Introduction
Pelvic organ prolapse (POP), a hernia in the
endopelvic fascia (1), is
associated with urinary incontinence and defecation dysfunction,
leading to an impaired quality of life for the affected individual
(2). Epidemiological studies have
revealed that numerous risk factors, including vaginal delivery,
senescence, obesity and pelvic surgery, contribute to the
development of the condition (3–5).
The pelvic organs are located in the pelvic floor,
which consists of connective tissue in a network of tough
extracellular matrix (ECM) protein fibers. To date, studies have
primarily focused on the molecular and biochemical changes in POP,
including ECM proteins (collagen, elastin and fibulin) (6,7) and
enzymes (5). The results have
indicated that the genes associated with the regulation of collagen
fiber assembly and impairment in elastic fibers play an important
role in the development of POP. Brizzolara et al (2) observed that several genes involved in
immunity and defense, including interleukin (IL)-6, Toll-like
receptors and interferon (IFN)-γ receptors (IFNGRs), were
upregulated, indicating that these genes enriched for ‘immunity and
defense’ contribute to POP.
IFNs were the first cytokines to be identified;
thus, have provided the fundamental base from which the
understanding of the functions, pathways, evolution and structure
of other class II cytokines and their receptors began. IFNs were
also the first cytokines to be used therapeutically (8). Based on their receptor specificity
and sequence homology, IFNs are classified into type I, II and III.
Type I IFNs consist of seven classes, including IFN-α, -β, -ɛ, -κ,
-σ, -ω and -τ, while type II IFNs consist of IFN-γ only (8). IFN-γ is an important cytokine in the
host defense against infection by viral and microbial pathogens.
Following the binding of IFN-γ to its heterodimeric cell surface
receptors, IFNGR1 and IFNGR2, the janus kinase (JAK)-signal
transducer and activator of transcription (STAT) signaling pathway
is activated. This activation is regulated by IFN regulatory
factors (IRFs) and nuclear factor (NF)-κB. IFN-γ has been
demonstrated to be involved in inflammatory disease progression,
for example in rheumatoid arthritis (9). Type III IFNs include IFN-λ1, -λ2 and
-λ3, which are also named as IL-29, IL-28A and IL-28B,
respectively.
A previous study demonstrated that severe
inflammation occurs during POP development (2). Thus, IFN-γ may be an optional
inflammatory marker during the development of POP. The expression
levels of IFN-γ may be associated with the levels of its
pathway-related genes. Thus, in the present study, the gene
expression levels and tissue localization of IFN-γ and its
pathway-associated genes, including IFNGR1, IFNGR2, JAK1 and STAT1,
were examined in the vaginal tissue of females with POP.
Materials and methods
Patient selection and tissue
collection
Premenopausal females (n=12) undergoing surgery for
POP were selected for involvement in the study according to their
POP-quantification (POP-Q) staging system result, which was
required to be stage II or greater (10). Females with a POP-Q score of stage
0 served as the controls (n=5). None of the participants in the POP
or control groups suffered from stress urinary incontinence. All
patient cases and controls were of Mongolian origin. The local
Ethics Committee of the Third Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) approved the experimental procedures
used in the study, and all female patients included provided
informed consent.
During surgery, following the removal of the uterus
~1 cm2 was obtained by sharp dissection down to the
avascular space of loose areolar tissue of the vagina using medical
scissors for all the POP cases and controls. Specimens used for the
quantitative polymerase chain reaction (qPCR) analysis were
immediately placed in TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) and stored at −70°C. For immunohistochemical
analysis, the specimens were fixed in 4% formaldehyde for 48 h.
qPCR
Total RNA was extracted by TRIzol reagent and
treated with 2.5 μl DNase I (Qiagen, Hilden, Germany). The RNA
concentration was determined by measuring the optical density
(OD)260/OD280 ratio until a value of 1.7 was
obtained (Eppendorf AG, Hamburg, Germany), and by electrophoresis
on 1.5% agarose gels. The RNA was reverse transcribed into cDNA
using SuperScript™ RNase H-Reverse Transcriptase
(Invitrogen Life Technologies), according to the manufacturer’s
instructions.
qPCR was conducted on an Applied Biosystems 7500
Real-Time PCR system (Invitrogen Life Technologies) using Applied
Biosystems SYBR® Green Master mix (Invitrogen Life
Technologies). All measurements were analyzed in triplicate on
96-well optical PCR-plates. Human ribosomal 18S rRNA (GenBank
accession no. 4310893) was used as an internal standard. The qPCR
protocol involved 40 cycles of denaturation-annealing. Following
qPCR, a dissociation curve was constructed by increasing the
temperature from 65 to 95°C in order to verify the specificity of
the PCR products. The threshold cycles (Cts) were recorded and the
relative quantitation (ΔΔCt method) was calculated to
compare gene expression (11). The
mRNA expression levels for POP were calculated as fold changes
(FCs) relative to the control mRNA expression levels.
Immunohistochemistry
Samples were sectioned at a thickness of 5 μm and
mounted on glass. Antigen retrieval was performed by treatment with
0.125% trypsin, followed by blocking with 5% normal bovine serum
and overnight incubation with the primary antibodies at 4°C; IFN-γ
antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA ),
IFNGR1 antibody (Abcam Inc, London, UK), IFNGR2 antibody (Santa
Cruz Biotechnology Inc.), JAK1 antibody (Santa Cruz Biotechnology
Inc.), STAT1 antibody (Santa Cruz Biotechnology Inc.) and NF κB
antibody (Cell Science, Sydney, Australia). This was followed by
incubation with the appropriate anti-rabbit secondary antibodies
(Wuhan Boster, Wuhan, China). For the negative control group,
nonspecific rabbit IgG was used at the same concentration as the
primary antibody (Santa Cruz Biotechnology Inc). The sections were
counterstained with 10% Mayer’s hematoxylin, mounted and observed
with a Leica microscope (Leica Microsystems, Ontario, Canada).
Statistical analysis
Data were analyzed by one-way analysis of variance
using the SAS 9.0 statistical software (SAS Institute, Inc., Cary
NC, USA). P<0.05 was considered to indicate a statistically
significant difference. Experimental error was reported as the
standard error of the mean.
Results
Patient sample
Vaginal tissue samples were obtained from a total of
17 patients, of which 12 were patients with POP and 5 were control
patients with vaginal myomas or other vaginal diseases. The
patients were matched for age (50.6 vs. 47.5 years, respectively)
and body mass index (25 vs. 26.8 kg/m2, respectively).
The statistically significant differences were observed between the
two groups in mean parity (3.8 vs. 1.9, respectively, P=0.001).
Gene expression
Gene expression levels were analyzed by qPCR. Gene
expression levels of IFN-γ (qPCR FC, 8.6), IFNGR1 (qPCR FC, 3.8),
IFNGR2 (qPCR FC, 2.6), JAK1 (qPCR FC, 2.2), STAT1 (qPCR FC, 3.4)
and NF-κB (qPCR FC, 5.1) were significantly increased in patients
with POP when compared with the control patients (P=0.001, 0.005,
0.04, 0.002, 0.001 and 0.007, respectively; Fig. 1). All the primers used in the
analysis are listed in Table
I.
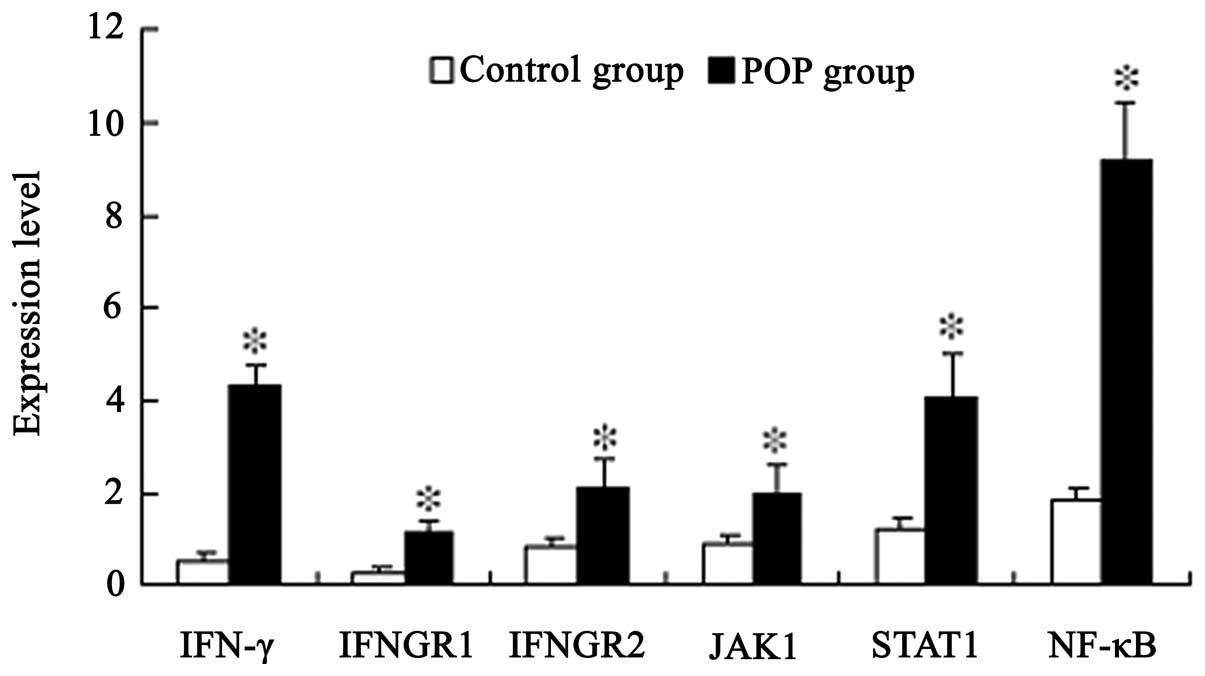 | Figure 1Quantitative polymerase chain reaction
analysis of the expression levels of IFN-γ, IFNGR1, IFNGR2, JAK1,
STAT1 and NF-κB in the control (n=10) and POP groups (n=12).
Results are presented as the mean ± standard error of the mean.
*P<0.05, vs. control group. IFN, interferon; IFNGR,
IFN-γ receptor; JAK, janus kinase; STAT, signal transducer and
activator of transcription; NF, nuclear factor; POP, pelvic organ
prolapse. |
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Sequence | Product size
(bp) | Annealing temperature
(°C) |
|---|
| IFN-γ | Forward
5′-GCAGGTCATTCAGATGTAGCGG-3′
Reverse 5′-GGCGACAGTTCAGCCATCACTT-3′ | 317 | 64 |
| IFNGR1 | Forward
5′-TATGTGAGAATGAACGGAAGTGA-3′
Reverse 5′-GATGAATACCAGGCTAAGCACTA-3′ | 276 | 59 |
| IFNGR2 | Forward
5′-AACATCTTTAGAGTCGGGCATTT-3′
Reverse 5′-TCTATCTGTAATGGGATGCATGG-3′ | 212 | 60 |
| JAK1 | Forward
5′-CCAGAACTGCCCAAGGACATCA-3′
Reverse 5′-ACGCTGCTGTCACAAATGGTCT-3′ | 158 | 63 |
| STAT1 | Forward
5′-GAGTGGAAGCGGAGACAGCAGA-3′
Reverse 5′-AGACTGAAGGTGCGGTCCCATA-3′ | 212 | 63 |
| NF-κB | Forward
5′-ACCAAGGAGATGGACCTCAGCG-3′
Reverse 5′-CCTTCCCAGACTCCACCATTTT-3′ | 278 | 63 |
| 18S RNA | Forward
5′-GTCTTCACCACCATGGAGAAGGCT-3′
Reverse 5′-CATGCCAGCGAGCTTCCCGTTCA-3′ | 393 | 64 |
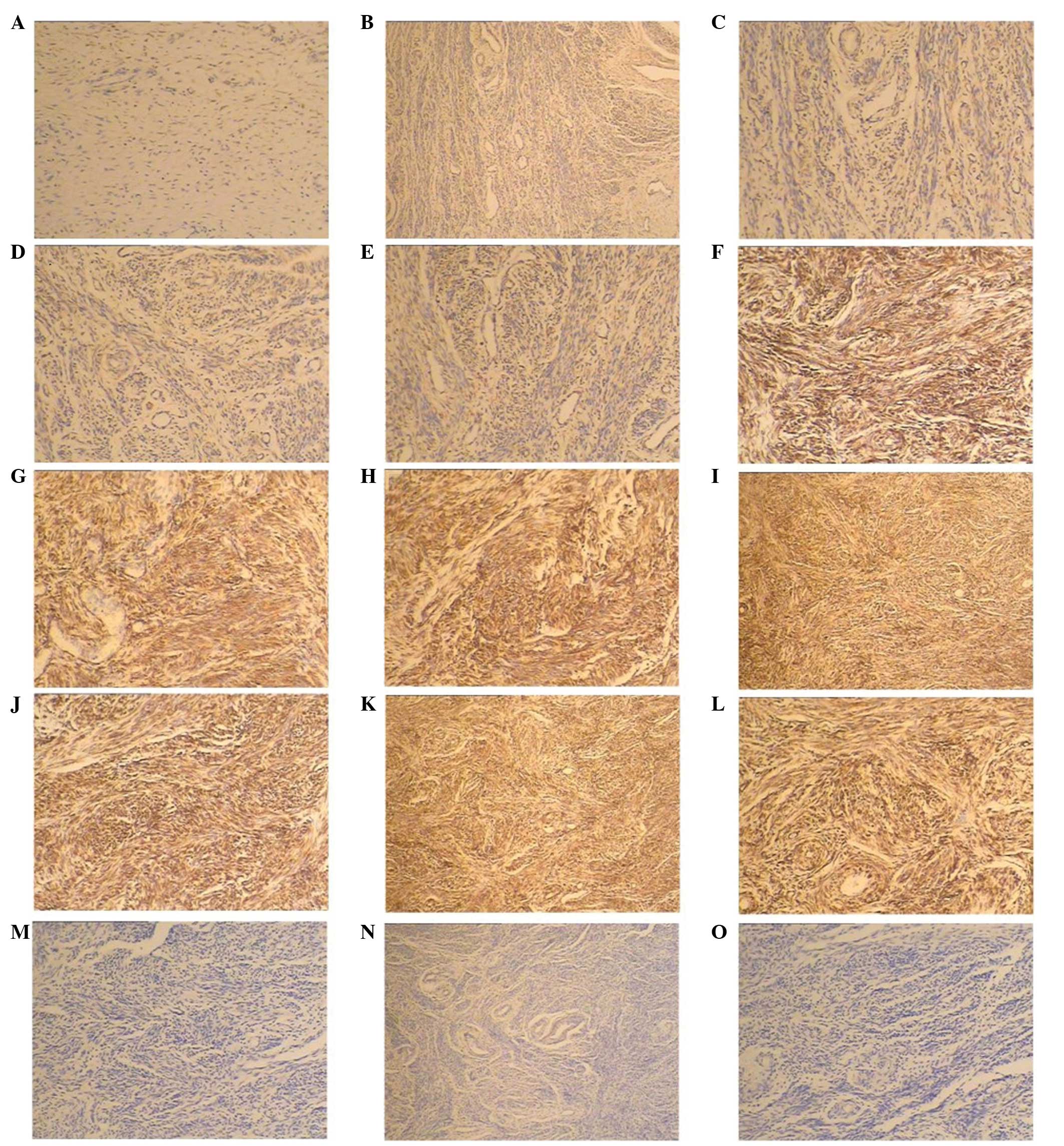
Immunohistochemistry
Levels of IFN-γ and its pathway-associated genes
(IFNGR1, IFNGR2, JAK1, STAT1 and NF-κB) were detected by
immunohistochemical analysis using specifically targeted
antibodies. Immunohistochemical staining revealed that all the
proteins were expressed in the ECM. Compared with the control
group, the protein expression levels were upregulated in the POP
group (Fig. 2).
Discussion
Numerous genes, including actin, myosin,
ECM-associated genes and transcription factors, have been studied
and shown to be associated with POP (11–13).
However, there have been a limited number of studies investigating
the role of immune-associated genes in POP. In the present study,
the mRNA and protein expression levels of IFN-γ, IFNGR1, IFNGR2 and
their pathway-associated genes, JAK1, STAT1 and NF-κB, were
demonstrated to increase in the ECM of the vaginal tissue of
patients with POP, as compared with the control group patients.
The JAK-STAT signaling pathway is a general pathway
involved in IFN activation. Initially, IFNs bind to their specific
receptors: IFN-α receptor 1 (IFNAR1) and IFNAR2 for type I IFNs;
IFNGR1 and IFNGR2 for IFN-γ; and IFN-λ receptor 1 and IL-10
receptor 2 for type III IFNs. Once the receptor complexes are fully
assembled, the JAK-STAT signaling pathway is activated. Differences
exist with regard to the interactions between STAT members and the
different types of IFNs; for example, STAT1 and STAT2 interact with
type I and type III IFNs, while STAT1 interacts with IFN-γ
(14). In addition to the JAK-STAT
signaling pathway, other pathways, including the NF-κB and
mitogen-activated protein kinase (MAPK) signaling pathways, mediate
the expression levels of IFNs (8).
The results of the present study demonstrated
significantly increased levels of IFN-γ, IFNGR1 and IFNGR2 in the
ECM of females with POP, which was in concordance with the
observations of the study by Brizzolara et al (2). Combined with the results of this
previous study, a hypothesis was proposed that the increase in the
expression levels of IFN-γ and its receptors in females with POP is
a result of the ECM NF-κB cascade. Conformational changes of the
ECM in females with POP have been shown to result in the
reorganization of the cellular cytoskeleton (15) and the activation of the MAPK
(16) and NF-κB signaling pathways
(17). To confirm this, the
present study investigated the expression levels of NF-κB in the
ECM using qPCR. The results clearly demonstrated that NF-κB
expression was upregulated in patients with POP (FC, 5.1). These
results were also supported by extensive analysis of the promoters
of IFN-γ and its receptors, which are controlled almost entirely by
IRFs and NF-κB.
The ECM component of connective tissue provides
structural integrity and a three-dimensional scaffold. During POP
etiopathology, inflammation was observed in the ECM. Although no
inflammatory cells were observed, microarray studies have revealed
that the expression levels of a number of inflammation-associated
genes are elevated, indicating that the immune system plays a role
in the etiopathology of POP and that there are alternative
functions of these inflammatory processes (2).
IFN-γ, a T helper-1 cytokine, is not only a key
mediator of antiviral defense, but also a mediator of inflammation.
The cytokine has been shown to induce chemokines, including
chemokine (C-X-C motif) ligand (CXCL)9 and CXCL10, which are
associated with inflammation and disease progression (18). Using microarray techniques,
Brizzolara et al (2)
demonstrated that numerous immune-associated genes, including CXCL2
and CXC receptor 4, were strongly induced in POP. However, whether
IFN-γ also plays a regulatory role in POP should be determined in
future studies. In conclusion, the present study confirmed that
IFN-γ and associated pathway genes were upregulated in patients
with POP, and that this phenomenon was associated with NF-κB.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 2010–2013).
References
|
1
|
Delancey JO: Anatomy and biomechanics of
genital prolapse. Clin Obstet Gynecol. 36:897–909. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brizzolara SS, Killeen J and Urschitz J:
Gene expression profile in pelvic organ prolapse. Mol Hum Reprod.
15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moalli PA, Jones Ivy S, Meyn LA and
Zyczynski HM: Risk factors associated with pelvic floor disorders
in women undergoing surgical repair. Obstet Gynecol. 101:869–874.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swift SE, Tate SB and Nicholas J:
Correlation of symptoms with degree of pelvic organ support in a
general population of women: what is pelvic organ prolapse? Am J
Obstet Gynecol. 189:372–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alarab M, Bortolini MA, Drutz H, Lye S and
Shynlova O: LOX family enzymes expression in vaginal tissue of
premenopausal women with severe pelvic organ prolapse. Int
Urogynecol J. 21:1397–1404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen B, Wen Y and Polan ML: Elastolytic
activity in women with stress urinary incontinence and pelvic organ
prolapse. Neurourol Urodyn. 23:119–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skala CE, Petry IB, Albrich S, Puhl A,
Naumann G and Koelbl H: The effect of genital and lower urinary
tract symptoms on steroid receptor expression in women with genital
prolapse. Int Urogynecol J. 22:705–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pestka S, Krause CD and Walter MR:
Interferons, interferon-like cytokines, and their receptors.
Immunol Rev. 202:8–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tukaj S, Kotlarz A, Jóźwik A, Smoleńska Z,
Bryl E, Witkowski JM and Lipińska B: Cytokines of the Th1 and Th2
type in sera of rheumatoid arthritis patients; correlations with
anti-Hsp40 immune response and diagnostic markers. Acta Biochim
Pol. 57:327–332. 2010.PubMed/NCBI
|
|
10
|
Bump RC, Mattiasson A, Bø K, Brubaker LP,
DeLancey JO, Klarskov P, Shull BL and Smith AR: The standardization
of terminology of female pelvic organ prolapse and pelvic floor
dysfunction. Am J Obstet Gynecol. 175:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Söderberg MW, Byström B, Kalamajski S,
Malmström A and Ekman-Ordeberg G: Gene expressions of small
leucine-rich repeat proteoglycans and fibulin-5 are decreased in
pelvic organ prolapse. Mol Hum Reprod. 15:251–257. 2009.PubMed/NCBI
|
|
12
|
Kökçü A, Yanik F, Cetinkaya M, Alper T,
Kandemir B and Malatyalioglu E: Histopathological evalution of the
connective tissue of the vaginal fascia and the uterine liagments
in women with and without pelvic relaxation. Arch Gynecol Obstet.
266:75–78. 2002.PubMed/NCBI
|
|
13
|
Chen B, Wen Y, Zhang Z, Guo Y, Warrington
JA and Polan ML: Microarray analysis of differentially expressed
genes in vaginal tissues from women with stress urinary
incontinence compared with asymptomatic women. Hum Reprod.
21:22–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vilcek J: Novel interferons. Nat Immunol.
4:8–9. 2003. View
Article : Google Scholar
|
|
15
|
Wang N, Bulter JP and Ingber DE:
Mechanotransduction across the cell surface and through the
cytoskeleton. Science. 260:1124–1127. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacKenna D, Summerour SR and Villarreal
FJ: Role of mechanical factors in modulating cardiac fibroblast
function and extracellular matrix synthesis. Cardiovasc Res.
46:257–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Zutter MM, Santoro SA and Clark RA:
A three-dimensional collagen lattice activates NF-kappaB in human
fibroblasts: role in integrin alpha2 gene expression and tissue
remodeling. J Cell Biol. 140:709–719. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abel K, La Franco-Scheuch L, Rourke T, Ma
ZM, De Silva V, Fallert B, Beckett L, Reinhart TA and Miller CJ:
Gamma interferon-mediated inflammation is associated with lack of
protection from intravaginal simian immunodeficiency virus
SIVmac239 challenge in simian-human immunodeficiency virus
89.6-immunized rhesus macaques. J Virol. 78:841–854. 2004.
View Article : Google Scholar
|