D).
Necrosis
Of all the examined samples, only 12.4% showed
single cells with necrosis (grade 1) in the fistula group, while no
necrosis was observed in any case from the fistula free group
(Fig. 2E).
Proteins involved in the wound healing
process
Quantitative multiplex analysis of the resection
margins from all the patients with pancreatic head resections or
distal pancreatectomies was performed for 38 proteins. The analyzed
factors were selected due to their essential roles in the wound
healing process and their regulatory functions in inflammation,
neovascularization, glucose metabolism and tissue remodeling. The
results of the individual parameters are summarized in Table II.
 | Table IIQuantitative multiplexing protein
analysis. |
Table II
Quantitative multiplexing protein
analysis.
| No POPF, pg/mg total
protein (n=16) | POPF, pg/mg total
protein (n=15) | |
|---|
|
|
| |
|---|
| Analyte | Min | Median | Max | Min | Median | Max | P-valuea |
|---|
| IL-1β | 2.2 | 59.0 | 2427 | 13.2 | 71.3 | 1665 | 0.7529 |
| IL-2 | 2.44 | 2.4 | 318.7 | 2.4 | 2.4 | 1845 | 0.8615 |
| IL-4 | 4.7 | 9.6 | 16.7 | 4.6 | 9.2 | 16.7 | 0.8260 |
| IL-5 | 2.2 | 3.1 | 8.9 | 2.2 | 3.2 | 3.62 | 0.5990 |
| IL-6 | 1.7 | 301.3 | 11705 | 0.7 | 64.6 | 20730 | 0.0345 |
| IL-7 | 2.3 | 366 | 4579 | 36.9 | 1788 | 3116 | 0.3106b |
| IL-8 | 3.1 | 218 | 23614 | 2.7 | 46 | 1114 | 0.0029b |
| IL-10 | 2.3 | 8.2 | 26.9 | 69.5 | 881 | 1901 | <0.0001b |
| IL-12 | 3.1 | 331 | 1191 | 3.3 | 10.4 | 33,93 | 0.0045b |
| IL-13 | 50.0 | 90 | 4553 | 49.5 | 103.5 | 4283 | 0.9538 |
| IL-17 | 1.6 | 128.9 | 264.5 | 94.0 | 167.8 | 335 | 0.0399b |
| G-CSF | 1.3 | 301.3 | 2796 | 1.3 | 65 | 2414 | 0.6050 |
| IFN-γ | 0.0 | 370.9 | 12585 | 0.0 | 659.5 | 2714 | 0.3099 |
| GM-CSF | 560.4 | 898 | 2892 | 757.8 | 1057 | 1466 | 0.0570 |
| MCP-1 | 0.1 | 2882 | 34386 | 986.1 | 2901 | 23659 | 0.6334 |
| MIP-1β | 0.0 | 2033 | 8178 | 0.0 | 648.4 | 22307 | 0.1548 |
| TNF-α | 1.2 | 1.9 | 131.6 | 1.2 | 3.3 | 141 | 0.7136 |
| Angiopoietin-2 | 21.8 | 205.6 | 648.1 | 0 | 61.3 | 11684 | 0.1382 |
| Follistatin | 64.7 | 272.9 | 782.4 | 41.0 | 200.3 | 801.2 | 0.2949 |
| HGF | 128.6 | 3216 | 10962 | 132.4 | 3385 | 9905 | 0.8279 |
| PDGF-BB | 8.3 | 21.6 | 381.7 | 2.9 | 17.0 | 158.6 | 0.3365 |
| PECAM-1 | 10751 | 22656 | 22656 | 1528 | 18530 | 22656 | 0.2770 |
| VEGF | 3.6 | 38.5 | 226.4 | 1.0 | 49.2 | 176.8 | 0.3845 |
| C-peptide | 8.5 | 2691 | 2691 | 1.1 | 31.3 | 2691 | 0.0709 |
| Ghrelin | 2.0 | 11.6 | 485.9 | 2.0 | 71.2 | 287.8 | 0.1638 |
| GIP | 0.5 | 2.4 | 6.5 | 0.8 | 4.3 | 6.1 | 0.0225b |
| GLP-1 | 0.5 | 1053 | 1645 | 0.5 | 502.8 | 1409 | 0.0402b |
| Insulin | 0.3 | 2027 | 2027 | 2.6 | 2027 | 2027 | 0.4873 |
| Leptin | 7.9 | 92.1 | 406.6 | 5.4 | 75.9 | 248.5 | 0.1015 |
| PAI-1 | 130 | 1134 | 5712 | 37.2 | 421.8 | 2201 | 0.1149 |
| MMP-1 | 1.0 | 44.69 | 914.8 | 9.886 | 33.58 | 46.91 | 0.0452b |
| MMP-2 | 1473 | 15924 | 23015 | 47.07 | 3344 | 21497 | 0.0049b |
| MMP-3 | 20.24 | 997.5 | 5747 | 0 | 159.8 | 1144 | 0.0012b |
| MMP-7 | 92.55 | 124.6 | 1034 | 95.11 | 113.1 | 136.2 | 0.0880 |
| MMP-8 | 519.2 | 15319 | 88596 | 0 | 10490 | 169078 | 0.6494 |
| MMP-9 | 3904 | 41120 |
5.26×106 | 774 | 29880 |
5.45×106 | 0.9842 |
| MMP-12 | 189.0 | 197.8 | 238.7 | 3.249 | 191.9 | 218.2 | 0.0369b |
| MMP-13 | 82.84 | 105.9 | 240 | 73.6 | 103.6 | 333.5 | 0.9202 |
In the cytokine panel, significantly higher
concentrations of the proinflammatory cytokines, IL-6, -8 and -12,
were observed in patients without a fistula, while significantly
higher concentrations of the anti-inflammatory cytokines, IL-10 and
-17, were observed in the fistula group. Analysis of neovascular
factors revealed elevated values for all the parameters in the
fistula-free group; however, the values were not statistically
significant.
In the diabetes panel, in addition to IL-6, the
GLP-1 concentration was found to be higher in patients without a
fistula, whereas the GIP level was observed at significantly higher
concentrations in the fistula group.
MMP analysis revealed highly expressed profiles for
MMP-1, -2, -3 and-12 in the group without a fistula (Table II).
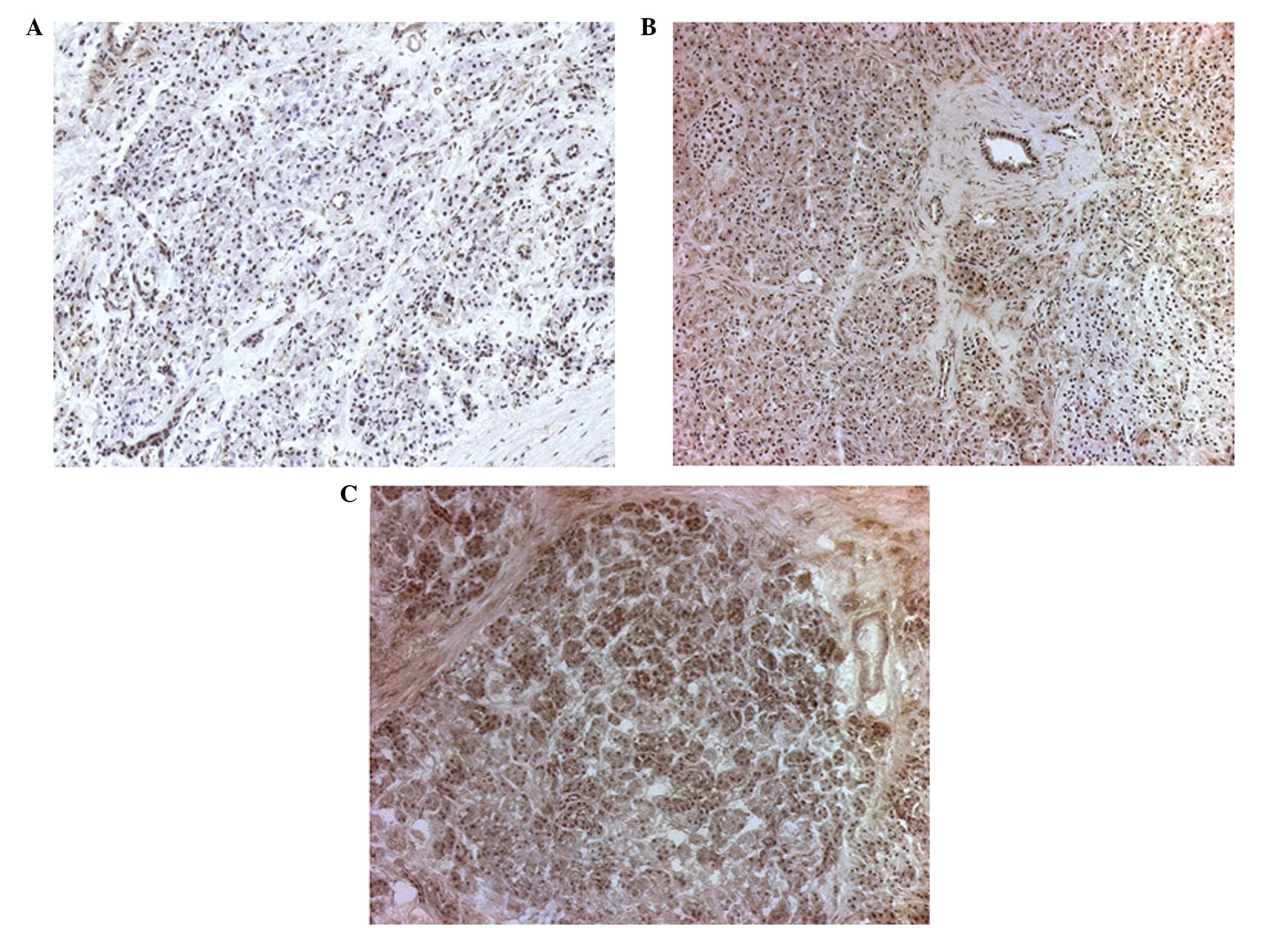
IHC
Based on the multiplex analysis, five factors,
including IL-6, IL-8, VEGF, MMP-1 and MMP-2, were additionally
analyzed using IHC. A total of 10 resection margins from the two
groups were stained for the five factors and semi-quantitative
scoring was performed. The staining distribution and intensity were
numerically graded and evaluated by an experienced pathologist. A
representative example of the MMP-1 intensity grading is shown in
Fig. 3. The scoring data were
statistically analyzed using Fisher’s exact test and the
Cochran-Armitage trend test. However, statistically significant
differences were only obtained in the tests for IL-8 and MMP-1
(Table III).
 | Table IIIIHC analysis of the frequency and
validation of the biochemical parameters. |
Table III
IHC analysis of the frequency and
validation of the biochemical parameters.
| IL-6 | IL-8 | VEGF | MMP-1 | MMP-2 |
|---|
|
|
|
|
|
|
|---|
| Parameter | Inten | Distrib | Inten | Distrib | Inten | Distrib | Inten | Distrib | Inten | Distrib |
|---|
| POPF |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 1 | 3 | 3 | 0 | 1 | 3 | 3 | 4 | 4 | 5 | 1 |
| 2 | 5 | 7 | 6 | 9 | 5 | 7 | 5 | 5 | 4 | 9 |
| 3 | 2 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| No POPF |
| 1 | 2 | 5 | 0 | 6 | 3 | 6 | 2 | 3 | 1 | 1 |
| 2 | 4 | 5 | 9 | 4 | 7 | 4 | 4 | 7 | 8 | 9 |
| 3 | 4 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 |
| P-valuea | 0.727 | 0.649 | 0.303 | 0.057 | 0.542 | 0.369 | 0.124 | 0.649 | 0.140 | 1.000 |
| P-valueb | 0.558 | 0.649 | 0.303 | 0.057 | 0.719 | 0.369 | 0.054 | 0.469 | 0.276 | 1.000 |
| Int. ×
distrba | 0.337 | 0.015 | 0.470 | 0.017 | 0.47 |
Discussion
Despite major progress in pancreatic surgery,
morbidity rates following pancreatic resection remain high, with
pancreatic fistulas being the most challenging postoperative
complication (2). The present
study provides a comprehensive analysis of the morphological and
biochemical parameters associated with healing of the pancreatic
remnant following resection.
Histological analysis of the tissues demonstrated a
high degree of fibrosis, elevated inflammatory activity and higher
inflammatory infiltration, as well as an absence of lipomatous
atrophy in the pancreatic tissue, correlating with a low incidence
of pancreatic fistulas and vice versa. These results are in
accordance with previous studies that found a non-fibrotic fragile
pancreas is likely to predispose individuals to the development of
POPF (5–7). Fibrotic pancreatic tissue is easier
to handle for surgeons, while soft pancreatic tissue is difficult
to sew, which partially explains the increased leakage rates of
soft pancreatic tissue. However, in addition to the technical
factor, tissue-remnant factors appear to have an important role in
the process of healing, as demonstrated in the present study.
Chronic inflammatory infiltration by macrophages,
B-cells and T-cells was more frequently observed in patients
without a fistula. The acute inflammatory activity (level of
neutrophil granulocytes) was slightly elevated following successful
resections. These observations correlate with the results from the
biochemical analysis using quantitative multiplex protein analysis.
The panel of 38 wound healing key proteins revealed higher
concentrations of proinflammatory factors, including IL-6, -8 and
-12 and MMP-1, -2, -3 and -12, and lower concentrations of IL-10
(anti-inflammatory), IL-17 (MMP modulating), and GIP in the
resection margins of patients without any incidence of fistula
formation compared with those tissues from patients with POPF.
A pancreatic fistula or anastomotic insufficiency
following pancreatic resection should be considered as insufficient
wound healing, which in a normal course, passes through four
defined phases: Bleeding, inflammatory, proliferative and
remodeling phases (8). These
phases have to be in perfect equilibration and are regulated by
cytokines and other mediators. Under- or overexpression, as well as
continuous ongoing expression, of these mediators is known to
induce failure of wound healing, which is observed in chronic
inflammatory diseases, including chronic ulcers (9–11).
In the present study, the increase in chronic inflammation and
proinflammatory cytokines (IL-6, -8 and -12), as well as the
decrease in anti-inflammatory cytokines (IL-10), was shown to
correlate with a decreased rate of pancreatic fistula. Thus, mild
to moderate, but not severe inflammation, appears to be a major
factor involved in the healing of pancreatic remnant and
anastomosis. The present study assessed the initial intraoperative
situation of the tissue and in this regard mirrored the tissue
condition prior to the healing process. IL-6 is a proinflammatory
cytokine that exhibits elevated levels in the first hours following
injury. The level of IL-6 correlates with acute phase reactions,
and the cytokine promotes the transition from unspecific to
specific immune defense (15–17).
IL-8, also a potent proinflammatory chemokine, recruits neutrophil
granulocytes and T-cells to the infection site (9,11,18).
The effects of IL-6 and IL-8 explain their positive effects on the
healing process. The main function of the anti-inflammatory
cytokine, IL-10, is the termination or limitation of inflammatory
responses by suppressing inflammatory reactions and cytokine
production and inhibiting macrophage activity. Higher
concentrations of the anti-inflammatory IL-10 in non-healing
anastomosis may reinforce a weaker immune defense of the tissue
(19,20).
Factors associated with angiogenesis or diabetes did
not exhibit statistically significant differences, with the
exception of two incretins, GLP-1, which was significantly higher
in the complication free group (without POPF), and GIP, which had
increased levels in the tissues with POPF. GIP, also known as
glucose-dependent insulinotropic peptide GIP, similar to GLP-1, is
expressed shortly following ingestion and exerts effects on β-islet
cells. Whether the higher levels of GIP in the POPF group are a
compensatory effect for lower GLP-1 levels is unclear. GLP-1 and
GIP are regarded as antidiabetic cytokines. It is well-known that
diabetic metabolic conditions inhibit wound healing (10); however, no association was observed
with the observations of the present study.
The remodeling phase of wound healing is dependent
on MMPs, which degrade the extracellular matrix to aid cells
migration and are engaged in remodeling processes in tissues
(21–23). The remodeling processes, as well as
MMP expression, are highly coordinated and maintained in a balance.
In the present study, tissue samples of patients without fistulas
were found to have higher concentrations of MMP-1, -2, -3 and -12
when compared with the tissues from patients with a fistula.
Therefore, high expression levels of MMP-1, -2, -3, and -12 appear
to promote adequate healing. The differential expression of MMPs in
the pancreas of patients with and without fistulas may be further
investigated using IHC.
The present study investigated the morphological and
biochemical predictive factors for anastomotic complications
following pancreatic resection. The histological results revealed a
higher degree of fibrosis and increased inflammatory activity, as
well as a lower degree of lipomatous atrophy, in the pancreatic
resection margins of patients without pancreatic fistulas or
anastomotic insufficiencies. Furthermore, the results from the
protein profiling indicated that a low predisposition to
inflammatory reaction results in lower concentrations of
proinflammatory cytokines, including IL-6, -8 and -12, as well as
high concentrations of anti-inflammatory cytokines, such as IL-10,
and decreased expression levels of MMP-1, -2, -3 and -12. These
conditions are associated with a higher postoperative complication
and fistula incidence rate. Accordingly, the MMP modulating
cytokine, IL-17, was found in higher concentrations when the
healing of the pancreatic remnant failed.
The development of a preoperative or intraoperative
test may eventually aid or assure the surgeons intraoperative
decision of performing an anastomosis in individual critical cases
where the pancreas texture is intraoperatively macroscopically
complex to evaluate.
Furthermore, a predictive statement to patients with
an increased risk of developing an anastomosis insufficiency or
fistula is possible, leading to close meshed patient’s monitoring
postoperatively. However, in order to develop a clinically
practical assay to predict pancreatic fistulas, further studies
investigating the role of inflammatory cytokines, chemokines and
MMPs are required. The present study, to the best of our knowledge,
is one of the only studies investigating this topic.
References
|
1
|
Bassi C, Dervenis C, Butturini G, et al;
International Study Group on Pancreatic Fistula Definition.
Postoperative pancreatic fistula: an international study group
(ISGPF) definition. Surgery. 138:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hackert T, Werner J and Buchler MW:
Postoperative pancreatic fistula. Surgeon. 9:211–217. 2011.
View Article : Google Scholar
|
|
3
|
Laaninen M, Bläuer M, Vasama K, et al: The
risk for immediate postoperative complications after
pancreaticoduodenectomy is increased by high frequency of acinar
cells and decreased by prevalent fibrosis of the cut edge of
pancreas. Pancreas. 41:957–961. 2012. View Article : Google Scholar
|
|
4
|
Friess H, Kleeff J, Fischer L, Muller M
and Büchler MW: Surgical standard therapy for cancer of the
pancreas. Chirurg. 74:183–190. 2003.(In German).
|
|
5
|
Pratt WB, Callery MP and Vollmer CM Jr:
Risk prediction for development of pancreatic fistula using the
ISGPF classification scheme. World J Surg. 32:419–428. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosso E, Casnedi S, Pessaux P, et al: The
role of ‘fatty pancreas’ and of BMI in the occurrence of pancreatic
fistula after pancreaticoduodenectomy. J Gastrointest Surg.
13:1845–1851. 2009.
|
|
7
|
Sulberg D, Chromik AM, Koster O and Uhl W:
Prevention and management of postoperative complications in
pancreatic surgery. Zentralbl Chir. 135:129–138. 2010.(In
German).
|
|
8
|
Watson T: Tissue repair: the current state
of the art. SportEx Medicine. 28:8–12. 2006.
|
|
9
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.PubMed/NCBI
|
|
10
|
Brem H and Tomic-Canic M: Cellular and
molecular basis of wound healing in diabetes. J Clin Invest.
117:1219–1222. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fivenson DP, Faria DT, Nickoloff BJ, et
al: Chemokine and inflammatory cytokine changes during chronic
wound healing. Wound Repair Regen. 5:310–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diener MK, Fitzmaurice C, Schwarzer G, et
al: Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus
pancreaticoduodenectomy (classic Whipple) for surgical treatment of
periampullary and pancreatic carcinoma. Cochrane Database Syst Rev.
CD0060532011.
|
|
13
|
Ho CK, Kleeff J, Friess H and Büchler MW:
Complications of pancreatic surgery. HPB (Oxford). 7:99–108. 2005.
View Article : Google Scholar
|
|
14
|
Abcam: Abcam protocols book.
822012/2013.
|
|
15
|
Jones SA: Directing transition from innate
to acquired immunity: defining a role for IL-6. J Immunol.
175:3463–3468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nijsten MW, Hack CE, Helle M, ten Duis HJ,
Klasen HJ and Aarden LA: Interleukin-6 and its relation to the
humoral immune response and clinical parameters in burned patients.
Surgery. 109:761–767. 1991.PubMed/NCBI
|
|
17
|
Gallucci RM, Simeonova PP, Matheson JM, et
al: Impaired cutaneous wound healing in interleukin-6-deficient and
immunosuppressed mice. FASEB J. 14:2525–2531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin F, Nguyen CM, Wang SJ, Saadi W, Gross
SP and Jeon NL: Effective neutrophil chemotaxis is strongly
influenced by mean IL-8 concentration. Biochem Biophys Res Commun.
319:576–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asadullah K, Sterry W and Volk HD:
Interleukin-10 therapy - review of a new approach. Pharmacol Rev.
55:241–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyaoka K, Iwase M, Suzuki R, et al:
Clinical evaluation of circulating interleukin-6 and interleukin-10
levels after surgery-induced inflammation. J Surg Res. 125:144–150.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar
|
|
22
|
Vu TH and Werb Z: Matrix
metalloproteinases: effectors of development and normal physiology.
Genes Dev. 14:2123–2133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armstrong DG and Jude EB: The role of
matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc.
92:12–18. 2002. View Article : Google Scholar : PubMed/NCBI
|