Introduction
In the past two decades, animal experiments have
indicated the protective effects of physical exercise on ischemic
stroke, including enhanced survival rates, a reduction in
neurological deficits, an alleviation of blood-brain barrier (BBB)
dysfunction and an improvement in neurovascular integrity (1–5).
Therefore, exercise preconditioning has begun to attract increasing
attention.
Our previous review summarized the association
between exercise preconditioning and brain ischemic tolerance,
involving a series of pathological changes following ischemic
stroke (6). However according to
the search results at that time, there was no study on the
antioxidative effect of pre-ischemic exercise following cerebral
ischemia.
Regular exercise training has been shown to
downregulate levels of free radicals (7), thus reducing the peroxidation levels
of lipids or proteins in the brain of the rat (8). Furthermore, regular exercise training
promoted the effect of antioxidant enzymes, including superoxide
dismutase (SOD) and glutathione peroxidase (9–11).
Since malondialdehyde (MDA) plays a key role in the process of
ischemic stroke, the concentration of MDA in brain tissues and
plasma has been used to assess the severity of neuronal ischemic
injury (12–14).
A recent study indicated that pre-ischemic treadmill
training for three weeks could alleviate brain oxidative damage by
suppressing 4-hydroxy-2-nonenal-modified proteins and
8-hydroxy-2′-deoxyguanosine following ischemic stroke (15). In the study, the SOD activity in
the exercise plus sham group was significantly higher than that in
the sham group. However, whether pre-ischemic exercise can regulate
the ischemic stroke-induced changes in SOD activity and MDA level
remains unknown. Thus, the aim of the present study was to explore
whether exercise preconditioning can regulate SOD and MDA and
thereby decrease oxidative stress following MCAO.
Materials and methods
Animals
Fifty-four male Sprague Dawley rats, each weighing
200–220 g, were obtained from the Hebei Province Laboratory Animal
Center (Shijiazhuang, China). All the rats were kept under a 12-h
light/dark cycle. Food and water were available ad libitum.
All the procedures in this study were approved by the Animal Care
and Use Committee of Hebei Medical University (Shijiazhuang,
China).
Treadmill training
Rats were randomly divided into three groups (n=18
per group): Sham surgery, middle cerebral artery occlusion (MCAO)
without exercise and MCAO with exercise. Prior to formal training,
rats in the MCAO with exercise group underwent adaptive running
exercise training for two days at a speed of 5–8 m/min for 30
min/day on a treadmill training machine (DSPT-202 Type 5-Lane
Treadmill; Litai Biotechnology Co., Ltd., Shishi, China). Following
the adaptive exercise training, the rats underwent formal treadmill
training at a speed of 20 m/min, 30 min/day for six days every
week. The rats in the sham and MCAO without exercise groups did not
receive exercise but were allowed to run freely in their living
cages during the same time period.
MCAO model
Following the treadmill training, rats received MCAO
surgery. Animals were anesthetized using 4% chloral hydrate (10
ml/kg, intraperitoneal) and were administered further doses if
necessary to maintain the anesthesia state during the process of
surgery. The body temperature of the rat was maintained at 37°C by
a heating pad. The surgical procedures were performed in accordance
with those described by Longa et al (16) with minor modification.
Briefly, the left external carotid artery (ECA),
common carotid artery (CCA) and internal carotid artery (ICA) were
firstly exposed. A monofilament (4–0 nylon suture) with a blunted
poly-L-lysine coated tip (Beijing Sunbio Biotech Co., Ltd.,
Beijing, China.) was lightly inserted into the ECA. The suture then
moved through the CCA and ICA, and finally occluded the MCA at its
origin. After 90 min of MCAO, reperfusion was performed by removing
the filament.
For the sham group, the CCA, ECA and ICA underwent
the same procedures without occlusion of the MCA. The associated
physiological parameters were monitored by a Blood Gas and
Electrolyte System (ABL505; Radiometer Medical ApS, Copenhagen,
Denmark). Rats were assessed 24 h after reperfusion according to a
widely accepted scale as follows: 0, no neurological symptoms; 1,
unable to completely extend the front jaw on the contralateral
side; 2, rotating while crawling and falling to the contralateral
side; 3, unable to walk without assistance; and 4, unconsciousness
(16).
Determination of brain infarct
volume
Twenty-four hours after reperfusion, animals were
sacrificed by decapitation under chloral hydrate (10%) anesthesia.
The whole brains were stored in a refrigerator at −20°C for 10 min,
following which each brain was cut into six coronal sections (2 mm
thick) between the anterior pole and the optic chiasm in the
center. All tissues were immediately infiltrated into a 2%
2,3,5-triphenyltetrazolium chloride solution at 37°C thermostat for
30 min, then fixed in 4% paraformaldehyde buffer. After 24 h, a
digital camera (DC240; Kodak, Rochester, NY, USA) and imaging
software (Adobe Photoshop 7.0; Adobe Systems Inc., San Jose, CA,
USA) were used to capture images and calculate the infarction area.
The total infarction volume was equal to the sum of the infarct
area in each section. In order to minimize the error caused by
brain edema, the corrected formula to calculate infarct volume was
as follows: Infarct volume = contralateral hemisphere region -
non-infarcted region in the ipsilateral hemisphere. The following
formula was used to calculate infarct percentage: Infarct
percentage = infarct volume/volume of the contralateral hemisphere
× 100%
Preparation of brain tissue
Twenty-four hours after reperfusion, the left
hemisphere of the rat brain was harvested following perfusion
transcardially with 0.9% saline under deep anesthesia. Briefly, the
tissues were immersed into radio-immunoprecipitation assay lysis
buffer and homogenized mechanically at 37°C. The homogenate was
centrifuged at 10,000 × g for 4 min at 4°C and the supernatant was
collected. Protein concentration was measured using an Enhanced
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China).
SOD activity detection
SOD activity in the brain tissue was detected using
the methodology described by Oyanagui (17) according to the kit instructions
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The
main principle was as follows: Superoxide anions were generated in
the xanthine and xanthine oxidase system. These superoxide anions
oxidized hydroxylamine, resulting in the formation of nitrite. This
nitrite reacted with sulfanilic acid and naphthalene diamine,
generating a colored product (18). SOD in the brain tissue decreased
the superoxide anion concentration, thus reducing the colorimetric
signal and absorbance, which was generally measured at 550 nm. One
unit of SOD activity was defined as the amount of enzyme that was
required to inhibit the 50% reduction of nitroblue tetrazolium in
the specified conditions. The procedures were repeated three times
in order to obtain the mean values.
MDA assay detection
The concentration of MDA was detected by a
thiobarbituric acid reaction method (19). Thiobarbituric acid reacts with MDA
in acidic medium resulting in a pink-colored pigment at 95°C. The
absorbance at a wavelength of 532 nm was detected by a microplate
reader (DU640; Beckman Coulter Inc., Miami, FL, USA). The
procedures were performed based on the kit instructions (Nanjing
Jiancheng Bioengineering Institute). MDA content (nmol/mg protein)
was calculated using the following formula: Absorbance of sample
tube/absorbance of standard tube × 2.5. The procedures were
repeated three times in order to obtain the mean values.
Statistical analysis
Statistical analysis was performed by SPSS for
Windows, version 15.0 (SPSS Inc., Chicago, IL, USA). The
neurological deficit scores and infarct volume between ischemic
rats with and without pre-ischemic exercise were compared by an
independent Student’s t-test. The differences in SOD enzyme
activity units and MDA concentration among the three groups were
analyzed by one-way analysis of variance followed by a post hoc
least significant difference test. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference in all statistical
assessments.
Results
Physiological variables
Fifty-four rats were divided into three groups (n=18
per group): Sham surgery, MCAO without exercise and MCAO with
exercise. No significant differences were observed in the partial
pressure of oxygen in arterial blood, the partial pressure of
carbon dioxide in arterial blood or pH (hydrogen ion concentration)
values among the three groups (P>0.05; data not shown).
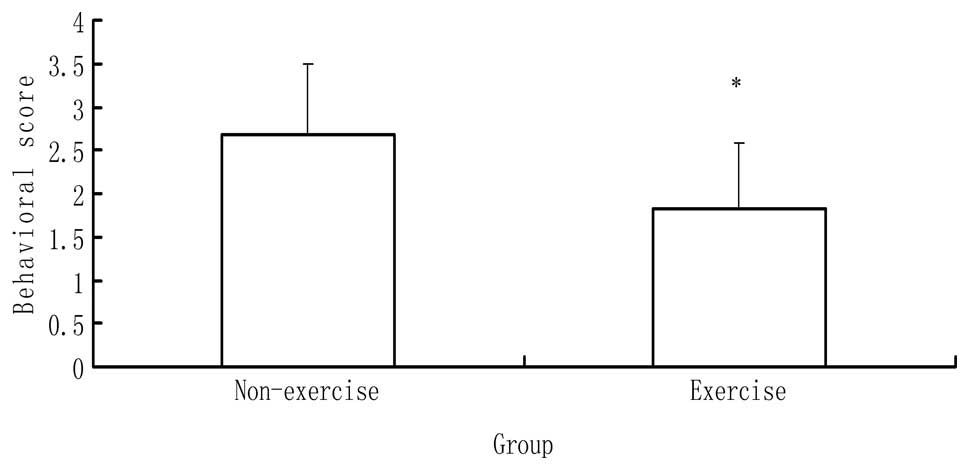
Behavioral scores
Rats in the three groups were evaluated 24 h after
reperfusion. In the sham surgery group, the rats exhibited no
neurological symptoms. By contrast, a significant difference in
behavioral scores was identified between the MCAO with and without
exercise groups (P<0.05), as shown in Fig. 1. The rats in the MCAO with exercise
group showed fewer neurological deficits than those in the MCAO
without exercise group.
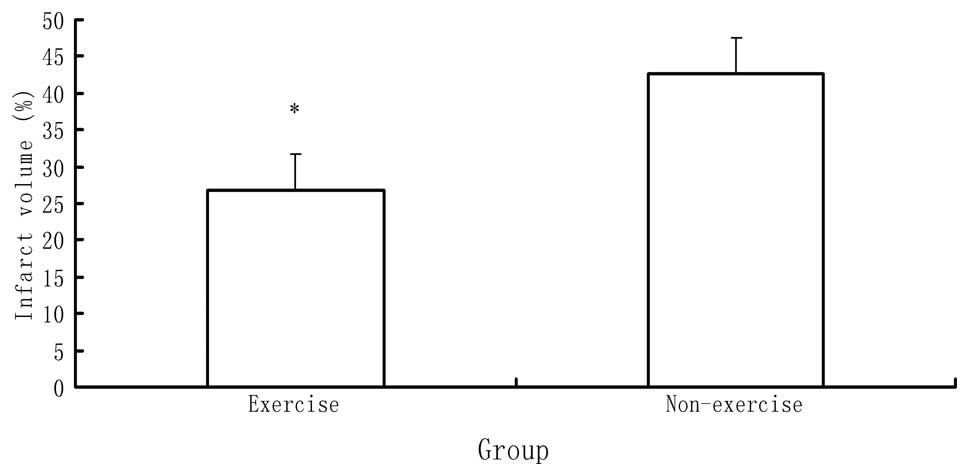
Infarct volume
Subsequent to behavioral evaluation, six rats in
each group were sacrificed by decapitation to determine the infarct
volume. The rats in the sham surgery group showed no ischemic
areas. By contrast, a significant difference in infarct volume was
identified between the MCAO with and without exercise groups
(P<0.05), as indicated in Fig.
2. The rats in the MCAO with exercise group showed a
significantly reduced ischemic area in the brain relative to those
in the MCAO without exercise group.
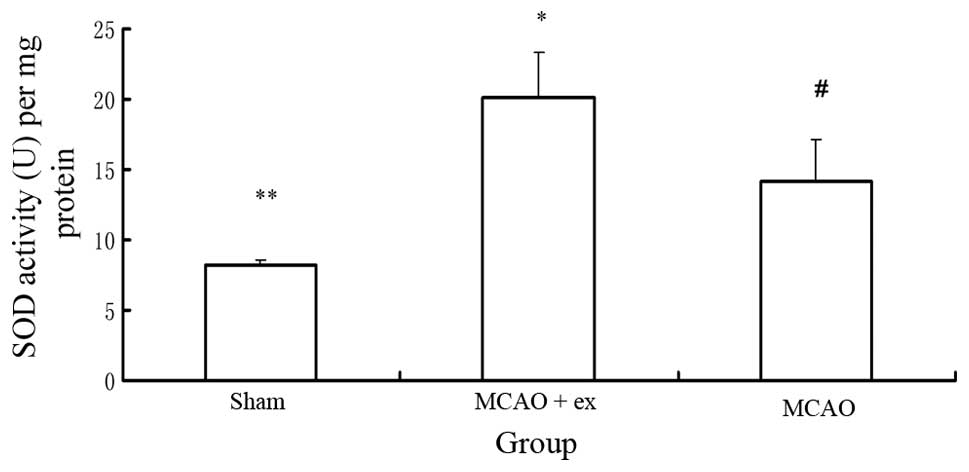
SOD activity
Six rats in each group were sacrificed by
decapitation to determine the SOD activity. A significant
difference in SOD activity was identified among the three groups
(P<0.05), as indicated in Fig.
3. The rats in the MCAO with exercise group showed a higher SOD
activity in the brain relative to those in the MCAO without
exercise group (P<0.05). The rats in the MCAO with and without
exercise groups showed higher SOD activity in the brain relative to
those in the sham surgery group (P<0.05).
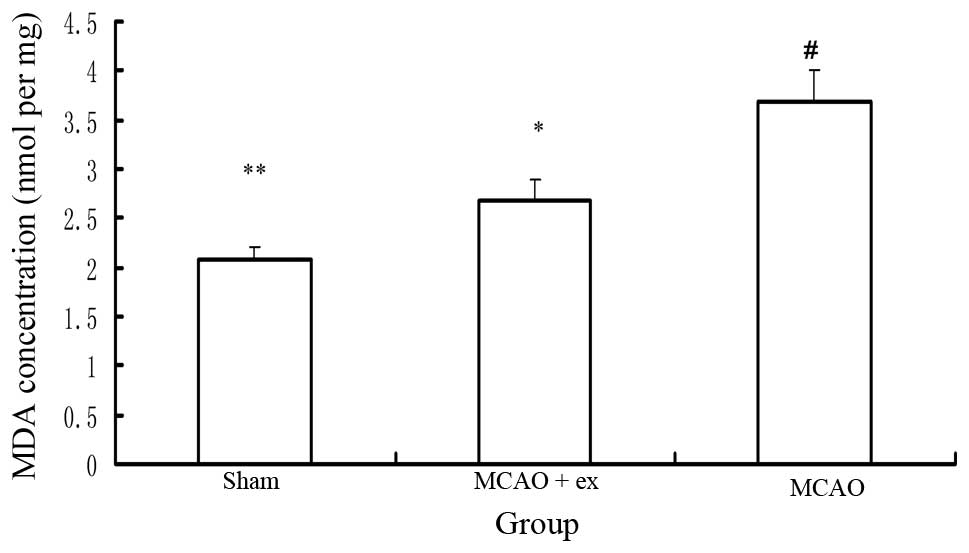
MDA concentration
Six rats in each group were sacrificed by
decapitation to determine the MDA concentration. A significant
difference in MDA concentration was identified among the three
groups (P<0.05), as indicated in Fig. 4. The rats in the MCAO with exercise
group exhibited a lower MDA concentration in the brain relative to
those in the MCAO without exercise group (P<0.05). The rats in
the MCAO with and without exercise groups showed a higher MDA
concentration in the brain relative to those in the sham surgery
group (P<0.05).
Discussion
Ischemic stroke is the third leading cause of
mortality in Western countries (20). Therefore, strategies to prevent
stroke and reduce brain damage following stroke have attracted
increasing attention. A number of interventions have been
investigated to achieve this, including physical activity (21).
A series of clinical studies on human subjects have
explored the association between pre-ischemic exercise and the risk
of ischemic stroke. These studies have produced different results.
Certain studies have demonstrated that pre-ischemic exercise
results in improved functional outcomes following stroke and a
reduction in the ischemic stroke risk (21–24).
The mechanisms underlying the neuroprotective effects of exercise
pretreatment on post-stroke function and the occurrence of stroke
are not clear according to these epidemiological studies, but have
been revealed to be associated with the effects of pre-ischemic
exercise on metabolic pathways, blood pressure, blood cholesterol
and glucose level (22–25). By contrast, a prospective clinical
study has indicated that pre-ischemic exercise may reduce the
occurrence of ischemic stroke, but does not alleviate dysfunction
following ischemic stroke (25).
Thus, on the basis of the above-mentioned clinical studies, it is
perhaps difficult to conclude that pre-ischemic exercise provides
beneficial effects on the pathogenesis of stroke; however, it
appears that the majority of the associated evidence demonstrates
that pre-ischemic exercise exerts a beneficial effect on both the
occurrence of and functional outcomes following ischemic
stroke.
With regard to the mechanism underlying the effect
of preconditioning exercise following ischemic stroke, animal
experiments reported that pre-ischemic exercise improved BBB
function and enhanced basal lamina integrity subsequent to ischemic
stroke (26). Additionally,
pre-ischemic exercise for three weeks increased cerebrovascular
integrity in the striatum of rats (27,28).
Our previous studies demonstrated that exercise preconditioning
reduced the over-release of glutamate and influenced glutamate
receptor changes, alleviating brain damage subsequent to stroke
(29,30).
All the above-mentioned neuroprotective effects of
pre-ischemic exercise may be indirectly associated with the
antioxidant ability of exercise pretreatment following ischemic
stroke. It is logically speculated that pre-ischemic exercise may
alleviate BBB dysfunction, reduce glutamate over-release and
increase cerebrovascular integrity so as to reduce oxidative stress
following cerebral ischemia.
Lipid peroxidation is induced by high levels of free
radicals, which can be caused by the cerebral ischemia-reperfusion
(31–33). Long-term exercise training
increases the antioxidant abilities of brain tissue (34). The present data also demonstrated
that pre-ischemic exercise increased SOD activity and decreased MDA
levels, thus providing a protective effect on oxidative injury
following MCAO. The pre-ischemic exercise also decreased infarct
volume and neurological deficits. However, further study is
required to explore the mechanism underlying the antioxidant effect
of pre-ischemic exercise training following ischemic stroke.
In conclusion, the results in the present study
indicated that treadmill training exercise prior to
MCAO/reperfusion increased antioxidant ability and decreased
oxidative damage in the brain. Therefore, the pre-ischemic exercise
alleviated brain damage and reduced motor dysfunction subsequent to
MCAO. These results could be beneficial for the development of
rational programs of prevention and treatment for ischemic
stroke.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81201512).
References
|
1
|
Stummer W, Baethmann A, Murr R, et al:
Cerebral protection against ischemia by locomotor activity in
gerbils. Underlying mechanisms. Stroke. 26:1423–1430. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ang ET, Wong PT, Moochhala S and Ng YK:
Neuroprotection associated with running: is it a result of
increased endogenous neurotrophic factors? Neuroscience.
118:335–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Endres M, Gertz K, Lindauer U, et al:
Mechanisms of stroke protection by physical activity. Ann Neurol.
54:582–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Luan X, Clark JC, et al:
Neuroprotection against transient cerebral ischemia by exercise
pre-conditioning in rats. Neurol Res. 26:404–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding YH, Ding Y, Li J, et al: Exercise
pre-conditioning strengthens brain microvascular integrity in a rat
stroke model. Neurol Res. 28:184–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang F, Wu Y and Jia J: Exercise
preconditioning and brain ischemic tolerance. Neuroscience.
17:170–176. 2011. View Article : Google Scholar
|
|
7
|
Radak Z, Toldy A, Szabo Z, et al: The
effects of training and detraining on memory, neurotrophins and
oxidative stress markers in rat brain. Neurochem Int. 49:387–392.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radák Z, Kaneko T, Tahara S, et al:
Regular exercise improves cognitive function and decreases
oxidative damage in rat brain. Neurochem Int. 38:17–23.
2001.PubMed/NCBI
|
|
9
|
Somani SM, Ravi R and Rybak LP: Effect of
exercise training on antioxidant system in brain regions of rat.
Pharmacol Biochem Behav. 50:635–639. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Somani SM and Husain K: Interaction of
exercise training and chronic ethanol ingestion on antioxidant
system of rat brain regions. J Appl Toxicol. 17:329–336. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lappalainen Z, Lappalainen J, Oksala NK,
et al: Diabetes impairs exercise training-associated thioredoxin
response and glutathione status in rat brain. J Appl Physiol
(1985). 106:461–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bromont C, Marie C and Bralet J: Increased
lipid peroxidation in vulnerable brain regions after transient
forebrain ischemia in rats. Stroke. 20:918–924. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo Y, Asanuma M, Nishibayashi S, et al:
Late-onset lipid peroxidation and neuronal cell death following
transient forebrain ischemia in rat brain. Brain Res. 772:37–44.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Re G, Azzimondi G, Lanzarini C, et al:
Plasma lipoperoxidative markers in ischaemic stroke suggest brain
embolism. Eur J Emerg Med. 4:5–9. 1997.PubMed/NCBI
|
|
15
|
Hamakawa M, Ishida A, Tamakoshi K, et al:
Repeated short-term daily exercise ameliorates oxidative cerebral
damage and the resultant motor dysfunction after transient ischemia
in rats. J Clin Biochem Nutr. 53:8–14. 2013. View Article : Google Scholar
|
|
16
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oyanagui Y: Reevaluation of assay methods
and establishment of kit for superoxide dismutase activity. Anal
Biochem. 142:290–296. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giannopolitis CN and Ries SK: Superoxide
dismutases: I. Occurrence in higher plants. Plant Physiol.
59:309–314. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Center for Health Statistics
(US). Health, United States, 2010: With Special Feature on Death
and Dying. Public Health Service. Report no. 2011–1232. US
Department of Health and Human Services; Hyattsville, MD: 2011
|
|
21
|
Krarup LH, Truelsen T, Gluud C, et al:
Prestroke physical activity is associated with severity and
long-term outcome from first-ever stroke. Neurology. 71:1313–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deplanque D, Masse I, Lefebvre C, et al:
Prior TIA, lipid-lowering drug use, and physical activity decrease
ischemic stroke severity. Neurology. 67:1403–1410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sacco RL, Gan R, Boden-Albala B, et al:
Leisure-time physical activity and ischemic stroke risk: the
Northern Manhattan Stroke Study. Stroke. 29:380–387. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stroud N, Mazwi TM, Case LD, et al;
Ischemic Stroke Genetics Study Investigators. Prestroke physical
activity and early functional status after stroke. J Neurol
Neurosurg Psychiatry. 80:1019–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rist PM, Lee IM, Kase CS, et al: Physical
activity and functional outcomes from cerebral vascular events in
men. Stroke. 42:3352–3356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo M, Cox B, Mahale S, et al:
Pre-ischemic exercise reduces matrix metalloproteinase-9 expression
and ameliorates blood-brain barrier dysfunction in stroke.
Neuroscience. 151:340–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Y, Li J, Luan X, et al: Exercise
pre-conditioning reduces brain damage in ischemic rats that may be
associated with regional angiogenesis and cellular overexpression
of neurotrophin. Neuroscience. 124:583–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding YH, Li J, Yao WX, et al: Exercise
preconditioning upregulates cerebral integrins and enhances
cerebrovascular integrity in ischemic rats. Acta Neuropathol.
112:74–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Jia J, Wu Y, et al: The effect of
treadmill training pre-exercise on glutamate receptor expression in
rats after cerebral ischemia. Int J Mol Sci. 11:2658–2669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Wu Y, Jia J and Hu YS:
Pre-ischemic treadmill training induces tolerance to brain
ischemia: involvement of glutamate and ERK1/2. Molecules.
15:5246–5257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheeseman KH and Slater TF: An
introduction to free radical biochemistry. Br Med Bull. 49:481–493.
1993.PubMed/NCBI
|
|
32
|
Esterbauer H: Estimation of peroxidative
damage. A critical review. Pathol Biol (Paris). 44:25–28.
1996.PubMed/NCBI
|
|
33
|
Romero FJ, Bosch-Morell F, Romero MJ, et
al: Lipid peroxidation products and antioxidants in human disease.
Environ Health Perspect. 106(Suppl 5): 1229–1234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radak Z, Kumagai S, Taylor AW, et al:
Effects of exercise on brain function: role of free radicals. Appl
Physiol Nutr Metab. 32:942–946. 2007. View
Article : Google Scholar : PubMed/NCBI
|