Introduction
Cough variant asthma (CVA) is a specific form of
asthma that presents solely with cough. A previous study has
revealed that ~30% of CVA may progress to classic asthma (1). CVA has many pathophysiological
features that are similar to those of classic asthma, including
airway hyper-responsiveness, atopy and chronic airway inflammation
(2). Airway remodeling is an
important feature of asthma and previous studies have confirmed its
existence in patients with CVA (3,4).
However, it remains to be determined whether there is a difference
between classic asthma and CVA.
The matrix metalloproteinase (MMP) family is a
protein family of zinc-dependent endopeptidases (5). MMPs are mainly involved in the
cleavage of the extracellular matrix (ECM). They also play critical
roles in a range of biological and pathological processes,
including fibrosis, inflammation and the healing of wounds
(6). A number of previous studies
have revealed the critical role of MMPs in chronic obstructive
pulmonary disease (COPD), interstitial lung disease (ILD), lung
cancer and acute lung injury (7–10).
Furthermore, several studies have demonstrated the important role
of MMP9 in classic asthma (11–13).
Patients with classic asthma have elevated levels of MMP9 in their
serum, sputum and bronchoalveolar lavage fluid (BALF). MMP9
immunoreactivity has been demonstrated to be associated with the
severity of classic asthma and MMP9-deficient animals exhibit
reduced airway inflammation (5).
However, one study reported heightened inflammation in
MMP9-deficient mice, which suggests a protective role of MMP9 in
classic asthma (14). Thus, it
remains unknown whether MMP9 participates in the airway
inflammation of CVA and whether this role is protective or
sensitizing.
Thus, in the present study, the levels of MMP9 in
the induced sputum of patients with CVA were detected. The effect
of treatment with a combination of inhaled corticosteroid and
long-acting β2-agonist (ICS/LABA) on MMP9 levels was also
observed.
Materials and methods
Patients
Twenty-four patients with a clinical diagnosis of
CVA were recruited from the Department of Respiratory Medicine of
Jining First People’s Hospital (Jining, China). Thirty-one healthy
individuals were simultaneously recruited as the control group. CVA
was diagnosed according to recommendations in the Chinese national
guidelines on the diagnosis and management of cough (15). None of the patients with CVA had
previously received inhaled or oral steroids. Pregnant women,
smokers and individuals who had had upper respiratory tract
infections during the preceding two weeks were excluded. The
individuals in the control group had no past history of asthma,
atopic diseases or other respiratory diseases. All the patients
with CVA received a therapy of ICS/LABA (salmeterol + fluticasone,
50 μg/250 μg bid; or fomoterol + budesonide, 4.5 μg/160 μg bid) for
at least six months. Ethical approval was provided by the medical
ethics committee of the Jining First People’s Hospital. Informed
consent was obtained either from the patients or the patients’
families.
Pulmonary function and bronchial
provocation tests
All patients with CVA were diagnosed via the
pulmonary function test (PFT) and bronchial provocation test (BPT).
In the present study, all patients and control subjects performed a
PFT. The PFT was carried out with a clinical spirometer (model
MS-10S; Jaeger, Magdeburg, Germany) using a previously described
method (11). Forced vital
capacity (FVC) and forced expiratory volume (FEV1) measurements
were taken and the FEV1/FVC ratio was calculated. The FEV1
percentage of predicted was calculated as the FEV1% predicted.
The BPT was performed using an aerodynamic
particle-sized aerosol provocation system (model MS-10S; Jaeger).
The provocation dose that caused a 20% reduction in FEV1
(PD20-FEV1) was measured through the inhalation of different
concentrations of histamine. The baseline FEV1 was first measured
using a spirometer. Patients inhaled a histamine aerosol from a
nebulizer with tidal breathing whilst wearing a nose clip for 2
min. The total inhalations at each histamine concentration were
administered and the FEV1 was measured three times following each
period of inhalation. The BPT test was ceased if there was a
reduction in the baseline FEV1 of 20% compared with that of the
control inhalation solution. The subject would then be considered
as airway hyper-responsive. Subjects received two puffs (200 μg) of
salbutamol from a metered dose inhaler following the BPT.
Induced sputum collection and counts
Induced sputum was collected with an aerosol of
hypertonic saline solution according to a previous method (11). Subjects inhaled hypertonic saline
(4%), which was delivered by an ultrasonic nebulizer (model 402AI,
Yuyue, Jiangsu, China). Subjects were encouraged to cough and
sputum was collected in clean polypropylene cups. The sputum
specimen was examined within 2 h and prepared as previously
described (11). A 1:10 dilution
of dithiothreitol (DTT) was added in a volume equal to four times
the weight of the selected sputum specimen. Samples were placed
into a shaking water bath at 37°C for 15 min and subsequently
further diluted with phosphate-buffered saline (PBS) to a volume
equal to that of the sputum plus DTT. The suspension was filtered
through a gauze to remove mucus and centrifuged at 120 × g for 8
min. The supernatant was aspirated and frozen at −80°C for
subsequent analysis. Cell suspensions were adjusted to
1×105/l and used for cytocentrifuge preparations. The
slides were stained with a Giemsa stain and cell counts were
performed under an optical microscope (model TS100, Nikon, Tokyo,
Japan). The percentage of eosinophils (EOS) in each slide was
calculated.
Enzyme-linked immunosorbent assay
(ELISA)
The MMP9 and interleukin (IL)-5 levels in the
supernatant of the induced sputum were detected by commercial ELISA
kits (R&D Systems, Minneapolis, MN, USA; Peprotech, Inc., Rocky
Hill, NJ, USA, respectively) according to the manufacturers’
instructions. The detection limits were 0.5 ng/ml and 2 pg/ml,
respectively.
Statistical analysis
Data are expressed as means ± standard deviations
and variables were assessed by the Kolmogorov-Smirnov test. The
Mann-Whitney U or Kruskal-Wallis one-way analysis of variance tests
were used for comparison in groups without a normal distribution.
The Student’s t-test or analysis of variance (ANOVA) were used for
comparison in groups with a normal distribution. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical data
The clinical characteristics of all subjects are
shown in Table I. There were no
significant differences in the age and gender of the two groups.
The PFT results (including FEV1/FVC and FEV1% predicted values) in
the two groups were similar. The percentage of EOS in the
peripheral blood samples was also similar in the control and CVA
groups. However, the total levels of immunoglobulin E (IgE) in the
induced sputum of patients with CVA were significantly higher than
those in the control group (565.2±46.5 vs. 133.5±15.4 IU/ml,
respectively; P<0.01).
 | Table IClinical characteristics in the two
groups. |
Table I
Clinical characteristics in the two
groups.
| Characteristic | Control group | CVA group | P-value |
|---|
| Number | 31 | 24 | |
| Gender (m/f) | 18/13 | 14/10 | >0.05 |
| Age (years) | 29.3±4.6 | 31.5±5.2 | >0.05 |
| FEV1/FVC | 89.5±7.1 | 82.8±6.5 | >0.05 |
| FEV1% predicted | 101.6±15.4 | 94.7±12.2 | >0.05 |
| Eosinophil (%) | 3.3±0.4 | 4.1±0.5 | >0.05 |
| Total IgE
(IU/ml) | 133.5±15.4 | 565.2±46.5 | <0.01 |
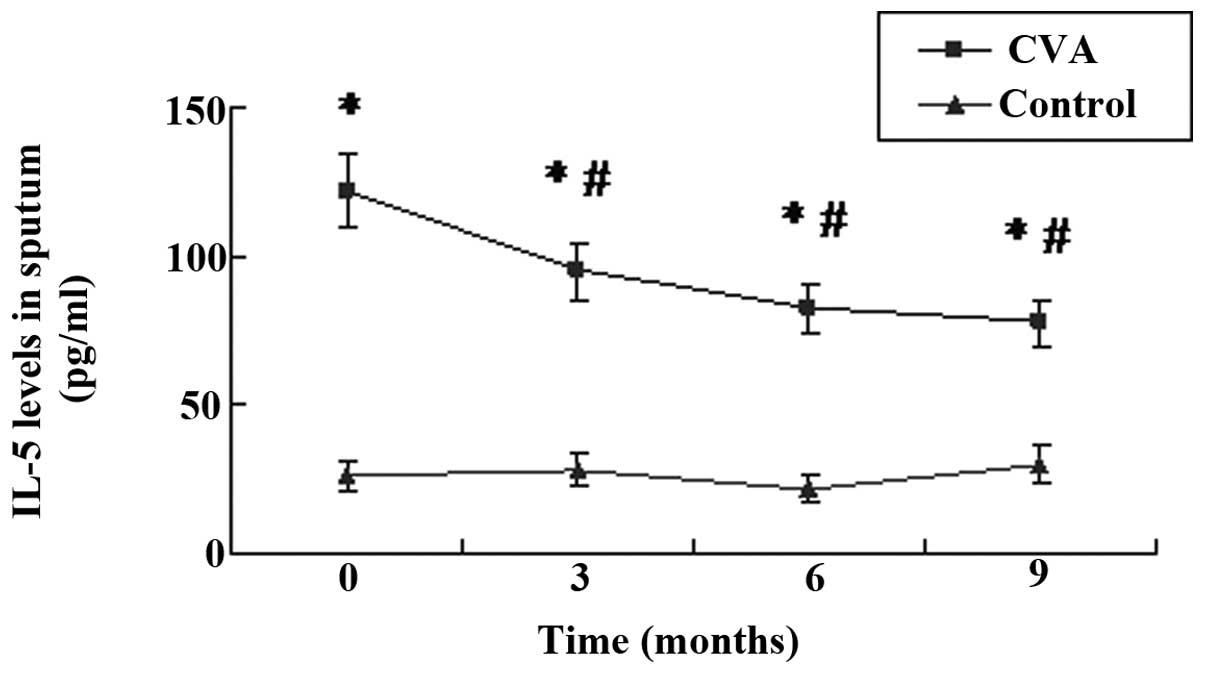
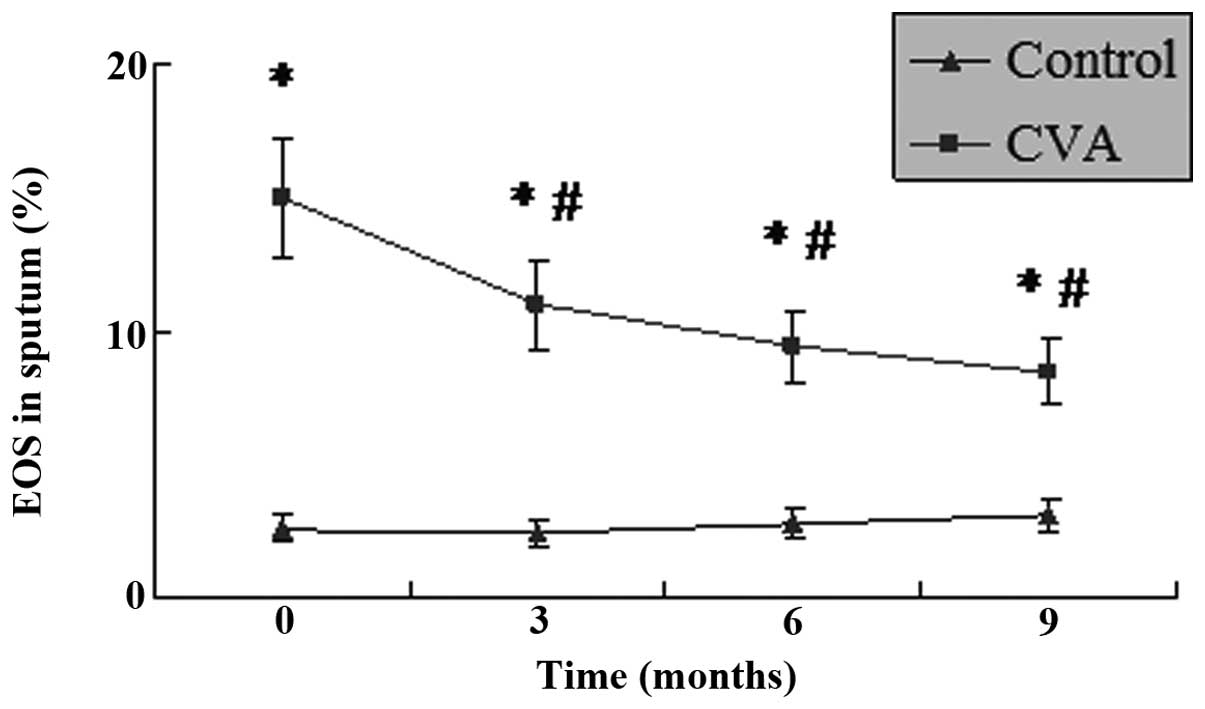
IL-5 levels and EOS percentage in the
induced sputum
As shown in Figs. 1
and 2, the levels of IL-5 and
percentage of EOS in the induced sputum of patients with CVA were
significantly higher than those in the controls (P<0.05). These
results indicate that EOS-induced airway inflammation in CVA
patients was similar to that in classic asthma. Following treatment
with ICS/LABA for 3 months, the levels of IL-5 in the induced
sputum of patients with CVA decreased significantly (P<0.05).
The strongest inhibitory effect on IL-5 was exhibited following
nine months of treatment. The EOS percentage followed a similar
trend to the IL-5 level, and was significantly decreased following
treatment with ICS/LABA (P<0.05). Although the IL-5 levels and
EOS percentage decreased during the treatment, they remained
significantly higher than those in the control group
(P<0.05).
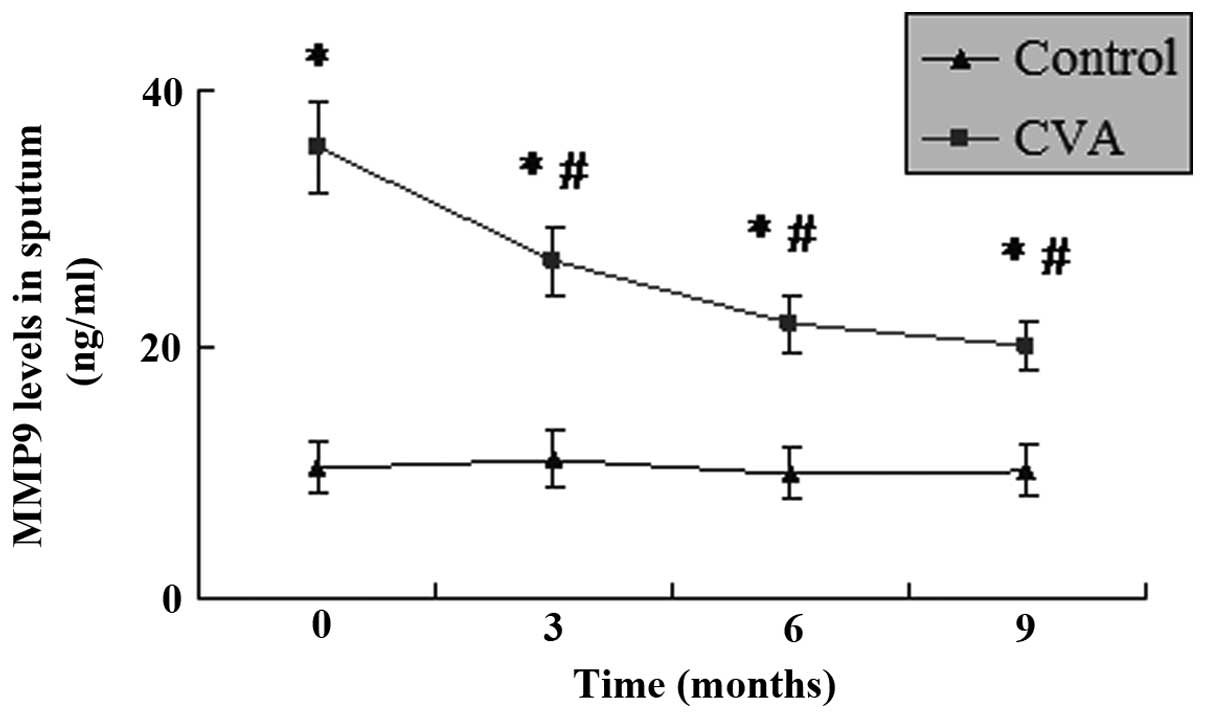
MMP9 levels in the induced sputum
As shown in Fig. 3,
the levels of MMP9 in the induced sputum of patients with CVA were
significantly higher than those in the control group (P<0.05).
Following treatment with ICS/LABA for different time periods, the
level of MMP9 in the induced sputum of the patients with CVA
decreased significantly. Although the level of MMP9 in the CVA
group had decreased following treatment for 3 months, the strongest
suppressing effect was revealed following 9–12 months. This range
is due to the fact that the MMP9 levels of all the patients were
collected at 9 months. However, several patients were lost at 12
months, therefore, the data at 9 months was shown but not at 12
months. However, the level of MMP9 remained higher than that in the
control group for the duration of the study (P<0.05).
Discussion
The present study revealed the presence of elevated
levels of MMP9 in the induced sputum of patients with CVA and that
treatment with ICS/LABA was able to significantly suppress airway
inflammation and the levels of MMP9. The results indicate that MMP9
participates in the airway inflammation of CVA.
As CVA is a specific form of asthma, chronic airway
inflammation is one of its most important features (2–4).
Previous studies have revealed that numerous types of inflammatory
factors or cytokines play a critical role in classic asthma and
CVA, including IL-5, IL-4 and eosinophilic cationic protein
(16,17). The present study demonstrated that
the levels of IL-5 and the percentage of EOS increased in the
induced sputum of patients with CVA. Treatment with ICS/LABA
successfully inhibited the airway inflammation of CVA. These
results confirmed the critical role of ICS/LABA in controlling the
airway inflammation associated with CVA.
MMPs are mainly involved in ECM cleavage. They also
play critical roles in a range of biological and pathological
processes, including fibrosis, inflammation and the healing of
wounds (6–10). A number of studies have
demonstrated the presence of increased levels of MMP9 in the serum
and sputum of patients with classic asthma (11–13).
In addition, these increased levels have been shown to be
associated with the FEV1 following allergen challenge. These
results demonstrate the important role of MMP9 in the airway
inflammation of classic asthma. The current study revealed elevated
levels of MMP9 in the induced sputum of patients with CVA.
Treatment with ICS/LABA was able to successfully reduce the
percentage of EOS and also the levels of MMP9 and IL-5 in the
sputum. This indicated that MMP9 may also participate in the
chronic airway inflammation of CVA.
However, the precise role of MMP9 in chronic airway
inflammation of CVA remains unclear. Certain studies have indicated
that the upregulation of MMP9 may be associated with eosinophilia
(18). The upregulation of MMP9
expression by EOS has also been demonstrated in nasal polyposis
(19) and this may reveal an
association between MMP9 and EOS. However, another study has
demonstrated that MMP9 is not associated with the numbers of EOS or
neutrophils (12). Therefore,
further studies are required to explore the role of MMP9 in EOS and
the airway inflammation of CVA.
References
|
1
|
Fujimura M, Ogawa H, Nishizawa Y and Nishi
K: Comparison of atopic cough with cough variant asthma: is atopic
cough a precursor of asthma? Thorax. 58:14–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai K, Chen R, Lin J, et al: A
prospective, multicenter survey on causes of chronic cough in
China. Chest. 143:613–620. 2013.PubMed/NCBI
|
|
3
|
Matsumoto H, Niimi A, Tabuena RP, et al:
Airway wall thickening in patients with cough variant asthma and
nonasthmatic chronic cough. Chest. 131:1042–1049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niimi A: Structural changes in the
airways: cause or effect of chronic cough? Pulm Pharmacol Ther.
24:328–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bratcher PE, Weathington NM, Nick HJ, et
al: MMP-9 cleaves SP-D and abrogates its innate immune functions
in vitro. PLoS One. 7:e418812012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vandenbroucke RE, Dejonckheere E and
Libert C: A therapeutic role for matrix metalloproteinase
inhibitors in lung diseases? Eur Respir J. 38:1200–1214. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaudhuri R, McSharry C, Brady J, et al:
Sputum matrix metalloproteinase-12 in patients with chronic
obstructive pulmonary disease and asthma: relationship to disease
severity. J Allergy Clin Immunol. 129:655–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oka S, Furukawa H, Shimada K, et al: Serum
biomarker analysis of collagen disease patients with acute-onset
diffuse interstitial lung disease. BMC Immunol. 14:92013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu C, Wang J, Xu Y, et al: Current
evidence on the relationship between five polymorphisms in the
matrix metalloproteinases (MMP) gene and lung cancer risk: a
meta-analysis. Gene. 517:65–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Craig VJ, Quintero PA, Fyfe SE, et al:
Profibrotic activities for matrix metalloproteinase-8 during
bleomycin-mediated lung injury. J Immunol. 190:4283–4296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko FW, Diba C, Roth M, et al: A comparison
of airway and serum matrix metalloproteinase-9 activity among
normal subjects, asthmatic patients, and patients with asthmatic
mucus hypersecretion. Chest. 127:1919–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castano R, Miedinger D, Maghni K, et al:
Matrix metalloproteinase-9 increases in the sputum from allergic
occupational asthma patients after specific inhalation challenge.
Int Arch Allergy Immunol. 160:161–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong Z, Lin YM, Qin X and Peng JL: Serum
MMP-9 is elevated in children with asthma. Mol Med Rep. 5:462–464.
2012.PubMed/NCBI
|
|
14
|
Page K, Ledford JR, Zhou P and Wills-Karp
M: A TLR2 agonist in German cockroach frass activates MMP-9 release
and is protective against allergic inflammation in mice. J Immunol.
183:3400–3408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asthma Workgroup, Chinese Society,
Respiratory, Diseases (CSRD), Chinese Medical, Association. The
Chinese national guidelines on diagnosis and management of cough
(December 2010). Chin Med J (Engl). 124:3207–3219. 2011.PubMed/NCBI
|
|
16
|
De Diego A, Martínez E, Perpiñá M, et al:
Airway inflammation and cough sensitivity in cough-variant asthma.
Allergy. 60:1407–1411. 2005.PubMed/NCBI
|
|
17
|
Yoo Y, Koh YY, Kang H, et al: Sputum
eosinophil counts and eosinophil cationic protein levels in
cough-variant asthma and in classic asthma, and their relationships
to airway hypersensitivity or maximal airway response to
methacholine. Allergy. 59:1055–1062. 2004. View Article : Google Scholar
|
|
18
|
Yang MS, Lee HS, Kim MH, et al: Rhinitis
patients with sputum eosinophilia show decreased lung function in
the absence of airway hyperresponsiveness. Allergy Asthma Immunol
Res. 5:232–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Borja Callejas F, Picado C,
Martínez-Antón A, et al: Differential expression of remodeling
markers by tissue structure in nasal polyposis. Am J Rhinol
Allergy. 27:e69–e74. 2013.PubMed/NCBI
|