Introduction
Lumbar vertebrae and femur bone metastases are
associated with an increased risk of fracture and a decreased
quality of life for the affected individual (1,2).
Zoledronic acid (ZA) is understood to prevent these risks by
inhibiting the function of osteoclast cells; thus, reducing bone
loss (3). Application of ZA and
irradiation to the bone metastases have been hypothesized to
facilitate ossification and cause an increase in bone mineral
density (BMD); however, to the best of our knowledge, these
phenomena have not yet been reported. In addition to conventional
irradiation, novel treatments, including systemic chemotherapy and
molecular target agents, such as erlotinib and bervacizumab, have
represented a significant advance in lung adenocarcinoma treatment
(4,5). These novel treatment strategies
combined with ZA are now recommended for the treatment of the
majority of patients with lung adenocarcinoma with bone metastasis
(3,6). The current study presents the case of
a patient with lung adenocarcinoma who exhibited an increased BMD
in the metastatic sites following ZA administration and
irradiation. An increase in ossification was also observed. The
patient was able to maintain a normal lifestyle for more than two
years, despite the lumbar vertebrae and femur bone metastases.
Written informed consent was obtained from the patient’s
family.
Case report
A 63-year-old female with no history of smoking was
admitted to Mito Medical Center, University of Tsukuba-Mito Kyodo
General Hospital (Mito, Japan) after presenting with lumbago and
pain around the hip joints for five months. At admission, chest
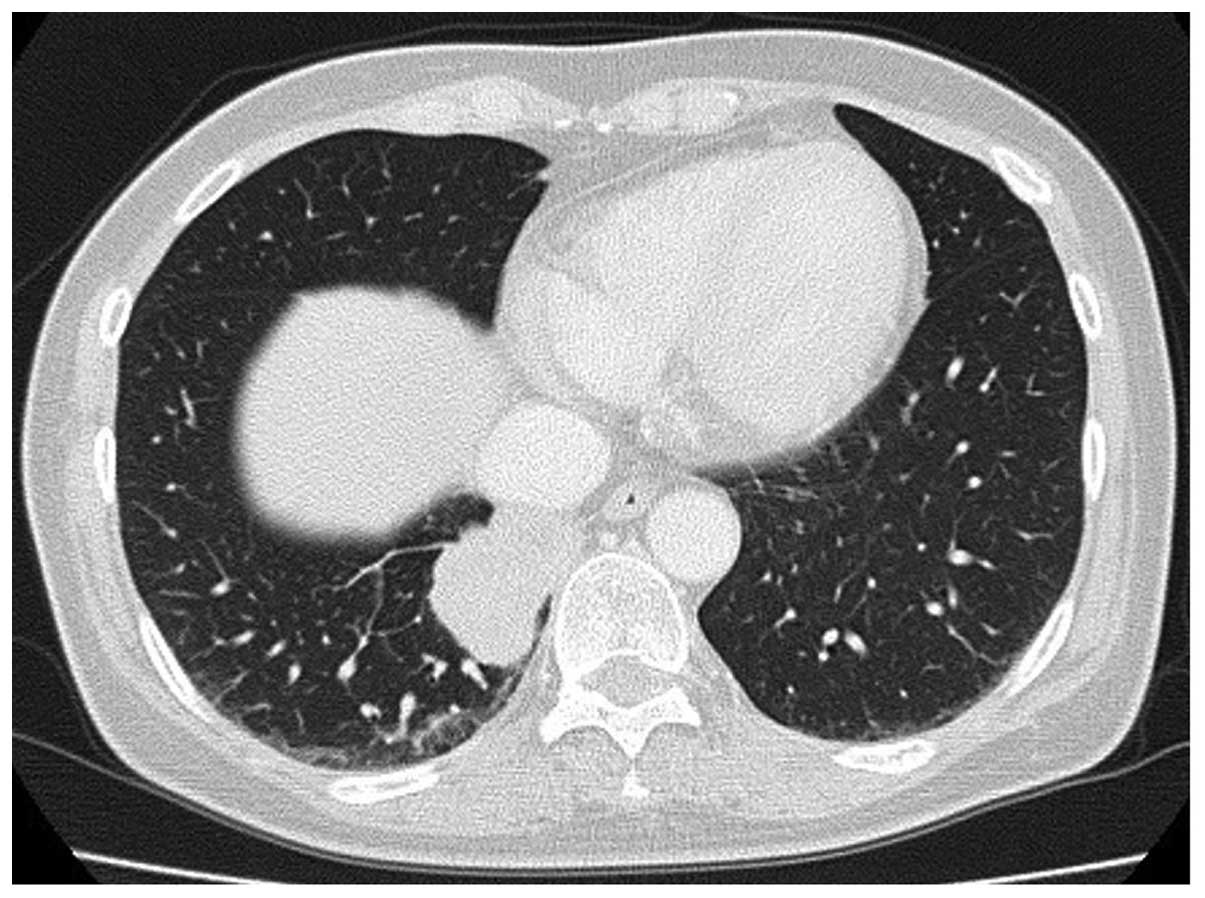
radiography and computed tomography (CT) scans revealed a 3.5×2.5
cm mass in the lower right lobe of the lungs, with multiple
intrapulmonary metastases (Fig.
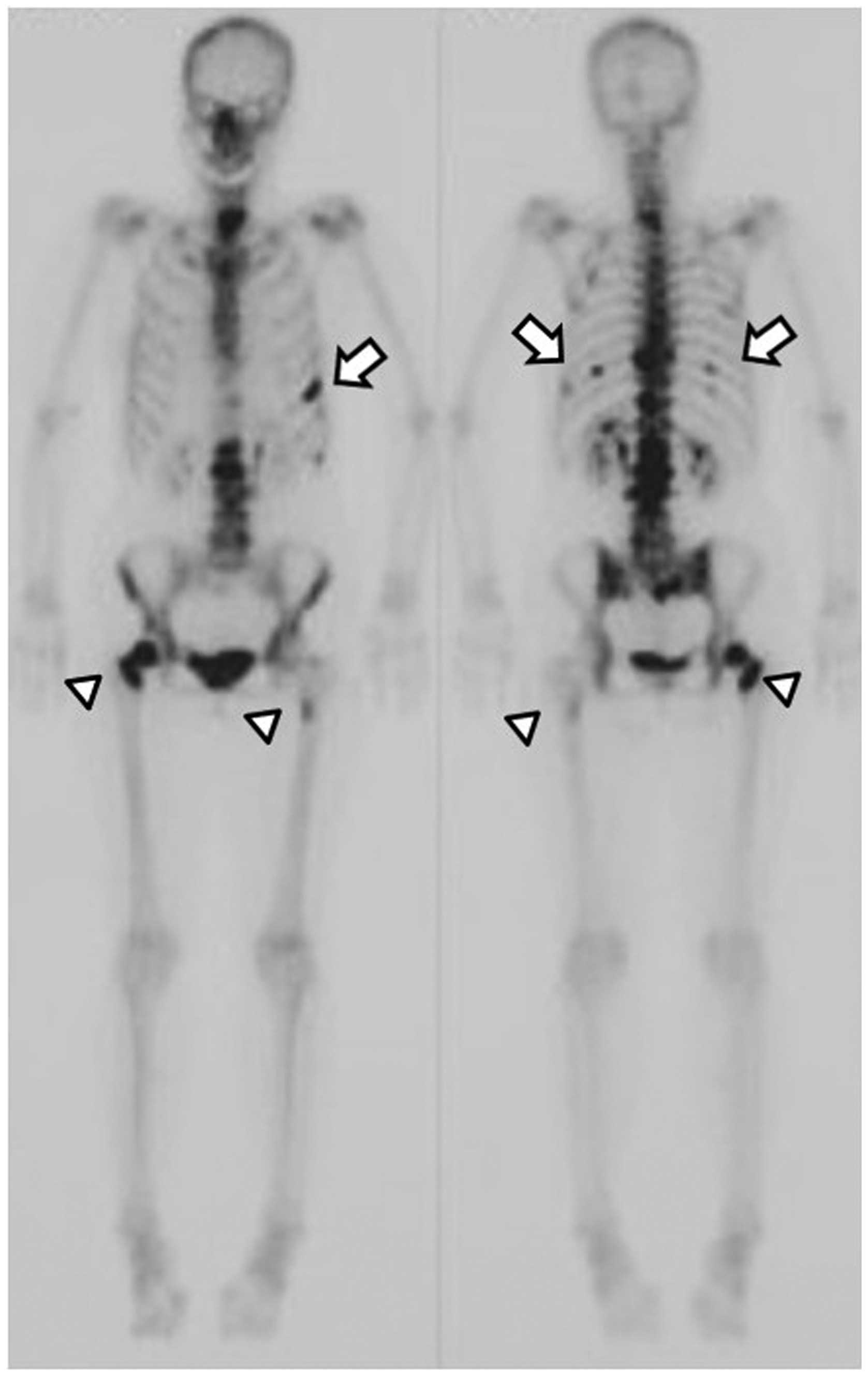
1). Bone scans demonstrated marked accumulation of
99mTc in the vertebrae (C7, Th9, Th11, L1 and L2),
femurs and ribs (Fig. 2). In
addition, an abdominal CT scan revealed a metastatic nodule in the
liver. Histopathological specimens, which were obtained
bronchoscopically from the mass in the right lung, revealed an
adenocarcinoma with an exon 19 deletion in the epidermal growth
factor receptor (EGFR) gene. The patient received a total hip
replacement due to a right femoral neck fracture. Subsequently,
irradiation was applied to the metastatic sites in the lumbar
vertebrae and femurs, and four courses of chemotherapy with
carboplatin, pemetrexed and bevacizumab were administered. The
patient received 4 mg ZA every month for 24 months. The response to
the chemotherapy was evaluated as a partial response; thereafter,
the patient received 20 courses of maintenance chemotherapy with
pemetrexed and bevacizumab.
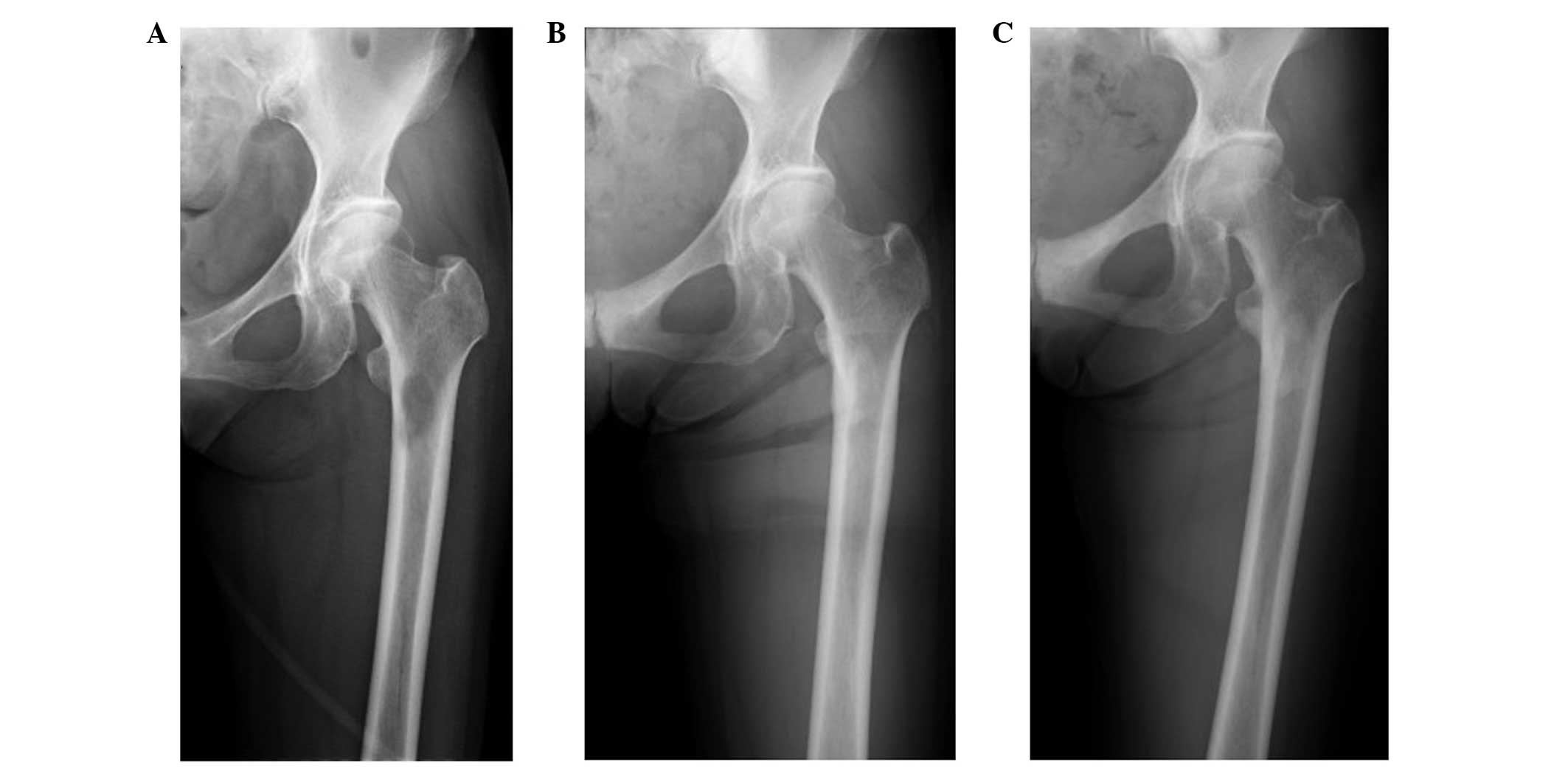
Ossification of the metastatic site in the left
femoral neck was observed using plain radiography three months
following the initiation of ZA treatment (Fig. 3). The BMD was measured by
dual-energy X-ray absorptiometry (DEXA; DPX-Bravo; GE Healthcare,
Tokyo Japan). Table I shows the
change in the BMD prior to, at 8 months and at 20 months following
the initiation of ZA administration. An increased BMD was observed
in the metastatic sites (L1, L2 and left femur), as well as the
non-metastatic sites (L3 and L4). The patient did not undergo any
adverse events following ZA treatment, including
biphosphonate-associated osteonecrosis of the jaw (BRONJ), fever or
renal toxicity. Following recurrence in the primary lesion and
liver metastasis, the patient received gefitinib therapy. The
patient survived with no evidence of bone metastasis in other sites
for two years. However, the patient had liver and pulmonary
recurrence and succumbed to the disease three years following the
diagnosis of lung adenocarcinoma.
 | Table IChange in the BMD prior to and
following the initiation of treatment with ZA. |
Table I
Change in the BMD prior to and
following the initiation of treatment with ZA.
| BMD
(g/cm2) |
|---|
|
|
|---|
| Site | Pretreatment | 8-months from ZA
initiation | 20-months from ZA
initiation |
|---|
| Lumbar spine |
| L1 | 0.659 | 1.260 | 1.363 |
| L2 | 0.654 | 1.706 | 1.970 |
| L3 | 0.655 | 1.029 | 1.231 |
| L4 | 0.767 | 0.917 | 0.949 |
| Neck of left
femur | 0.772 | 0.779 | 0.849 |
Discussion
ZA is a third-generation nitrogen-containing
parenteral bisphosphonate prescribed for the treatment of bone
metastases caused by solid tumors (7). In patients with lung cancer, ZA
exhibits a favorable protective effect against skeletal-associated
events, including bone fracture and severe pain requiring
irradiation and opioids (7).
Although ZA has a well-established tolerability profile and may be
administered safely as a long-term therapy, preventive measures are
required to avoid a number of severe side effects, including BRONJ
and renal toxicity, which have been observed in a small number of
patients receiving long-term therapy (8). BRONJ is characterized by the
unexpected appearance of necrotic bone in the oral cavity (8). In the current patient, cyclic
administration of intravenous ZA resulted in ossification and an
increase in the BMD of the lumbar spine and femur bone. The
observations of the present study revealed that the use of ZA was
associated with a clinical and radiological benefit. The patient
also underwent irradiation therapy on the metastatic sites,
platinum-based chemotherapy and gefitinib therapy. Apparent
ossification with an improvement in the BMD was observed following
the completion of irradiation, and the treatment was continued with
ZA and systemic chemotherapy. Although the most effective treatment
strategies for metastases remain unknown, it was hypothesized that
ZA had an important effect on a favorable outcome without any
adverse side effects, including BRONJ. Bone metastasis has been
identified as one of the unfavorable prognostic factors in patients
with lung cancer (9). However, in
the present study, the patient was found to have a mutation in the
EGFR gene, which was evaluated as a favorable prognostic factor.
Long-term survivors with bone metastasis have rarely been reported
(10), and the incidence rate of
complete remission from bone metastasis in patients with
non-small-cell lung carcinoma (NSCLC) is low (11). Although currently rare, treatment
with ZA for lung cancer patients with bone metastasis, particularly
for those that are indicative for other intensive therapies,
including the patient in the present study, should be considered
for future cases.
There have been a few reported cases of ossification
at the metastatic site following administration of ZA (12). Kawai et al reported a
patient with large cell lung cancer who received ZA treatment and
subsequently exhibited marked regression of the thoracic vertebrae
following irradiation applied to the metastatic site (12). Ikeda et al also presented
two cases of patients with NSCLC who were successfully treated with
irradiation applied to the metastatic sites, systemic chemotherapy
and ZA (13). Ossification in the
thoracic spine of one patient and in the rib of the other patient
were observed (13). The BMD, as
measured by DEXA, increases with the administration of ZA in
post-menopausal osteoporosis patients (14), pediatric patients with spinal cord
injury (15) and children with
type III osteogenesis imperfecta (16). However, to the best of our
knowledge, no study has revealed an improved BMD in the metastatic
sites following the administration of ZA.
In conclusion, the results of the present study
indicated that ZA enhanced ossification in the metastatic sites. In
addition, an increase in the BMD occurred as a result of the
formation of the metastatic bone lesions by irradiation, systemic
chemotherapy and ZA. As the patient was able to maintain a normal
lifestyle following treatment, the new bone appeared to have
similar mechanical properties to that of normal non-metastatic
bone. However, whether the increase in BMD with ossification in
metastatic sites depends only on the efficacy of ZA remains
inconclusive. Nonetheless, therapies that include ZA have been
shown to improve the quality of life of patients; thus, should be
considered for the treatment of bone metastasis, whilst considering
potential side effects, such as BRONJ.
References
|
1
|
Lote K, Walløe A and Bjersand A: Bone
metastasis. Prognosis, diagnosis and treatment. Acta Radiol Oncol.
25:227–232. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aydinli U, Ozturk C, Bayram S, Sarihan S,
Evrensel T and Yilmaz HS: Evaluation of lung cancer metastases to
the spine. Acta Orthop Belg. 72:592–597. 2006.PubMed/NCBI
|
|
3
|
Gnant M: Bisphosphonates in the prevention
of disease recurrence: current results and ongoing trials. Curr
Cancer Drug Targets. 9:824–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang CC, Chi KH, Kao SJ, et al: Upfront
gefitinib/erlotinib treatment followed by concomitant radiotherapy
for advanced lung cancer: a mono-institutional experience. Lung
Cancer. 73:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inomata M, Shukuya T, Takahashi T, et al:
Continuous administration of EGFR-TKIs following radiotherapy after
disease progression in bone lesions for non-small cell lung cancer.
Anticancer Res. 31:4519–4523. 2011.PubMed/NCBI
|
|
6
|
Takagi M, Arizumi T, Tanio Y, et al:
Multidisciplinary strategy for lung cancer patients with bone
metastasis. Gan To Kagaku Ryoho. 35:1783–1786. 2008.(In
Japanese).
|
|
7
|
McKeage K and Plosker GL: Zoledronic acid:
a pharmacoeconomic review of its use in the management of bone
metastases. Pharmacoeconomics. 26:251–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papapetrou PD: Bisphosphonate-associated
adverse events. Hormones (Athens). 8:96–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakazawa K, Kurishima K, Tamura T, et al:
Specific organ metastases and survival in small cell lung cancer.
Oncol Lett. 4:617–620. 2012.PubMed/NCBI
|
|
10
|
Hirano Y, Oda M, Tsunezuka Y, Ishikawa N
and Watanabe G: Long-term survival cases of lung cancer presented
as solitary bone metastasis. Ann Thorac Cardiovasc Surg.
11:401–404. 2005.PubMed/NCBI
|
|
11
|
Taniwaki M, Kakusui M, Imoto M, Takashima
T, Misawa S and Kashima K: Disease-free survival in a patient with
squamous cell lung carcinoma following complete response with the
combination chemotherapy of cisplatin and etoposide. Gan To Kagaku
Ryoho. 20:949–952. 1993.(In Japanese).
|
|
12
|
Kawai S, Yamaura G, Yasuda K and Suzuki T:
Remarkable regression of an osteolytic lesion of large cell lung
cancer treated with zoledronic acid: a case report. Case Rep Oncol.
5:233–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeda T, Nakanishi H and Hayata A: Use of
zoledronic acid for lung cancer with bone metastases - a study on
reossification of osteolytic bone metastases. Gan To Kagaku Ryoho.
40:871–875. 2013.(In Japanese).
|
|
14
|
Reid IR, Brown JP, Burckhardt P, et al:
Intravenous zoledronic acid in postmenopausal women with low bone
mineral density. N Engl J Med. 346:653–661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ooi HL, Briody J, McQuade M and Munns CF:
Zoledronic acid improves bone mineral density in pediatric spinal
cord injury. J Bone Miner Res. 27:1536–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panigrahi I, Das RR, Sharda S, Marwaha RK
and Khandelwal N: Response to zolendronic acid in children with
type III osteogenesis imperfecta. J Bone Miner Metab. 28:451–455.
2010. View Article : Google Scholar : PubMed/NCBI
|