Introduction
Prostate cancer, one of the most common types of
cancer in males, has become a major public health concern (1). The molecular pathogenesis of prostate
cancer is complicated and remains poorly understood (2). Therefore, the identification of novel
molecular mechanisms may help to develop strategies for its
diagnosis, treatment and prognosis.
Recent studies have shown that microRNAs (miRNAs)
have a critical role in the development of numerous different types
of human cancer (3,4). miRNAs regulate multiple genes by
targeting mRNAs, resulting in mRNA degradation or translation
repression (5). For example,
miR-888 is a miRNA secreted by prostate cells that promotes
prostate cell growth and migration through the repression of the
levels of protein produced by the tumor suppressor genes RBL1 and
SMAD4 (6).
Previous studies have demonstrated that the
upregulation of hepatic miR-181 promotes the growth, clonogenic
survival, migration and invasion of hepatocellular carcinoma cells
(7,8). Furthermore, the expression level of
miR-181 is significantly associated with overall survival in
hematological malignancies and may be an important clinical
prognostic factor for patients with hepatocellular carcinoma
(9). However, the expression and
function of mi-181 in prostate cancer has yet to be elucidated.
Therefore, in the present study, the expression of
miR-181 was determined in prostate cancer tissues. In addition, the
proliferation of prostate cancer cells overexpressing mi-R181 was
analyzed in vivo and in vitro. Furthermore, the
targets of miR-181 were investigated in order to determine the
underlying mechanism of miR-181 in prostate cancer.
Materials and methods
Tissue samples and cell culture
A total of 20 prostate cancer samples and adjacent
normal tissues were obtained from patients who underwent surgery at
Huashan Hospital Affiliated to Fudan University (Shanghai, China).
The present study was approved by the hospital institutional review
board and written informed consent was obtained from each patient.
LNCaP cells were provided by the Institute of Biochemistry and Cell
Biology of Chinese Academy of Science (Shanghai, China). The cells
were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (Invitrogen Life Technologies), 100 IU/ml penicillin
and 100 μg/ml streptomycin sulfate. Cells were incubated at 37°C
with 5% CO2.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA containing miRNA and mRNA was extracted
from tissues or cells using TRIzol® reagent (Invitrogen
Life Technologies), in accordance with the manufacturer’s
instructions. To analyze miR-181 expression, specific stem-loop
reverse transcription primers (Invitrogen Life Technologies) were
used. In order to determine the transcripts of the interest genes,
qPCR was performed using a SYBR Green Premix Ex Taq (Takara,
Dalian, China). The primer sequences were listed as follows: DAX-1
sense, 5′-AGCACAAATCAAG CGCAGG-3′, antisense, 5′-GAAGCGCAGCGTCTTC
AAC-3′; PSA sense, 5′-CTGCTGCACGTCAGTCAACTA-3′, antisense:
5′-GAGGACTACACTGGTCTGGAAT-3′; CDK1 sense,
5′-AAACTACAGGTCAAGTGGTAGCC-3′, antisense:
5′-TCCTGCATAAGCACATCCTGA-3′ and CDK2 sense:
5′-CCAGGAGTTACTTCTATGCCTGA-3′, antisense:
5′-TTCATCCAGGGGAGGTACAAC-3′.
qPCR was performed by TaqMan MicroRNA assay (Qiagen,
Shanghai, China) using the Applied Biosystems 7300 system (Applied
Biosystems, Foster City, CA, USA). The PCR conditions included an
initial holding period at 95°C for 5 min, followed by a two-step
PCR program consisting of 95°C for 5 sec and 60°C for 30 sec for 45
cycles. All samples were normalized against the internal control
(U6 small nuclear RNA) and analyzed using the 2−ΔΔCt
method.
Cell proliferation and cell-cycle
assays
The viability of LNCaP cells was determined by
assaying the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2,
5-di-phenylte-trazolium bromide (MTT; Beyotime Company, Shanghai,
China) to formazan. For the analysis of cell proliferation, cells
were seeded onto 24-well plates. For the BrdU incorporation assays,
a cell proliferation enzyme-linked immunosorbent assay (ELISA;
Beyotime, Shanghai, China) was used to analyze the incorporation of
BrdU during DNA synthesis, in accordance with the manufacturer’s
instructions. Absorbance was measured at 450 nm using the Spectra
Max 190 ELISA reader (Molecular Devices, Sunnyvale, CA, USA). For
analysis of the cell cycle, cells were suspended in 0.5 ml solution
containing 20 μg/ml propidium iodide and 50 μg/ml RNase, and then
analyzed using flow cytometry (Becton Dickinson, San Jose, CA,
USA). Histograms were used to represent the percentage of cells in
each phase of the cell cycle (G0/G1, S and G2/M).
microRNA mimics and transfection
Human miR-181 mimics and negative controls (NC) were
purchased from Qiagen (Shanghai, China). All transfections of LNCaP
cells were performed using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA), following the manufacturer’s
instructions.
Western blot analysis
Total cell protein extracts were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and
transferred onto a polyvinylidene difluoride membrane. After
blocking with 10% nonfat milk in phosphate-buffered saline, the
membranes were immunoblotted with antibodies as indicated, followed
by horseradish peroxidase-linked secondary antibodies (Cell
Signaling Technology, Inc., Danvers, MA, USA). The signals were
detected using a chemiluminescence detection kit (Millipore,
Billerica, MA, USA). Anti-dosage-sensitive sex reversal, adrenal
hypoplasia critical region, on chromosome X, gene 1 (DAX-1) and
anti-GAPDH antibodies were purchased from Abcam (Cambridge, MA,
USA). Anti-PSA, CDK1 and CDK2 antibodies were purchased from Santa
Cruz Biotechnology Inc. (Santa Cruz Biotechnology, CA, USA).
Protein levels were normalized against those of GAPDH (Santa Cruz
Biotechnology, Inc.).
Luciferase reporter assay
cDNA fragments corresponding to the entire
3′-untranslated region (UTR) were amplified by qPCR from the total
RNA extracted from LNCaP cells with KpnI and EcoRI
linkers. The PCR products were cloned downstream of the Renilla
luciferase open reading frame of the pMir-Report (Qiagen), which
also contained a constitutively expressed firefly luciferase gene
that was used to normalize the transfections. For the luciferase
reporter assays, the cells were seeded in 24-well plates and
harvested 48 h after transfection. The wild-type and mutant
3′-untranslated region fragments from the human DAX-1 gene were
cloned into pMir-Report (Qiagen). Mutations were introduced in
potential miR-181 binding sites using a site-directed mutagenesis
kit (Qiagen). Luciferase values were determined using the
Dual-Luciferase Reporter assay system (Promega Corporation,
Madison, WI, USA).
Tumor growth assay
Male BALB/c nude mice, aged 4 weeks, were purchased
from the animal center of the Second Military Medical University
(Shanghai, China). A total of 2×105 LNCaP cells stably
expressing miR-181 or NC were injected subcutaneously into the
dorsal flank of the mice. The mice were observed over 5 weeks for
tumor formation. The mice were then sacrificed and the tumors were
recovered and the wet weight of each tumor was determined. The
tumor volume (mm3) was calculated according to the
following formula: Volume (mm3) = 1/2 × length ×
width2. The experimental protocol was approved by the
Experimental Animal Care Commission of Huashan Hospital Affiliated
to Fudan University.
Statistical analysis
Differences between groups were analyzed using a
Student’s t-test and expressed as the mean ± standard deviation
from ≥three independent experiments. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using GraphPad Prism version .0 software
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
miR-181 is upregulated in prostate cancer
tissues
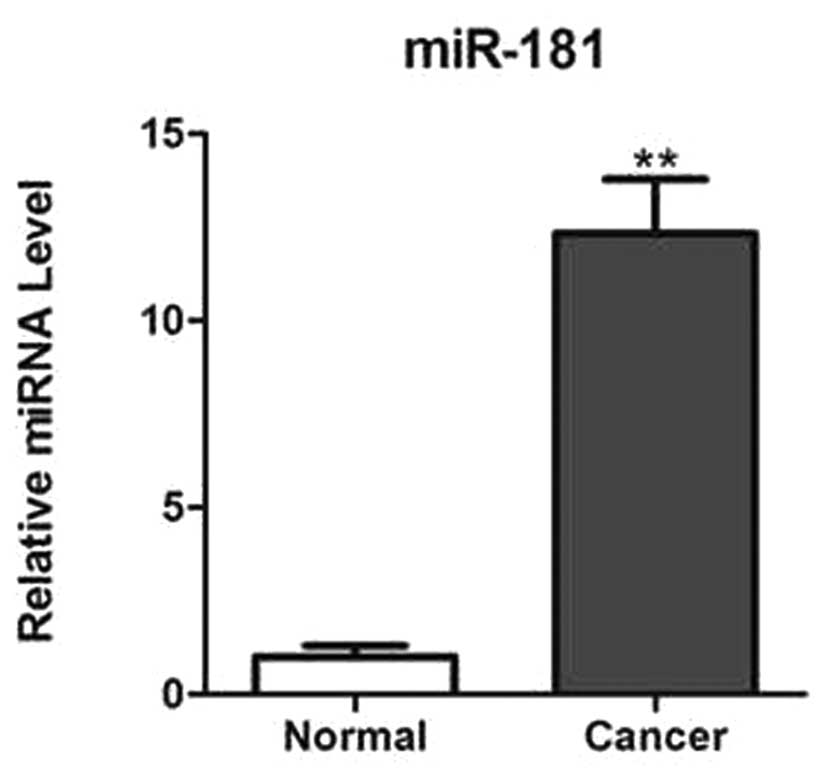
The expression of miR-181 was analyzed in prostate
cancer tissues and adjacent normal tissues using qPCR. It was found
that miR-181 is significantly upregulated in cancer tissues
compared with that in normal adjacent tissues, as shown in Fig. 1.
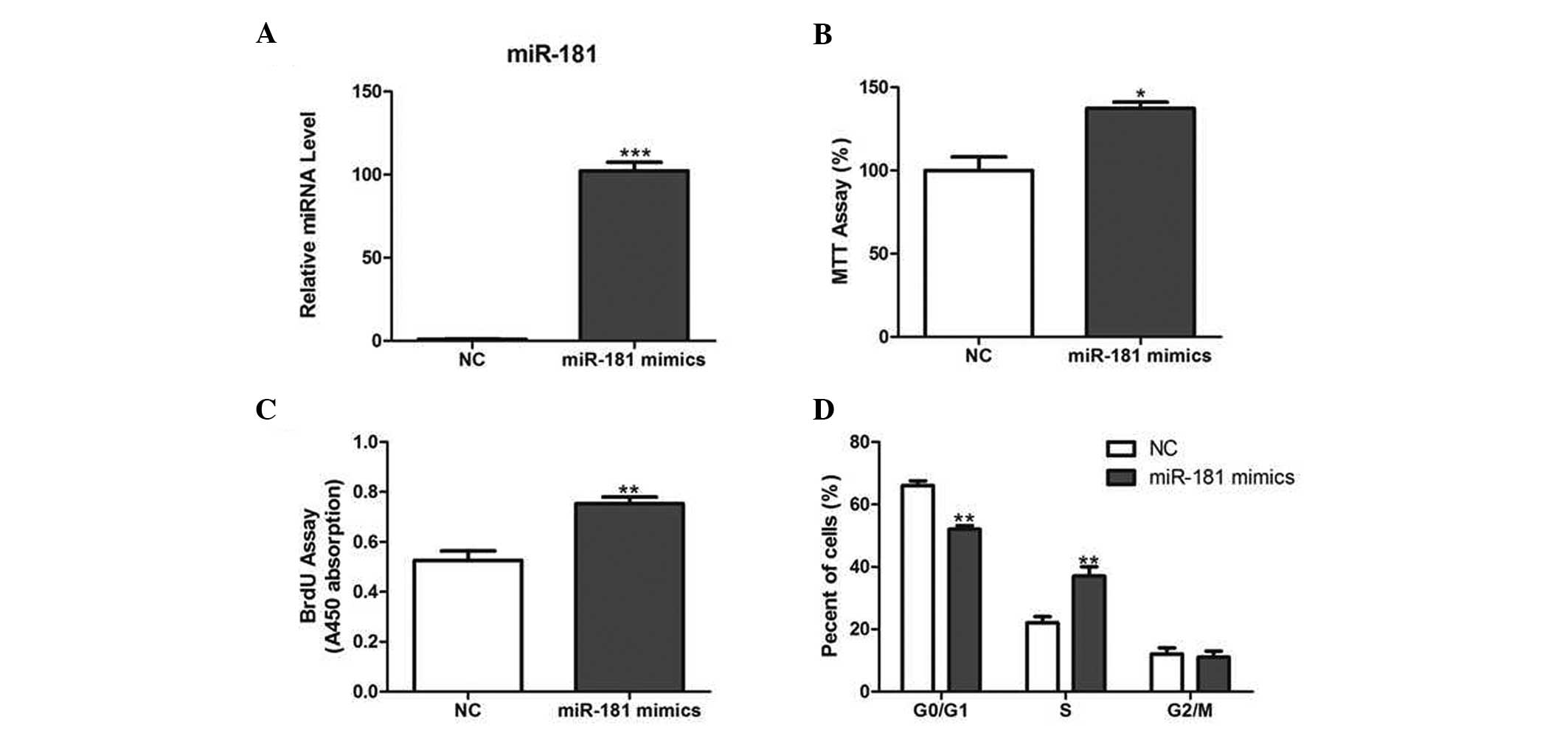
miR-181 overexpression promotes prostate
cancer cell proliferation in vitro
Since miR-181 was found to be upregulated in
prostate cancer tissues, the effect of miR-181 on prostate cancer
cell growth was investigated. LNCaP cells were transfected with
miR-181 mimics or NC (Fig. 2A).
The results demonstrated that cell growth was significantly
increased in miR-181-overexpressing cells compared with that of
their corresponding controls, measured using the MTT and BrdU
assays (Fig. 2B and C).
Furthermore, miR-181 overexpression decreased the percentage of
cells in the G1 phase and increased the percentage of cells in the
S phase (Fig. 2D).
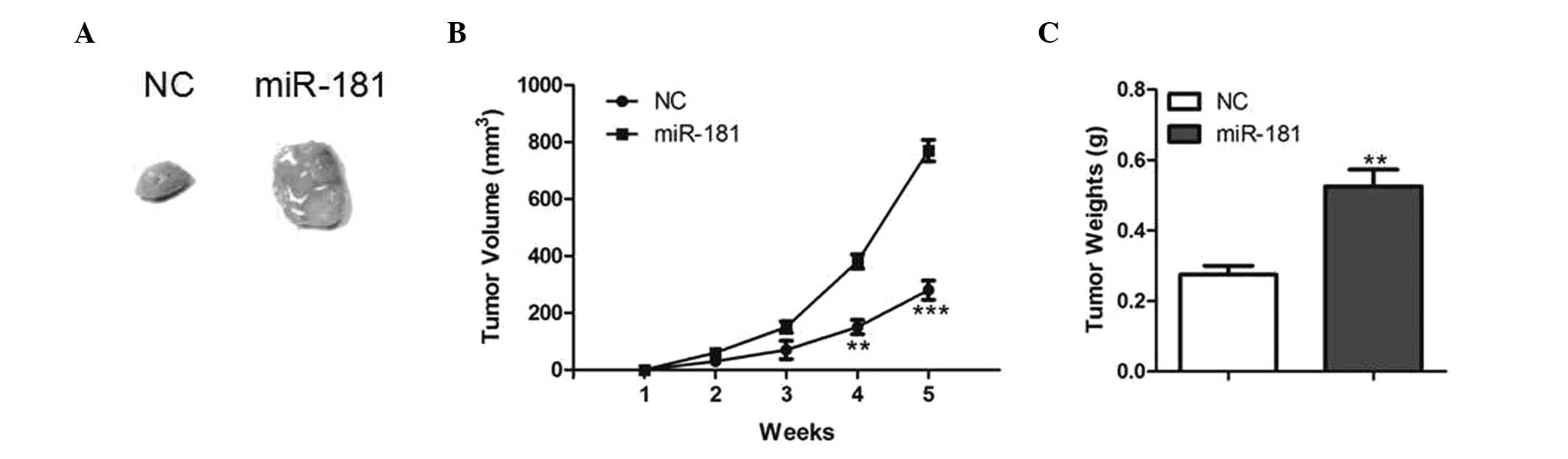
miR-181 overexpression promotes tumor
growth in vivo
To further investigate the function of miR-181 on
tumor growth in vivo, LNCaP cells with stable overexpression
of miR-181 were generated and injected subcutaneously into the
dorsal flank of nude mice. Tumor growth was closely monitored for 5
weeks. The tumor size and volume were markedly increased in mice
injected with LNCaP cells overexpressing miR-181 compared with
those in control mice (Fig. 3A and
B). In addition, the average tumor weight was significantly
increased by miR-181 overexpression (Fig. 3C), suggesting that miR-181 may
promote tumor growth in vivo.
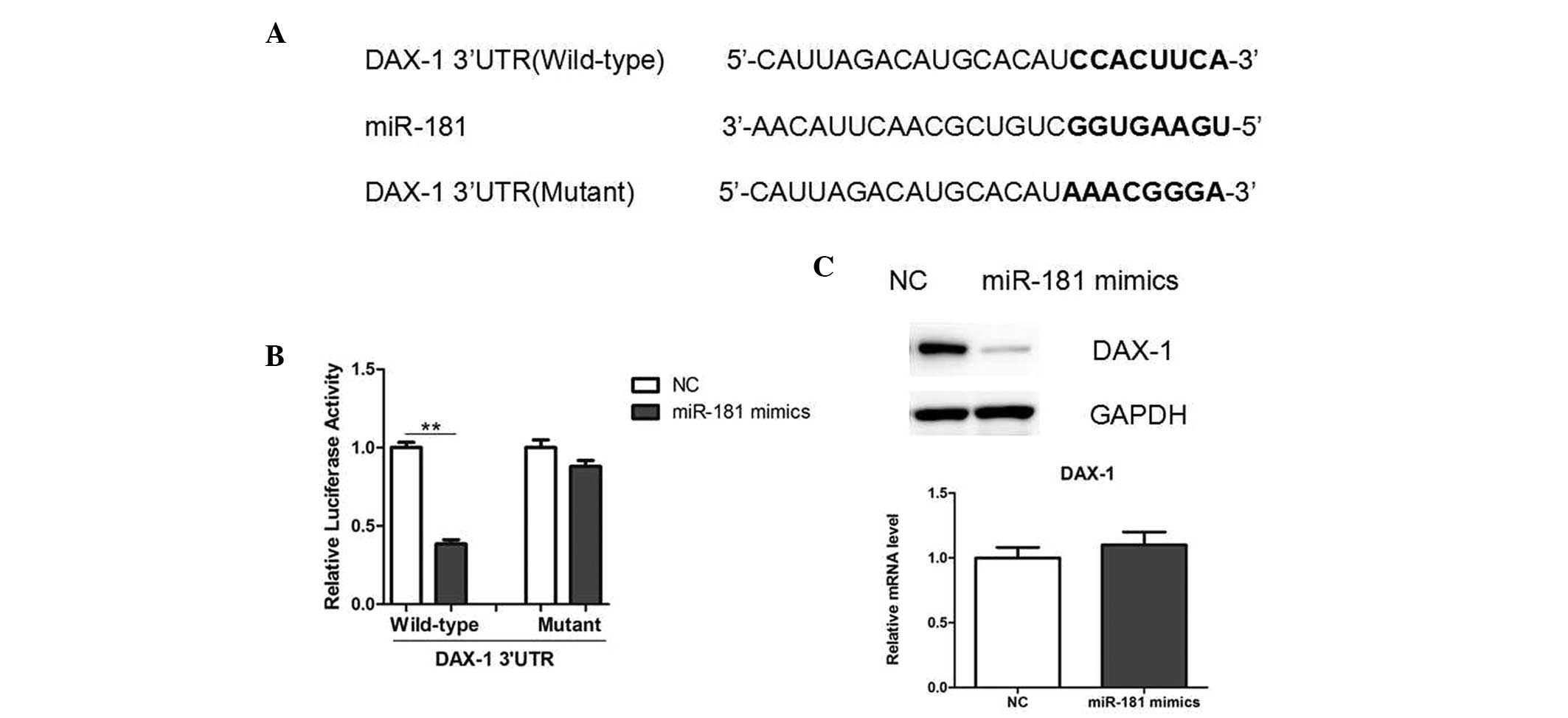
miR-181 targets the DAX-1 3′-untranslated
region (3′-UTR) and downregulates its expression
In order to understand the underlying mechanism,
potential targets of miR-181 were determined using TargetScan
software. DAX-1 was identified as a potential target of miR-181.
Notably, the 3′-UTR of DAX-1 mRNA was observed to contain a
complementary site for the seed region of miR-181 (Fig. 4A). To investigate whether DAX-1 may
be directly targeted by miR-181, a luciferase reporter vector was
constructed, containing the putative miR-181 binding sites within
the DAX-1 3′-UTR. The results showed that miR-181 overexpression
significantly decreased the luciferase activity, and mutations in
the miR-181 binding site from the DAX-1 3′-UTR abolished this
effect, suggesting that miR-181 directly inhibited DAX-1 expression
by targeting the 3′-UTR (Fig. 4B).
Furthermore, miR-181 mimics decreased the endogenous protein levels
of DAX-1, as indicated by western blot analysis (Fig. 4C), while the DAX-1 mRNA levels
remained unchanged (Fig. 4D).
Therefore, these results suggest that miR-181 may negatively
regulate DAX-1 expression at the translational level in LNCaP
cells.
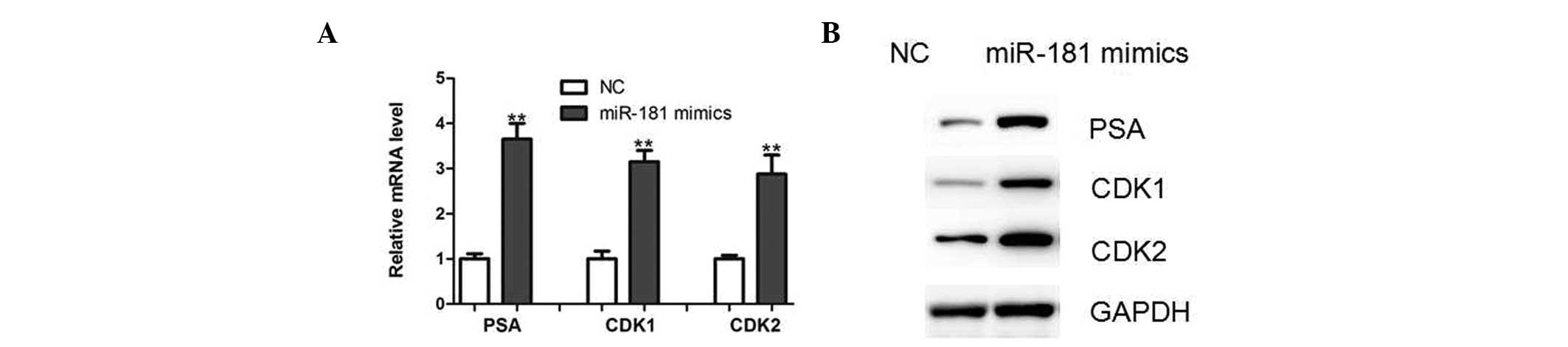
DAX1 has been previously demonstrated to repress the
transcriptional activity of the androgen receptor (AR) in LNCaP
cells (10). In the present study,
elevated expression levels of AR target genes and proteins,
including prostate-specific antigen, cyclin-dependent kinase (CDK)
1 and CDK2, was observed in LNCaP cells overexpressing miR-181
(Fig. 5). In combination, these
results further confirm that DAX-1 is an important target gene of
miR-181 in prostate cancer cells.
Discussion
It has been previously demonstrated that several
miRNAs are dysregulated in prostate cancer tissues or cell lines,
and they have been shown to be associated with prostate cancer
progression and disease outcome (11–13).
In the present study, it was demonstrated for the first time, to
the best of our knowledge, that miR-181 overexpression may promote
cell proliferation and cell-cycle progression in LNCaP cells. In
addition, miR-181 overexpression was observed to promote the growth
of LNCaP tumors in nude mice. Therefore, miR-181 may be an
onco-miRNA in the development of prostate cancer.
Furthermore, in the present study DAX-1 was
identified as a direct target of miR-181 in prostate cancer cells.
DAX-1, a member of the orphan nuclear receptor family, is known to
have an important role during development, particularly in gender
determination and steroidogenesis (14,15).
In humans, DAX-1 gene mutations usually lead to the X-linked
congenital adrenal hypoplasia and primary adrenal insufficiency
associated with hypogonadotropic hypogonadism (16,17).
With regard to the types of cancer observed in
humans, DAX-1 expression has been reported in endocrine and sex
steroid-dependent neoplasms, including adrenocortical, pituitary,
endometrial and ovarian tumors (18–20).
For example, DAX-1 overexpression has been demonstrated to repress
estrogen-dependent breast cancer cell proliferation via the
inhibition of aromatase expression (19). In addition, DAX-1 expression has
been observed to be significantly downregulated in prostate cancer
(10). In a previous study, at the
molecular level, DAX-1 was demonstrated to interact with the AR and
inhibit its nuclear localization. As a result, DAX-1 was found to
repress androgen-dependent gene transcription in prostate cancer
cells (10). The results from the
present study are in accordance with these findings. They
demonstrate that miR-181 overexpression causes the upregulation of
AR target genes, suggesting that the proliferative role of miR-181,
at least in part, may be dependent on androgen signaling.
In conclusion, the present study provides a novel
role for miR-181 in prostate cancer cell proliferation. The results
suggest that miR-181 may be a potential therapeutic target for the
treatment of prostate cancer in the future.
References
|
1
|
Emery JD, Shaw K, Williams B, et al: The
role of primary care in early detection and follow-up of cancer.
Nat Rev Clin Oncol. 11:38–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hutchinson L: Genetics: tracking clonal
origin of prostate cancer. Nat Rev Clin Oncol. 11:42014. View Article : Google Scholar
|
|
3
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011.PubMed/NCBI
|
|
5
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis H, Lance R, Troyer D, et al: miR-888
is an expressed prostatic secretions-derived microRNA that promotes
prostate cell growth and migration. Cell Cycle. 13:227–239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji J, Yamashita T, Budhu A, et al:
Identification of microRNA-181 by genome-wide screening as a
critical player in EpCAM-positive hepatic cancer stem cells.
Hepatology. 50:472–480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Hsu SH, Majumder S, et al:
TGFbeta-mediated upregulation of hepatic miR-181b promotes
hepatocarcinogenesis by targeting TIMP3. Oncogene. 29:1787–1797.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin S, Pan L, Guo S, et al: Prognostic
role of microRNA-181a/b in hematological malignancies: a
meta-analysis. PLoS One. 8:e595322013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holter E, Kotaja N, Mäkela S, et al:
Inhibition of androgen receptor (AR) function by the reproductive
orphan nuclear receptor DAX-1. Mol Endocrinol. 16:515–528. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hessels D and Schalken JA: Urinary
biomarkers for prostate cancer: a review. Asian J Androl.
15:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sita-Lumsden A, Dart DA, Waxman J and
Bevan CL: Circulating microRNAs as potential new biomarkers for
prostate cancer. Br J Cancer. 108:1925–1930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim WT and Kim WJ: MicroRNAs in prostate
cancer. Prostate Int. 1:3–9. 2013. View Article : Google Scholar
|
|
14
|
McCabe ER: DAX1: Increasing complexity in
the roles of this novel nuclear receptor. Mol Cell Endocrinol.
265–266:179–182. 2007.PubMed/NCBI
|
|
15
|
El-Khairi R, Martinez-Aguayo A,
Ferraz-de-Souza B, Lin L and Achermann JC: Role of DAX-1 (NR0B1)
and steroidogenic factor-1 (NR5A1) in human adrenal function.
Endocr Dev. 20:38–46. 2011.PubMed/NCBI
|
|
16
|
Li J, Lu Y, Liu R, et al: DAX1 suppresses
FXR transactivity as a novel co-repressor. Biochem Biophys Res
Commun. 412:660–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jadhav U, Harris RM and Jameson JL:
Hypogonadotropic hypogonadism in subjects with DAX1 mutations. Mol
Cell Endocrinol. 346:65–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chae BJ, Lee A, Bae JS, Song BJ and Jung
SS: Expression of nuclear receptor DAX-1 and androgen receptor in
human breast cancer. J Surg Oncol. 103:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lanzino M, Maris P, Sirianni R, et al:
DAX-1, as an androgen-target gene, inhibits aromatase expression: a
novel mechanism blocking estrogen-dependent breast cancer cell
proliferation. Cell Death Dis. 4:e7242013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lalli E and Alonso J: Targeting DAX-1 in
embryonic stem cells and cancer. Expert Opin Ther Targets.
14:169–177. 2010. View Article : Google Scholar : PubMed/NCBI
|