Introduction
Idiopathic congenital nystagmus (ICN) is an
oculomotor disorder characterized by involuntary oscillation of the
eyes, with an onset at birth or within the first six months of life
and an estimated global prevalence of 24 in 10,000 births (1). ICN is genetically heterogeneous, and
X-linked ICN is the most common mode of inheritance. Three genetic
loci responsible for X-linked ICN have been mapped to chromosomes
Xp11.3–p11.4 (2), Xp22 (3,4) and
Xq26–Xq27. The FERM domain-containing protein 7 (FRMD7) at
Xq26–Xq27 has been identified as responsible for X-linked ICN
(5,6). Two novel missense mutations of the
FRMD7 gene in the Chinese population have been reported
(7), and to date, >40 mutations
have been identified (8,9). A previous study indicated that
FRMD7 may play an important role in neurite outgrowth, and
downregulation of FRMD7 results in a strong reduction of the
neurite length (10). However, the
biochemical role of FRMD7 in neural development and the
mechanisms whereby mutations of FRMD7 lead to X-linked ICN
remain unclear.
Alternative splicing produces multiple protein
products with variable domain compositions from a single gene. The
brain and testes show more prevalent alternative splicing compared
with other tissues, indicating that these organs possess an
unusually high number of splicing-associated genes (11–14).
The FRMD7 gene has been found to be subject to alternative
splicing.
Previously, the first FRMD7 splice variant,
(FRMD7-S), containing a 45-bp truncation in the fourth exon
(GenBank accession number, FJ717411), was cloned and identified.
FRMD7 and FRMD7-S were found to colocalize and
coimmunoprecipitate. Furthermore, overexpression of FRMD7 in
NT2 cells resulted in altered neurite development and the
upregulation of FRMD7-S (15). Based on these observations, a novel
splice variant of human FRMD7, with missing exons 2, 3 and
4, was detected and a severely truncated protein was generated,
termed FRMD7_splice variant 2 (FRMD7_SV2). The
aim of the present study was to investigate whether
FRMD7_SV2 plays a role in neuronal development.
Materials and methods
Human embryonic brain tissue
Human fetal brain tissues at 14, 19 and 24 weeks
post conception (wpc; gestational age was calculated from the first
day of the last menstrual period; 14 wpc, n=2; 19 wpc, n=3; 24 wpc,
n=2) were obtained from the Women’s Hospital affiliated to Zhejiang
University School of Medicine (Hangzhou, China). All the samples
were obtained and used in compliance with the Code of Ethics of the
World Medical Association (Declaration of Helsinki) and the study
was approved by the Second Affiliated Hospital of Zhejiang
University School of Medicine (Hangzhou, China). Written informed
consent was obtained from all the female participants of this
study. The post-mortem interval for obtaining the samples was <6
h. Brain tissue samples (size, 1–3 mm3) were cut from
each fetal brain, and the fresh tissues were stored in cryogenic
vials (Corning; Sigma-Aldrich, St. Louis, MO, USA) containing
liquid nitrogen. RNA extraction experiments were performed within
three days.
Cell culture, retinoic acid (RA)/bone
morphogenetic protein-2 (BMP-2)-induced differentiation and
immunofluorescence
Human NTERA-2/cl.Dl (NT2) cells were obtained from
the Cell Culture Center of Peking Union Medical College (Peking,
China) and cultured in Dulbecco’s modified Eagle’s medium/F12
(Gibco Life Technologies, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS; HyClone™; Thermo Fisher Scientific,
Waltham, MA, USA), 100 U/ml penicillin and 100 μg/ml streptomycin
(Gibco Life Technologies). A stock solution (10 mM) of all-trans RA
(Sigma-Aldrich Trading Co., Ltd., Shanghai, China) was prepared in
dimethyl sulfoxide and stored at −75°C. Recombinant human BMP-2 was
dissolved in normal saline (50 μg/ml) and stored at −20°C. Prior to
the induction of differentiation, the NT2 cells were incubated
(temperature, 37°C; high humidity; 5% CO2) in
25-cm2 cell culture flasks (Corning; Sigma-Aldrich)
containing 4 ml culture medium.
In the differentiation experiments, the NT2 cells
were divided into two groups: Group 1 was treated with RA (16,17),
while group 2 was treated with BMP-2 (18,19).
On day 0, the culture medium was replaced with Opti-MEM I
(Invitrogen Life Technologies, Grand Island, NY, USA), containing
4% FBS and 10 μM RA (group 1) or 50 ng/ml BMP-2 (group 2). The
cells were collected for RNA isolation after 12, 24 and 48 h
incubation (early time points) or after 5, 8, 12 and 14 days (later
time points). For prolonged cultures (>3 days), the culture
medium was refreshed every three days in the continued presence of
RA or BMP-2.
RNA extraction, reverse transcription
polymerase chain reaction (RT-PCR) and quantitative PCR (qPCR)
analyses
Total RNA was extracted from the human embryonic
brain tissue samples and NT2 cells using TRIzol reagent (Invitrogen
Life Technologies), according to the manufacturer’s instructions.
Total RNA (5 μg) and 1 μl oligo(dT)18 primer (0.5
μg/μl), with a total volume of ≤15 μl, were mixed in water. The
test tube holding the mixture was heated to 70°C for 5 min and
immediately immersed in ice-cold water for 5 min. Next, 1 μl M-MLV
reverse transcriptase (200 U/μl; Promega Corporation, Madison, WI,
USA), 5 μl M-MLV 5X reaction buffer, 2 μl dNTPmix (10 mM), 25 units
recombinant RNasin® ribonuclease inhibitor (Promega
Biotech Co., Ltd, Beijing, China) and diethylpyrocarbonate water
were added to the sample solution (total volume, 50 μl). The
mixture was incubated for 1 h at 42°C, followed by 10 min at 70°C.
For the PCR amplification, specific oligonucleotide primer pairs
(10 pmol each) were incubated with 2 μl cDNA template in 25 μl PCR
reaction mixtures, containing 2.5 μl 10X PCR buffer, 1.5 mM
MgSO4, mixed deoxynucleotides (1 mM each) and 0.5 units
KOD PLUS polymerase (Toyobo Corporation, Osaka, Japan). For
amplification of the full-length FRMD7 gene and its splice
variant, PCR was performed for 40 cycles at 95°C for 2 min, 95°C
for 20 sec, 56°C for 20 sec and 72°C for 150 sec, followed by a
final elongation step at 72°C for 5 min. The primer sequences were
as follows: P1-forward, 5′-ATGCTACATTTAAAAGTGCAGTTT-3′, and
P1-reverse, 5′-TTAAGCTAAAAAGTAATTACATGGT-3′.
Relative gene expression levels were measured with
qPCR on a Light Cycler™ Real-time PCR thermocycler (Roche
Diagnostics Corporation, Basel, Switzerland), using
SYBR® Premix Ex Taq™ (Perfect Real Time; Takara
Biotechnology Co., Ltd., Dalian, China). For the amplification of
FRMD7_FL, the specific primers used were as follows:
P2-forward, 5′-CAAAGCAGGTAAAAAATCCTAAGG-3′ [melting temperature
(Tm), 62°C], and P2-reverse, 5′-ATGTGAGATACCATCAACGCTGT-3′ (Tm,
60°C). For the amplification of FRMD7_SV2, the following
primers were used: P3-forward, 5′-CCAGAA GATTTTTGTGGTTGATGTAT-3′
(Tm, 70°C) and P3-reverse, 5′-GAGTTTGTGCCAGATGCTTCCTAT-3′ (Tm,
70°C). Each reaction amplified a single product, and all PCR
amplifications were conducted in triplicate. The mean fold change
in the expression of the target gene at each time point was
calculated using the 2−ΔΔCt method (20), with GAPDH as the endogenous
control. The PCR products were run on 2% agarose gels to confirm
the amplification size and identify the single PCR product.
FRMD7 and FRMD7_SV2 detection in NT2
cells using northern blotting
Total RNA was extracted from NT2 cells that had been
induced by RA, according to the manufacturer’s instructions (Ambion
Life Technologies, Carlsbad, CA, USA). The total RNA was separated
by electrophoresis on a 1% agarose gel in 1X TBE buffer [90 mM
Tris-boric acid and 2 mM EDTA (pH 8.0)] and transferred to a nylon
membrane (Amersham Biosciences, Amersham, UK). Following
crosslinking under ultraviolet light, the membrane was
prehybridized in DIG Easy Hyb Granules buffer (Roche Diagnostics
Operations, Inc., Indianapolis, IN, USA) for 30 min. Subsequently,
the membrane was hybridized with a DIG-labeled probe (FRMD7
probe, 5′-TAC TGAGATGGGTAATGTTTCCTTTCAAATGGCAAGCTC TTCAG-3′) at
42°C overnight. A DIG-labeled actin probe
(5′-CAAACATGATCTGGGTCATCTTCTC-3′) was used as the control, while
RNA Molecular Weight Marker I labeled with DIG was used as the
marker. Immunological detection was performed using the DIG High
Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics
Corporation), according to the manufacturer’s instructions.
Statistical analysis
All the experiments were conducted in triplicate and
the data are represented as the mean ± standard error of mean.
Statistically significant differences between groups were
identified using the Kolmogorov-Smirnov test and Student’s t-test,
as implemented using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
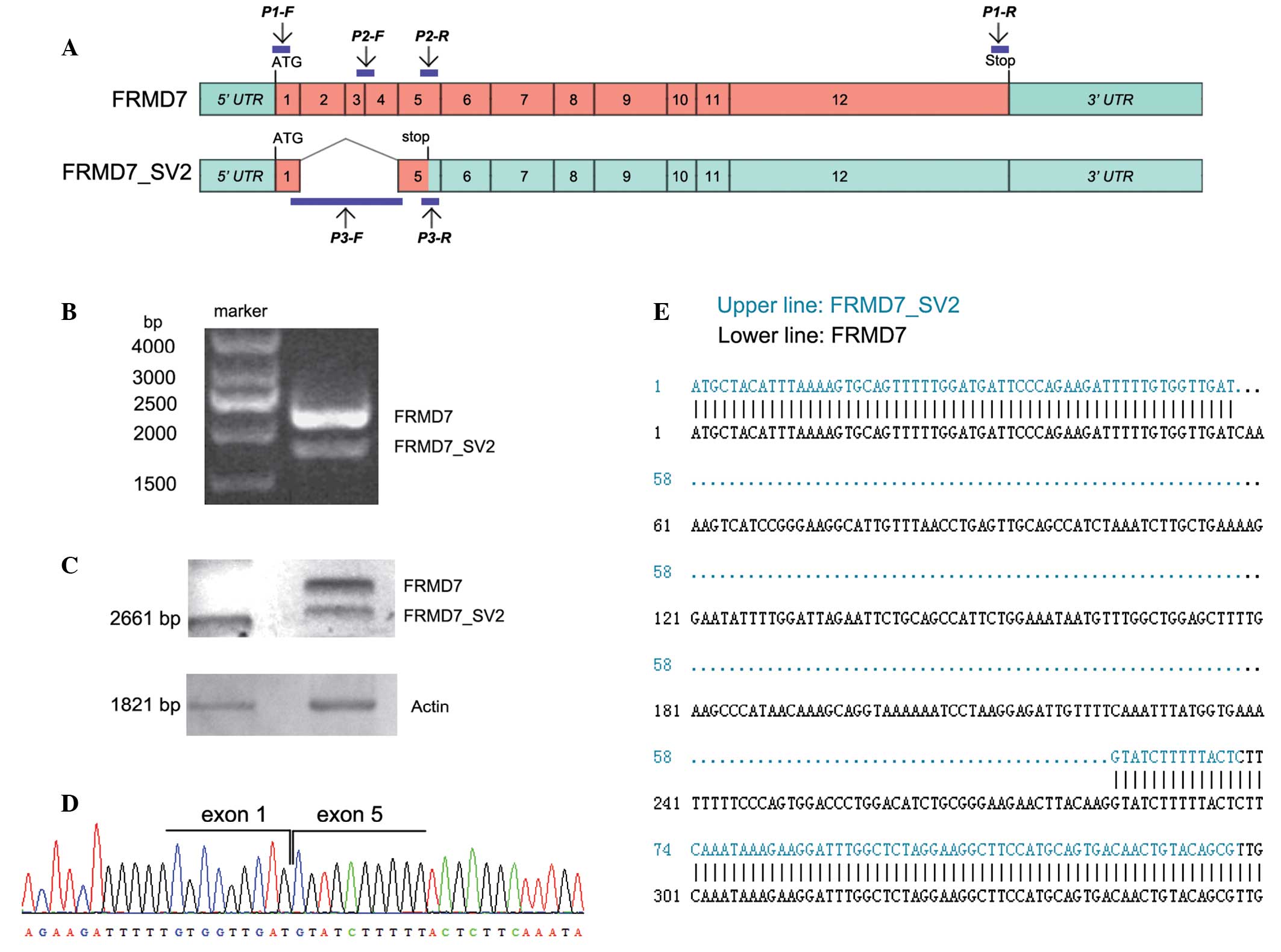
Identification of a novel alternative
splicing variant of the human FRMD7 gene
To obtain the full-length human FRMD7
(FRMD7_FL) cDNA, RT-PCR experiments were performed on
the RNA extracted from the NT2 cells using a pair of specific
primers. DNA fragments were isolated by electrophoresis and two
distinct fragments were observed on the gel (Fig. 1B). Sequence analyses were performed
and a multiple exon-skipping mRNA splice variant of FRMD7
was identified, which was missing exons 2, 3 and 4, and was assumed
to generate a severely truncated variant. The splice variant was
termed FRMD7_SV2 (Fig.
1C–E). This multiple exon-skipping event eliminated 227
nucleotides of the FRMD7_FL gene and resulted in a
frameshift mutation that altered 19 amino acids prior to the
premature termination at codon 39 (TGA), predicted to lead to the
synthesis of a severely truncated protein based on the sequence
(Fig. 1A).
Expression of FRMD7_SV2 is spatially and
temporally restricted in human brain development
A previous study revealed a restricted expression of
FRMD7 in the human embryonic brain and development of the
neural retina (10). In addition,
high expression levels of FRMD7_FL and FRMD7-S
were found in 16-wpc human fetal cerebellum samples (15). In the present study, the expression
levels of the three variants at the three stages (14, 19 and 24
wpc) in the development of the human fetal brain were detected by
RT-PCR, using isoform-specific primers.
The results revealed that the mRNA expression levels
of FRMD7_FL and FRMD7-S were high in the
cerebral cortex, cerebellum and brainstem for all three stages;
however, expression was not detected in the optic nerve or bulbus
oculi. Similarly, FRMD7_SV2 was expressed in the
cerebral cortex, cerebellum and brainstem at 14 and 19 wpc;
however, the expression level was slightly decreased in the
cerebral cortex and brainstem at 19 wpc. At 24 wpc,
FRMD7_SV2 was only detected in the cerebellum
(Fig. 2).
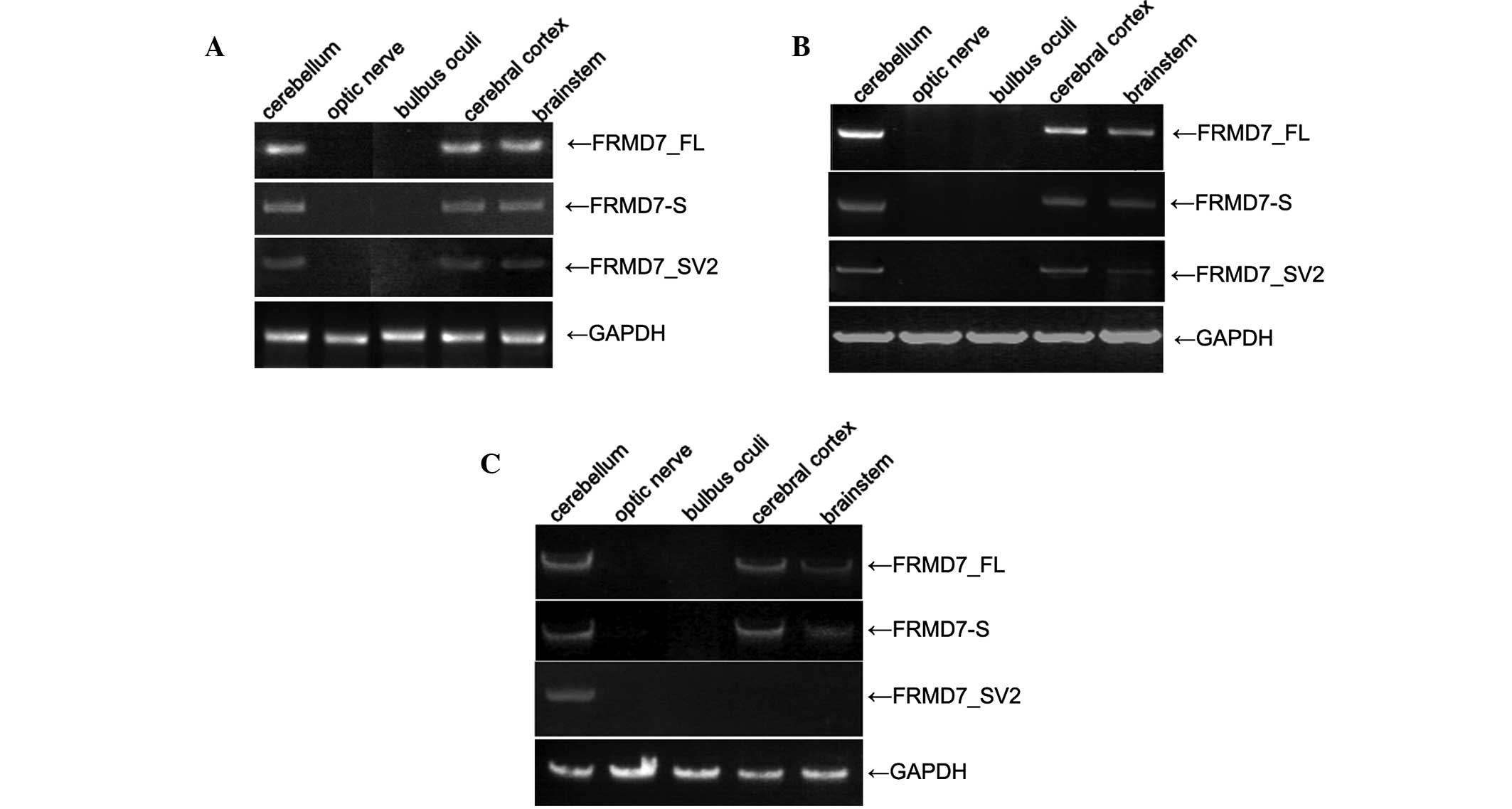 | Figure 2PCR analysis of FRMD7_FL,
FRMD7-S and FRMD7_SV2 in the developing human fetal
brain, with the products separated by electrophoresis on 2% agarose
gels. (A) At 14 wpc, the three variants were detected in the
cerebral cortex, cerebellum and brainstem, but not in the optic
nerve or bulbus oculi. (B) At 19 wpc, FRMD7_FL and
FRMD7-S were detected in the cerebral cortex, cerebellum and
brainstem, while the expression of FRMD7_SV2 decreased
slightly in the cerebral cortex and brainstem. (C) At 24 wpc, the
expression of FRMD7_FL and FRMD7-S decreased in the
brainstem, and FRMD7_SV2 was only detected in the
cerebellum. PCR, polymerase chain reaction; FRMD7_FL,
full-length FRMD7; FRMD7_S, FRMD7 splice;
FRMD7_SV2, FRMD7 splice variant 2; wpc, weeks post
conception. |
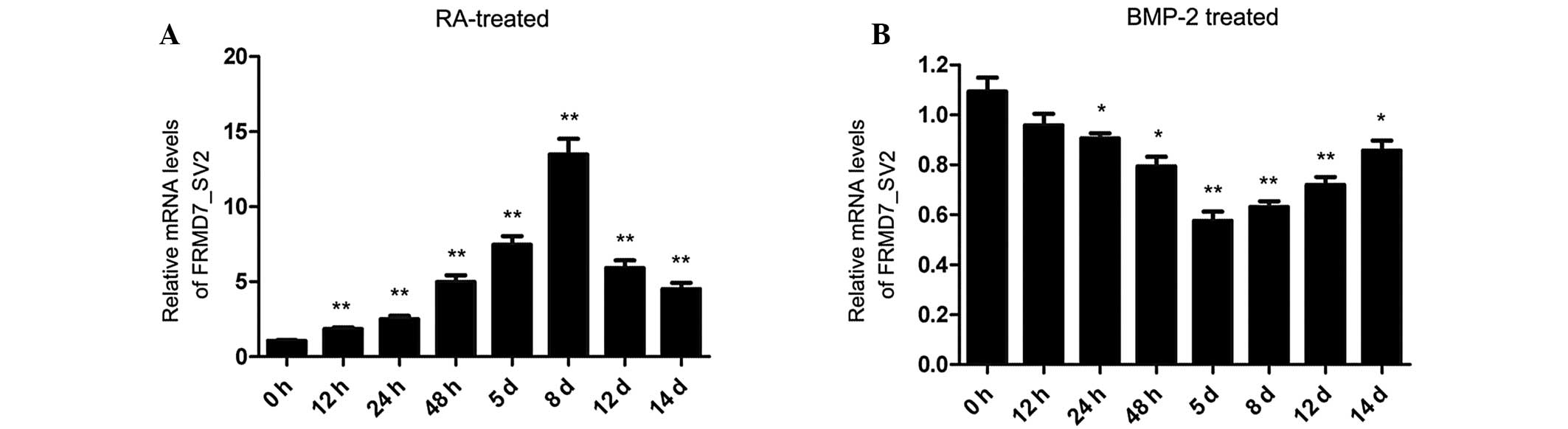
mRNA expression levels of FRMD7_SV2
increase during RA-induced, but not BMP-2-induced differentiation
of NT2 cells
To investigate whether FRMD7_SV2 is
involved in differentiation, the mRNA expression level of
FRMD7_SV2 in NT2 cells was examined during RA-induced or
BMP-2-induced differentiation.
A time-course experiment was performed and the
results revealed that the mRNA expression levels of
FRMD7_SV2 increased markedly during RA-induced
neuronal differentiation of NT2 cells. Within 12 h of treatment
with RA, the relative expression levels of FRMD7_SV2
exhibited a 1.8-fold increase. The expression levels of
FRMD7_SV2 gradually increased over time, with the
highest expression level increase (13.5-fold) detected within eight
days of treatment with RA, in accordance with the developmental
time course of neurite outgrowth. However, the expression levels of
FRMD7_SV2 showed an evident decline following eight
days of treatment with RA (Fig.
3A). By contrast, FRMD7_SV2 expression levels exhibited
a small but significant decreasing trend between 24 h and five days
of treatment with BMP-2, which stopped at day 5 and subsequently
began to increase (Fig. 3B).
Discussion
Alternative splicing is an established mechanism for
gene diversification and increasing the complexity of mammalian
transcriptomes. International genome and transcript sequencing
projects have shown that the frequency of alternative splicing is
associated with organism complexity, with up to 94% of human
multi-exon genes alternatively spliced (21). The significance of alternative
splicing is evident in highly specialized nerve cells, and
highlighted in a number of neurological disorders (22).
In the present study, a novel FRMD7 splice
variant was identified through RT-PCR and qPCR analyses, and was
termed FRMD7_SV2. This splice variant of human
FRMD7 was missing exons 2, 3 and 4, and presumably encoded a
severely truncated protein. FRMD7_SV2 eliminated 227
nucleotides of the full-length FRMD7 gene and resulted in a
frameshift mutation, altering 19 amino acids prior to premature
termination at codon 39 (TGA). These changes were hypothesized to
lead to the synthesis of a severely truncated protein based on the
sequence.
Alternative splicing may change the structure of
transcripts and their encoded proteins, determining the binding
properties, intracellular localization, enzymatic activity, protein
stability and post-translational modifications of a large number of
proteins (23). Typically,
alternative splicing shows tissue- and/or development-specific
distribution, resulting in different expression levels in different
cell lines or developmental stages (24). A previous study indicated that the
COOH-terminus of FRMD7 plays a key role in the subcellular
localization of FRMD7 in Neuro-2A and HEK 293T cells
(25). In the present study, the
splicing event occurred within the NH2-terminal FERM
domain, and the resulting loss of the COOH-terminus of FRMD7
may alter the function of the full-length FRMD7 protein.
FRMD7 is a member of the FERM family that
causes X-linked ICN. Initial studies using in situ
hybridization and immunohistochemistry revealed that the expression
of FRMD7 is spatially and temporally regulated in the human
brain during embryonic and fetal development (10). In the present study, RT-PCR
analysis was used to detect the expression of
FRMD7_SV2 mRNA in the developing human fetal brain
(14, 19 and 24 wpc), and the results revealed that the expression
level of FRMD7_SV2 was also spatially and temporally
restricted. At 14 and 19 wpc, FRMD7_SV2 was detected
in the cerebral cortex, cerebellum and brainstem; however, the
expression level was slightly decreased in the cerebral cortex and
brainstem at 19 wpc. At 24 wpc, FRMD7_SV2 was only
detected in the cerebellum. The cerebellum is involved in the
coordination and precision of voluntary motor movement, balance and
equilibrium and muscle fine-tuning functions. The
vestibulocerebellum primarily regulates balance and spatial
orientation. Any damage in this area causes disturbances in balance
and gait (26), as well as
nystagmus (27,28). These results indicate that the
expression of the FRMD7_SV2 gene exhibits spatially
and temporally restricted distribution in the human fetal brain,
indicating that the majority of FRMD7_SV2 functions
are associated with cerebellum development.
In a previous study, Betts-Henderson et al
demonstrated that the mRNA and protein expression levels of mouse
FRMD7 were elevated during RA-induced differentiation of
Neuro-2A neuroblastoma cells (10). Due to the lack of specific primer
set data, it is unclear whether only the full-length FRMD7
was detected or a combination of two or more transcripts. The NT2
cell line is a characterized human embryonic carcinoma cell line,
and NT2 cells can be induced to differentiate into postmitotic
central nervous system neurons when treated with RA (16,17).
In addition, the cell line differentiates into non-neural
epithelial lineages when treated with BMP-2 (18,19).
Therefore, NT2 cells offer a valuable model for observing different
trends in mRNA expression levels during RA-induced and
BMP-2-induced differentiation processes. qPCR analysis revealed
that the expression levels of the FRMD7_SV2 gene
gradually increased in RA-induced differentiating NT2 cells. A
13.5-fold increase in the expression level was detected within
eight days of treatment with RA, in accordance with the
developmental time course of neurite outgrowth. Therefore,
FRMD7_SV2 is hypothesized to be involved in the early
stages of RA-induced neural differentiation in NT2 cells.
Previous observations provide evidence that
FRMD7 plays a critical role in neuronal morphogenesis,
synapse function and neurite growth. Therefore,
FRMD7_SV2 is hypothesized to play a role in the
function of FRMD7; however, further studies are required to
confirm this hypothesis.
In summary, FRMD7_SV2 was identified
as a splice variant of the FRMD7 gene.
FRMD7_SV2 may play a role in neuronal development and
provide further evidence on the function of FRMD7.
Acknowledgements
The study was supported by grants from the Nature
Science Foundation of China (no. 81070903) and by the Zhejiang
Provincial Natural Science Foundation(no. Y2090256). The authors
thank the participants of the study.
References
|
1
|
Sarvananthan N, Surendran M, Roberts EO,
et al: The prevalence of nystagmus: the Leicestershire nystagmus
survey. Invest Ophthalmol Vis Sci. 50:5201–5206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cabot A, Rozet JM, Gerber S, et al: A gene
for X-linked idiopathic congenital nystagmus (NYS1) maps to
chromosome Xp11.4–p11.3. Am J Hum Genet. 64:1141–1146.
1999.PubMed/NCBI
|
|
3
|
Schiaffino MV, Bassi MT, Galli L, et al:
Analysis of the OA1 gene reveals mutations in only one-third of
patients with X-linked ocular albinism. Hum Mol Genet. 4:2319–2325.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bassi MT, Schiaffino MV, Renieri A, et al:
Cloning of the gene for ocular albinism type 1 from the distal
short arm of the X chromosome. Nat Genet. 10:13–19. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tarpey P, Thomas S, Sarvananthan N, et al:
Mutations in FRMD7, a newly identified member of the FERM family,
cause X-linked idiopathic congenital nystagmus. Nat Genet.
38:1242–1244. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schorderet DF, Tiab L, Gaillard MC, et al:
Novel mutations in FRMD7 in X-linked congenital nystagmus. Mutation
in brief #963. Online. Hum Mutat. 28:5252007.PubMed/NCBI
|
|
7
|
Zhang B, Liu Z, Zhao G, et al: Novel
mutations of the FRMD7 gene in X-linked congenital motor nystagmus.
Mol Vis. 13:1674–1679. 2007.PubMed/NCBI
|
|
8
|
Shiels A, Bennett TM, Prince JB and
Tychsen L: X-linked idiopathic infantile nystagmus associated with
a missense mutation in FRMD7. Mol Vis. 13:2233–2241.
2007.PubMed/NCBI
|
|
9
|
Thomas S, Proudlock FA, Sarvananthan N, et
al: Phenotypical characteristics of idiopathic infantile nystagmus
with and without mutations in FRMD7. Brain. 131:1259–1267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Betts-Henderson J, Bartesaghi S, Crosier
M, et al: The nystagmus-associated FRMD7 gene regulates neuronal
outgrowth and development. Hum Mol Genet. 19:342–351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ET, Sandberg R, Luo S, et al:
Alternative isoform regulation in human tissue transcriptomes.
Nature. 456:470–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Grange P, Gratadou L, Delord M,
Dutertre M and Auboeuf D: Splicing factor and exon profiling across
human tissues. Nucleic Acids Res. 38:2825–2838. 2010.PubMed/NCBI
|
|
13
|
Li Q, Lee JA and Black DL: Neuronal
regulation of alternative pre-mRNA splicing. Nat Rev Neurosci.
8:819–831. 2007. View
Article : Google Scholar
|
|
14
|
Venables JP: Alternative splicing in the
testes. Curr Opin Genet Dev. 12:615–619. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Pu J, Liu Z, et al: Identification
of a novel FRMD7 splice variant and functional analysis of two
FRMD7 transcripts during human NT2 cell differentiation. Mol Vis.
17:2986–2996. 2011.PubMed/NCBI
|
|
16
|
Andrews PW: Retinoic acid induces neuronal
differentiation of a cloned human embryonal carcinoma cell line in
vitro. Dev Biol. 103:285–293. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pleasure SJ, Page C and Lee VM: Pure,
postmitotic, polarized human neurons derived from NTera 2 cells
provide a system for expressing exogenous proteins in terminally
differentiated neurons. J Neurosci. 12:1802–1815. 1992.
|
|
18
|
Chadalavada RS, Houldsworth J, Olshen AB,
et al: Transcriptional program of bone morphogenetic
protein-2-induced epithelial and smooth muscle differentiation of
pluripotent human embryonal carcinoma cells. Funct Integr Genomics.
5:59–69. 2005. View Article : Google Scholar
|
|
19
|
Caricasole A, Ward-van Oostwaard D,
Zeinstra L, et al: Bone morphogenetic proteins (BMPs) induce
epithelial differentiation of NT2D1 human embryonal carcinoma
cells. Int J Dev Biol. 44:443–450. 2000.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
21
|
Johnson JM, Castle J, Garrett-Engele P, et
al: Genome-wide survey of human alternative pre-mRNA splicing with
exon junction microarrays. Science. 302:2141–2144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dredge BK, Polydorides AD and Darnell RB:
The splice of life: alternative splicing and neurological disease.
Nat Rev Neurosci. 2:43–50. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stamm S, Ben-Ari S, Rafalska I, et al:
Function of alternative splicing. Gene. 344:1–20. 2005. View Article : Google Scholar
|
|
24
|
Yeo G, Holste D, Kreiman G and Burge CB:
Variation in alternative splicing across human tissues. Genome
Biol. 5:R742004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pu J, Li Y, Liu Z, et al: Expression and
localization of FRMD7 in human fetal brain, and a role for F-actin.
Mol Vis. 17:591–597. 2011.PubMed/NCBI
|
|
26
|
Martin JH, Cooper SE, Hacking A and Ghez
C: Differential effects of deep cerebellar nuclei inactivation on
reaching and adaptive control. J Neurophysiol. 83:1886–1899.
2000.PubMed/NCBI
|
|
27
|
Dieterich M and Brandt T: Functional brain
imaging of peripheral and central vestibular disorders. Brain.
131:2538–2552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harris CM, Walker J, Shawkat F, et al: Eye
movements in a familial vestibulocerebellar disorder.
Neuropediatrics. 24:117–122. 1993. View Article : Google Scholar : PubMed/NCBI
|