Introduction
Hepatitis C is an infectious disease that is
prevalent around the world. Hepatitis C virus (HCV) induces chronic
infection in ~80% of infected individuals. Approximately 10–20% of
patients develop cirrhosis and nearly 5% will eventually suffer
from liver cancer (1). The aim of
HCV treatment is to clear the virus and the symptoms associated
with hepatitis C infection (2,3). At
present, combined treatment with pegylated interferon α (PEG-IFN)
and the antiviral drug ribavirin is the standard approach for HCV
treatment (4–6). In a previous study, the sustained
virological response (SVR) was found to be 8, 15 and 30% in
patients with chronic hepatitis C and cirrhosis following treatment
with IFN, 90 μg PEG-IFN and 180 μg PEG-IFN, respectively (7). In the present study, the effect of
Fuzheng Huayu capsule combined with Pegasys (peginterferon α-2a)
was investigated on cell apoptosis in rats with dimethylnitrosamine
(DMN)-induced liver injury and on liver function and fibrosis in
patients with hepatitis C and hepatic cirrhosis. These results may
provide a novel therapeutic strategy for the treatment of patients
with hepatic cirrhosis.
Materials and methods
Animals
A total of 60 Wistar male rats (200±10 g) were
provided by Jiangsu Laboratory Animal Center (Suzhou, China). This
study was performed in accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (8th edition, Bethesda, MD, USA, 2010). The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of the Fifth People’s Hospital of
Wuxi (Wuxi, China). The rats were randomly divided into two groups,
a normal control group (n=20) and a model group (n=40). After four
weeks, the model group was subdivided into a model control group
and a treatment group (Fuzheng Huayu capsule combined with
Pegasys). The rats were administered a gavage of distilled water or
a solution of the combined drugs [4.5 g Fuzheng Huayu (6 capsules)
and 180 μg Pegasys in 10 ml distilled water] respectively, at a
dosage of 10 ml/kg, six times a week for four weeks. Blood was then
taken from the inferior vena cava and the serum was obtained
through centrifugation. In addition, liver tissues were prepared
for further experiments.
The model group was intraperitoneally injected with
0.5% DMN (2 ml/kg). The injections were administered on three
successive days with two-thirds of a full dose and then no
injections were administered for 4 days. This was continued for
four weeks and the last two injections were performed with one shot
of two-thirds of a full dose and one shot of half of a full dose,
respectively. An equal volume of saline was injected in the normal
group. Once the DMN model was established, two rats were randomly
sacrificed and the pathological condition of the liver was analyzed
using an α-SMA monoclonal antibody (Dako, Agilent Technologies,
Santa Clara, CA, USA). The model was successfully established since
liver fibrosis was observed. Following fixation of the liver
tissues with 10% formalin, dehydration, transparency and wax
immersion, cell apoptosis indices [apoptotic hemopoietic stem cell
(HSC), apoptotic hepatocyte and α-smooth muscle actin (SMA) levels]
were analyzed using terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL; Roche, Basel, Switzerland), α-SMA and Masson
triple staining (both from Shanghai Bogoo Biotechnology Co., Ltd.,
Shanghai, China). These experimental procedures were performed in
accordance with the manufacturers’ instructions.
Patients
A total of 100 patients with genotype 1 hepatitis C
and hepatic cirrhosis from the Fifth People’s Hospital of Wuxi
(Wuxi, China) were analyzed. The patients were randomly divided
into a treatment group (group A) and a control group (group B) with
50 patients in each group. In the treatment group, there were 28
males and 22 females, aged 42.3±10.2 years old and with a disease
course of 5.7±1.1 years. In the control group, there were 26 males
and 24 females aged 40.3±3.4 years old and with a disease course of
7.2±2.2 years. All patients were HCV antibody positive, had an HCV
RNA level of ≥500 cps/ml and an Ishak fibrosis score of 2, 3 or 4
(8). This study was performed in
accordance with the Declaration of Helsinki and with approval from
the Ethics Committee of the Fifth People’s Hospital of Wuxi.
Written informed consent was obtained from all participants.
Clinical trials
The two groups of patients were treated with 180 μg
Pegasys (Shanghai Roche Pharmaceuticals Co., Ltd, Shanghai, China)
combined with 1,000–1,200 μg ribavirin for 48 weeks. The patients
in group A were also treated with a 4.5 g Fuzheng Huayu capsule
(Shanghai Yellow Sea Pharmaceutical Co., Ltd., Shanghai, China)
each day. The patients in group B were given a placebo. The levels
of aminotransferase (ALT), total bilirubin (TBiL), aspartate
aminotransferase (AST), albumin, α-fetoprotein (AFP) and total bile
acids (TBA) were determined using a Beckman-Coulter 3XL flow
cytometry instrument (Beckman-Coulter, Miami, FL, USA), a CEQ-8000
sequencer instrument (Beckman-Coulter, Miami, FL, USA), a PE-9600
PCR instrument (Perkin-Elmer, Fremont, CA, USA) and an 7600
automatic biochemistry analyzer (Hitachi Ltd., Tokyo, Japan). Liver
fibrosis indicators, including serum hyaluronic acid (HA), laminin
(LN), type IV collagen (IVC) and type III procollagen (PCIII) were
detected using an ELISA kit (Euroimmun, Lübeck, Germany) and a
TUNEL apoptosis kit (Roche, Basel, Swiss). Fas and FasL levels of
PBMCs were detected with a Fas and FasL testing kit (Shenzhen
Jingmei Biotech Co., Ltd., Shenzhen, China) according to the
manufacturer’s instructions.
Statistical analysis
The quantitative data was presented as the mean ±
standard deviation and analyzed using SPSS software, version 16.0
(SPSS Inc., Chicago, IL, USA). Statistical analysis was performed
using a Student’s t-test for comparison and a Spearman’s rank
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
Animal results
The TUNEL, α-SMA and Masson staining results
revealed that there were no apoptotic HSCs in the normal group.
However, in DMN rats, a number of apoptotic HSCs were observed
around the hepatic sinusoid. The number of apoptotic HSCs was
greater in the DMN model rats than in the DMN group treated with
Fuzheng Huayu capsule combined with Pegasys. A small number of
hepatocytes became apoptotic in the normal group, while there was a
significant increase in the number of apoptotic hepatocytes in the
DMN group. The number of apoptotic cells was markedly reduced in
the rats treated with Fuzheng Huayu capsule combined with that in
the model rats (Table I).
 | Table INumber of apoptotic HSCs and
hepatocytes and the expression of α-SMA in the three groups of
rats. |
Table I
Number of apoptotic HSCs and
hepatocytes and the expression of α-SMA in the three groups of
rats.
| Group | Apoptotic HSCs (per
high power field, magnification, ×40) | Apoptotic hepatocytes
(per high power field, (magnification, ×40) | α-SMA (percentage of
positive area) |
|---|
| Normal | 0 | 0.95±0.27 | 1.46±0.13 |
| Model | 0.15±0.05 | 3.22±0.25 | 8.42±0.53 |
| Treatment | 0.27±0.08 | 2.49±0.17 | 4.11±0.45 |
| F-value | 627.5 | 2.51 | 0.12 |
| P-value | <0.05 | <0.05 | <0.05 |
Clinical observations
Significant differences in ALT, TBiL, AST, ALB, AFP
and TBA were observed between the patients treated with Fuzheng
Huayu capsule combined with Pegasys and those treated with Pegasys
alone (P<0.01; Table II). In
addition, the levels of markers for hepatic fibrosis, including HA,
LN, IVC and PCIII were significantly reduced in the combined
treatment group (P<0.01; Table
III). These results indicate that Fuzheng Huayu capsule
combined with Pegasys improves the efficacy of Pegasys in
protecting the liver from fibrosis. The Fas and FasL levels in
PBMCs were detected in the blood. The expression of Fas and FasL
was attenuated following treatment with Fuzheng Huayu capsules
(Table IV). The results
demonstrate that the function of the immune system was influenced
by liver fibrosis following treatment. This suggests that liver
injury and hepatocyte apoptosis were significantly improved
following treatment with Fuzheng Huayu capsule combined with
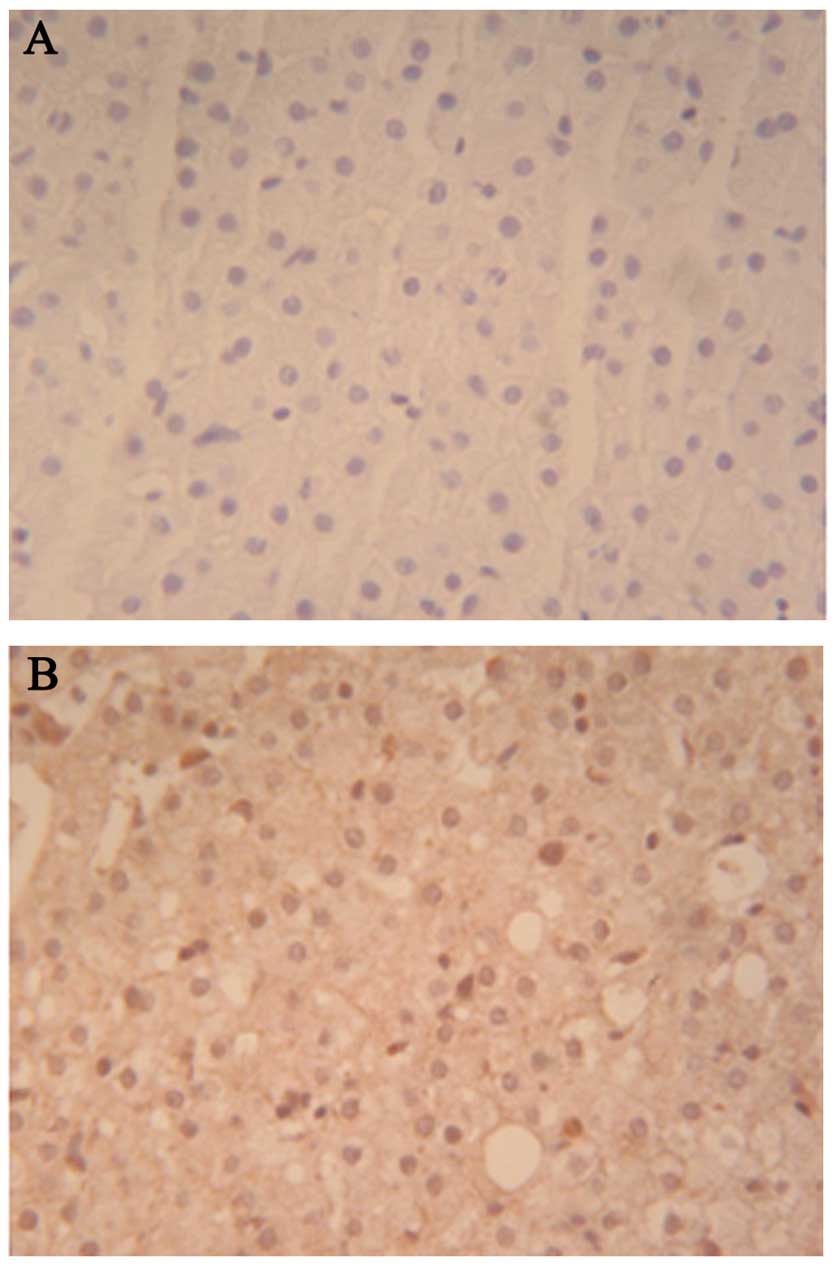
Pegasys, as indicated by liver tissue pathology (Fig. 1).
 | Table IILiver function following treatment
with Fuzheng Huayu and Pegasys (treatment) or Pegasys alone
(control). |
Table II
Liver function following treatment
with Fuzheng Huayu and Pegasys (treatment) or Pegasys alone
(control).
| Group | ALT (U/l) | TBiL (μmol/l) | AST (U/l) | ALB (G/l) | AFP (ng/ml) | TBA (μmol/l) |
|---|
| Control | 88.65±20.75 | 37.85±9.65 | 213.48±44.61 | 25.29±1.65 | 56.17±12.91 | 52.89±21.61 |
| Treatment | 33.21±14.65 | 12.38±6.45 | 65.26±21.47 | 39.46±2.47 | 19.23±5.63 | 89.34±11.78 |
| t-value | 4.30 | 2.78 | 4.51 | 5.12 | 8.56 | 4.24 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
 | Table IIISerum fibrosis indices following
treatment with Fuzheng Huayu and Pegasys (treatment) or Pegasys
alone (control). |
Table III
Serum fibrosis indices following
treatment with Fuzheng Huayu and Pegasys (treatment) or Pegasys
alone (control).
| Group | HA (μg/l) | LN (μg/l) | IVC (μg/l) | PCIII (μg/l) |
|---|
| Control | 287.04±67.27 | 145.38±67.35 | 135.19±68.79 | 202.52±49.87 |
| Treatment | 245.38±52.19 | 122.73±71.19 | 110.32±42.16 | 177.27±72.26 |
| t-value | 4.77 | 3.87 | 4.65 | 7.67 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
 | Table IVExpression of Fas and FasL in the
peripheral blood mononucleated cells of patients following
treatment with Fuzheng Huayu and Pegasys (treatment) or with
Pegasys alone (control). |
Table IV
Expression of Fas and FasL in the
peripheral blood mononucleated cells of patients following
treatment with Fuzheng Huayu and Pegasys (treatment) or with
Pegasys alone (control).
| Group | Fas | FasL |
|---|
| Control | 0.69±0.25 | 0.82±0.17 |
| Treatment | 0.42±0.15 | 0.57±0.19 |
| t-value | 6.43 | 4.78 |
| P-value | <0.05 | <0.05 |
Discussion
Chronic liver fibrosis is a common pathological
process in various chronic liver diseases. If chronic hepatitis C
is not treated effectively, it increases the risk of liver
cirrhosis and cancer (9). The
treatment and prevention of liver fibrosis has been investigated
clinically. Fuzheng Huayu capsule has been shown to alleviate blood
stasis and have a beneficial effect on the liver (10). In the present study, the effect of
Fuzheng Huayu capsule combined with Pegasys was investigated by
analyzing liver indicators following treatment.
Hepatocyte apoptosis was found to be significantly
reduced in rats with DMN-induced liver injury following treatment
with Fuzheng Huayu capsule combined with Pegasys. The decreased
activation of HSCs, alleviation of endothelial cell damage and
reduction of hepatocytes may be involved in this process. Reduced
secretion of TGF-β and TNF-α, which promote the hepatocyte
apoptosis, may be important for the function of these combined
drugs (11,12). These results demonstrated that
Fuzheng Huayu capsule combined with Pegasys improved liver fibrosis
induced by hepatitis C, which are consistent with the previously
reported studies (13–15). Fuzheng Huayu capsule includes:
Salviae miltiorrhizae, which promotes blood circulation and
alleviates blood stasis, as well as cooling and nourishing the
blood; peach seed, which improves ‘circulation of qi’, alleviates
blood stasis and activates enzymes required in bilirubin
metabolism; Gynostemma pentaphyllum, which ‘replenishes qi’
to invigorate the spleen and regulates the immunity function;
Cordyceps sinensis, which ‘tonifies the spleen’ and
strengthens humoral immunity; and Schisandra, which
‘supplements qi’ and promotes the production of body fluid to
increase the function of cell immunity (16,17,18).
The metabolism of CCl4 is also inhibited by
Schisandra to protect hepatocytes (19). Based on this, Fuzheng Huayu capsule
combined with Pegasys was used in the present study for the
treatment of liver fibrosis, and it was hypothesized that the drug
would improve the blood flow to the liver, strengthen liver
detoxification and enhance hepatocyte regeneration.
In the present study, hepatocyte apoptosis was
investigated in a DMN-induced rat model of liver fibrosis and
clinical patients following treatment with Fuzheng Huayu capsule
combined with Pegasys. The results demonstrate that there was a
significant improvement in cell apoptosis following the treatment.
The observed effects indicate that Fuzheng Huayu capsule and
Pegasys efficiently enhance the body’s immune function and promote
liver regeneration (20–22). There are two aspects of liver
fibrosis treatment: etiological treatment of the primary disease
and treatment of liver fibrosis itself. The Fuzheng Huayu capsule
combined with Pegasys treated the two aspects of the disease and
decreased serum fibrosis indexes to inhibit or delay the
development of liver fibrosis. This is the first study, to the best
of our knowledge, to use the Fuzheng Huayu capsule combined with
Pegasys for the treatment of patients with hepatitis C and hepatic
cirrhosis. The clinical results demonstrate that the combined drugs
exert certain curative effects in patients with hepatitis C and
increase their quality of life. In conclusion, the present study
provides a method for the optimization of existing treatment
strategies and for the establishment of potentially effective
combination therapies.
Acknowledgements
This study was supported by the Social Development
Project of Wuxi Administration of Science & Technology (no.
CSE01009).
References
|
1
|
Lavanchy D: The global burden of hepatitis
C. Liver Int. 29(Suppl 1): 74–81. 2009. View Article : Google Scholar
|
|
2
|
Bota S, Sporea I, Popescu A, Sirli R,
Neghina AM, Danila M and Strain M: Response to standard of care
antiviral treatment in patients with HCV liver cirrhosis - a
systematic review. J Gastrointestin Liver Dis. 20:293–298.
2011.PubMed/NCBI
|
|
3
|
Michielsen P, Ho E and Francque S: Does
antiviral therapy reduce the risk of hepatocellular carcinoma in
patients with chronic hepatitis C? Minerva Gastroenterol Dietol.
58:65–79. 2012.PubMed/NCBI
|
|
4
|
Ghany MG, Strader DB, Thomas DL and Seeff
LB: American Association for the Study of Liver Diseases:
Diagnosis, management, and treatment of hepatitis C: an update.
Hepatology. 49:1335–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asian Pacific Association for the Study of
the Liver (APASL) Hepatitis C Working Party. McCaughan GW, Omata M,
Amarapurkar D, et al: Asian Pacific Association for the Study of
the Liver consensus statements on the diagnosis, management and
treatment of hepatitis C virus infection. J Gastroenterol Hepatol.
22:615–633. 2007. View Article : Google Scholar
|
|
6
|
Guedj H, Guedj J, Negro F, et al: The
impact of fibrosis and steatosis on early viral kinetics in HCV
genotype 1-infected patients treated with Peg-IFN-alfa-2a and
ribavirin. J Viral Hepat. 19:488–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heathcote EJ, Shiffman ML, Cooksley WG, et
al: Peginterferon alfa-2a in patients with chronic hepatitis C and
cirrhosis. N Engl J Med. 343:1673–1680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordon SC: Treatment of viral
hepatitis-2001. Ann Med. 33:385–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu JW and Wang W: The relationship of rat
fibrosis and hyaluronic acid and related proteins. Chinese Journal
of Anatomy. 23:2682010.(In Chinese).
|
|
10
|
Cui AL: Treatment of 54 cases of active
hepatocirrhosis with lamivudine combined with Fu Zheng Hua Yu
capsule. Journal of China Pharmaceutical. 21:74–75. 2012.(In
Chinese).
|
|
11
|
De Bleser PJ, Niki T, Xu G, Rogiers V and
Geerts A: Localization and cellular sources of activins in normal
and fibrotic rat liver. Hepatology. 26:905–912. 1997.PubMed/NCBI
|
|
12
|
Brenndörfer ED, Weiland M, Frelin L, et
al: Anti-tumor necrosis factor α treatment promotes apoptosis and
prevents liver regeneration in a transgenic mouse model of chronic
hepatitis C. Hepatology. 52:1553–1563. 2010.
|
|
13
|
Tan CY, Tan SZ, Jiang J and Xu LM: Effects
of ‘Fuzheng Huayu Decoction’ on hepatocellular apoptosis in rats
with DMN liver fibrosis. Shanghai Journal of Traditional Chinese
Medicine. 39:47–49. 2005.(In Chinese).
|
|
14
|
Prati GM, Aghemo A, Rumi MG, et al:
Hyporesponsiveness to PegIFNα2B plus ribavirin in patients with
hepatitis C-related advanced fibrosis. J Hepatol. 56:341–347.
2012.PubMed/NCBI
|
|
15
|
Bzowej N, Nelson DR, Terrault NA, et al:
PHOENIX: A randomized controlled trial of peginterferon alfa-2a
plus ribavirin as a prophylactic treatment after liver
transplantation for hepatitis C virus. Liver Transpl. 17:528–538.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park CH, Kim DH, Park MH, et al: Chinese
prescription Kangen-karyu and Salviae Miltiorrhizae radix
improve age-related oxidative stress and inflammatory response
through the PI3K/Akt or MAPK pathways. Am J Chin Med. 42:987–1005.
2014.
|
|
17
|
Teng H and Lee WY: Antibacterial and
antioxidant activities and chemical compositions of volatile oils
extracted from Schisandra chinensis Baill. seeds using
simultaneous distillation extraction method, and comparison with
Soxhlet and microwave-assisted extraction. Biosci Biotechnol
Biochem. 78:79–85. 2014.PubMed/NCBI
|
|
18
|
Yu HY, Chen ZY, Sun B, et al: Lignans from
the fruit of Schisandra glaucescens with antioxidant and
neuroprotective properties. J Nat Prod. 77:1311–1320. 2014.
|
|
19
|
Cheng N, Ren N, Gao H, Lei X, Zheng J and
Cao W: Antioxidant and hepatoprotective effects of Schisandra
chinensis pollen extract on CCl4-induced acute liver damage in
mice. Food Chem Toxicol. 55:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li TF, Qin MZ and Hu SS: The expression of
Fas and FasL in PBMC of multiple sclerosis patients. Shanghai
Journal of Immunology. 22:1312002.(In Chinese).
|
|
21
|
Alkahtani S: Hepatitis C infection and
apoptosis in hepatocellular carcinoma. Pak J Biol Sci. 12:804–808.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaki Mel S, Auf FA, Ghawalby NA and Saddal
NM: Clinical significance of serum soluble Fas, Fas ligand and fas
in intrahepatic lymphocytes in chronic hepatitis C. Immunol Invest.
37:163–170. 2008.PubMed/NCBI
|