Introduction
Heat shock protein 90 (Hsp90) has been identified to
be a potential therapeutic target for cancer. It is an abundant
molecular chaperone involved in the folding, assembly, maturation
and stabilization of specific target proteins that are critical for
the proliferation and survival of cancer cells. Target proteins
dependent on Hsp90 have been implicated in all six hallmarks of
cancer, including growth signal self sufficiency, anti-growth
signal insensitivity, evasion of apoptosis, unlimited replicative
potential, metastasis and tissue invasion and sustained
angiogenesis (1–3). Therefore, inhibition of Hsp90
provides a combinatorial attack on multiple signaling pathways
responsible for malignant cell growth. Hsp90 also has an important
role in the proliferation and apoptosis of cervical carcinoma cells
(4–7). The phosphatidylinositol 3-kinase
(PI3K) and mitogen-activated protein kinase (MAPK) pathways are
known to be involved in the vascular endothelial growth factor-C
(VEGF-C)-induced proliferation and apoptosis of HeLa cells, with
the overexpression of downstream genes, including B-cell lymphoma 2
(Bcl-2) and cyclin D1 (8).
However, the role of Hsp90 in the proliferation and apoptosis of
HeLa cells induced by VEGF-C has yet to be elucidated. Therefore,
in the present study, Hsp90 expression in HeLa cells treated with
Hsp90-specific inhibitor geldanamycin (GA) acting in concert with
VEGF-C was investigated.
Materials and methods
Materials
The human cervical carcinoma cell line, HeLa, was
provided by the Tianjin Institute of Hematology (Chinese Academy of
Medical Science; Tianjin, China). GA was purchased from Alexis
Biochemicals (San Diego, CA, USA) and Hsp90 rabbit anti-human
polyclonal antibody was obtained from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Bcl-2, Bcl-2-associated X protein (Bax),
GAPDH mouse monoclonal antibodies and cyclin D1 polyclonal antibody
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Goat anti-rabbit and goat anti-mouse secondary antibodies
were purchased from ZSGB Bio. Co. (Beijing, China), and recombinant
VEGF-C protein was obtained from R&D Systems (Minneapolis, MN,
USA).
Overview of methods
Hsp90 expression levels were first evaluated in
VEGF-C-treated HeLa cells. HeLa cells (1×106/ml) in the
logarithmic growth phase were incubated without serum for 24 h, and
then treated with VEGF-C (at a final concentration of 50 ng/μl) for
3, 6, 12 and 24 h. The cells were harvested and subjected to
SDS-PAGE and western blot analysis in order to determine Hsp90
protein expression.
The pathways involved were then investigated. HeLa
cells (1×106/ml) in the logarithmic growth phase were
incubated without serum for 24 h, and then pretreated with kinase
insert domain receptor antibody (KDR)-Ab (20 μg/ml; Tianjin
Institute of Hematology, Chinese Academy of Medical Science), PI3K
inhibitor LY294002 (3 μmol/l; Promega Corp. Madison, WI, USA), MAPK
inhibitor PD98059 (30 μmol/l; Promega Corp.) or Hsp90-specific
inhibitor GA (10 μmol/l) for 30 min. The cells were then treated
with VEGF-C (50 ng/μl) for a further 24 h. The cells were harvested
and subjected to SDS-PAGE and western blot analysis for Hsp90
protein expression.
Finally, the role of Hsp90 in the proliferation and
apoptosis of HeLa cells induced by VEGF-C was investigated. HeLa
cells (1×106/ml) in logarithmic growth phase were
incubated without serum for 24 h, pretreated with GA (0.02 μmol/l)
for 30 min, and then treated with VEGF-C (50 ng/μl) for 24 h. The
cells were harvested for MTT analysis, annexin V-FITC/propidium
iodide (PI) double staining for early apoptosis, and SDS-PAGE and
western blot analysis to determine the levels of Bcl-2, Bax and
cyclin D1 expression.
MTT cell proliferation assay
HeLa cells were seeded onto 96-well culture plates
and subjected to the different treatments. Following treatment, the
media were removed and 20 μl MTT was added to each well. The cells
were further cultured at 37°C for 4 h, prior to the removal of the
cell culture and the addition of 100 μl dimethyl sulfoxide to
dissolve the formazan completely. The absorbance of formazan was
determined at 546 nm by the microplate reader and the survival rate
was evaluated. All experiments were repeated in triplicate.
Flow cytometry
HeLa cells were washed three times with
phosphate-buffered saline (PBS) and stained with
Annexin-V-fluorescein and Puffer (Bio-Rad, Philadelphia, PA, USA),
according to the manufacturer’s instructions. Cell fluorescence was
determined with a flow cytometer. All experiments were repeated in
triplicate.
Western blot analysis
HeLa cells were collected and lysed with
radioimmunoprecipitation assay buffer (Sigma, St. Louis, MO, USA),
and the total proteins were evaluated using bicinchoninic acid
protein assay reagent (Pierce, Rockford, IL, USA). The protein
samples were separated by 10% SDS-PAGE and then transferred to
polyvinylidene difluoride membranes. Subsequent to blocking in
non-fat dry milk at 37°C for 1 h, the membranes were incubated
overnight at 4°C with antibodies against Bcl-2 (1:50), Bax (1:50),
Bcl-2 (1:200), cyclin D1 (1:200) and GAPDH (1:500), respectively.
The membranes were then washed with PBS with Tween 20 at 10-min
intervals, prior to incubation with the secondary diluted
anti-mouse or anti-rabbit antibody (1:1,000; Santa Cruz
Biotechnology, Inc., Paso Robles, CA, USA) for 1 h at room
temperature in the dark. The membranes were developed using
enhanced chemiluminescence (Pierce) and exposed to X-ray film.
Tanon Gel Imaging System Version 4.00 software (Tanon Science &
Technology Co., Ltd., Shanghai, China) was used to analyze the
images. The GAPDH was regarded as an internal reference for
relative quantification.
Statistical analysis
All analyses were performed using SPSS statistical
software (version 13.0; SPSS Inc., Chicago, IL). Results are
expressed as mean ± standard deviation. Significance of difference
between the groups was assessed by t-test or ANOVA, and regression
analysis was performed. One-tailed P-value of <0.05 was
considered as statistically significant.
Results
Hsp90 expression in VEGF-C-treated HeLa
cells
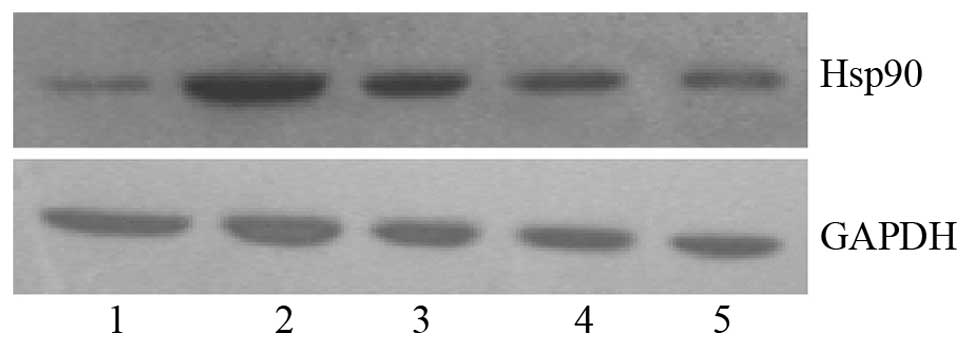
VEGF-C was found to induce Hsp90 protein expression
in HeLa cells at various treatment time-points, as shown in
Table I. Hsp90 protein expression
was increased 3.84-fold 3 h following VEGF-C stimulation
(1.93±0.17; P<0.05), peaked at 12 h (2.46±0.04; P<0.05) and
decreased slightly at 24 h (1.47±0.13; P<0.05). Fig. 1 shows the Hsp90 protein bands in
HeLa cells treated with VEGF-C for 3, 6, 12 and 24 h, respectively,
using GAPDH as the internal reference.
 | Table IHeat shock protein 90 expression in
VEGF-C treated HeLa cells. |
Table I
Heat shock protein 90 expression in
VEGF-C treated HeLa cells.
| Treatment
duration | Expressiona | Ratiob | P-value |
|---|
| 0 h | 0.50±0.07 | 1 | |
| 3 h | 1.93±0.17 | 3.84 | <0.05c |
| 6 h | 2.08±0.26 | 4.15 | <0.05c,d |
| 12 h | 2.46±0.04 | 4.9 | <0.05c,d |
| 24 h | 1.47±0.13 | 2.92 | <0.05c,d |
Signaling pathways for the induction of
Hsp90
To investigate whether VEGF-C induced Hsp90
expression via the VEGFR-2 (KDR), MAPK and PI3K pathways, HeLa
cells were treated with VEGF-C (50 ng/μl), VEGF-C (50 ng/μl) +
KDR-Ab (20 μg/ml), VEGF-C (50 ng/μl) + LY294002 (3 μmol/l), VEGF-C
(50 ng/μl) + PD98059 (30 μmol/l) or VEGF-C (50 ng/μl) + GA (10
μmol/l). Results are shown in Table
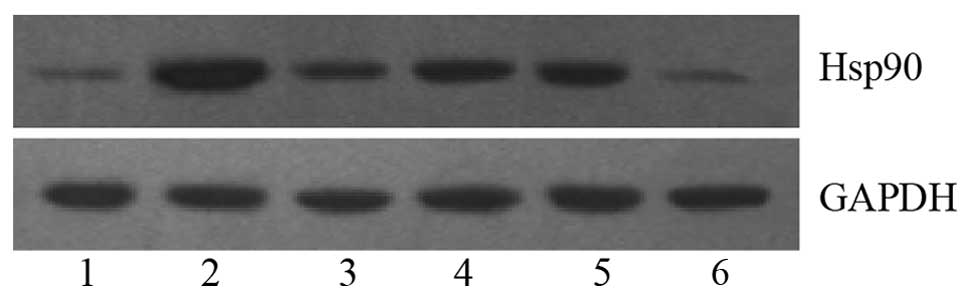
II. Hsp90 expression was increased 3.31-fold in VEGF-C treated
HeLa cells; whilst Hsp90 expression was attenuated in the other
groups (2.17-, 1.69-, and 1.82-fold in the VEGF-C + KDR-Ab, VEGF-C
+ PD98059 and VEGF-C + LY294002 groups, respectively; P<0.05),
indicating that KDR-Ab, PD98059 and LY294002 partially inhibited
Hsp90 expression in HeLa cells induced by VEGF-C. However, there
was no significant difference between the expression of Hsp90
between the GA-treated and control cells (0.70±0.05 vs. 0.62±0.21,
respectively; P>0.05). Fig. 2
shows the Hsp90 protein bands in HeLa cells following the various
treatments, using GAPDH as the internal reference.
 | Table IIEffect of inhibitors of various
signaling pathways on the induction of heat shock protein 90 by
VEGF-C. |
Table II
Effect of inhibitors of various
signaling pathways on the induction of heat shock protein 90 by
VEGF-C.
| Treatment | Expressiona | Ratiob | P-value |
|---|
| Control | 0.62±0.21 | 1 | |
| VEGF-C | 2.04±0.15 | 3.31 | <0.05c |
| VEGF-C+KDR-Ab | 1.33±0.26 | 2.17 | <0.05d |
| VEGF-C+LY294002 | 1.04±0.08 | 1.69 | <0.05d |
| VEGF-C+PD98059 | 1.11±0.11 | 1.82 | <0.05d |
| VEGF-C+GA | 0.70±0.05 | 1.14 | >0.05c |
Role of Hsp90 in the proliferation of
HeLa cells induced by VEGF-C
GA was demonstrated to completely inhibit the
proliferation of HeLa cells induced by VEGF-C. The results from the
MTT analysis demonstrated that the proliferation of the HeLa cells
was increased ~2.13-fold following treatment with VEGF-C, whilst
the proliferation of VEGF-C + GA-treated HeLa cells decreased
0.87-fold (P<0.05; Table
III). In addition, the proliferation of HeLa cells treated with
GA alone was significantly lower compared with that of control
cells (P<0.05), indicating that GA completely inhibited the
proliferation of HeLa cells induced by VEGF-C.
 | Table IIIEffect of VEGF-C and GA on the
proliferation and apoptosis of HeLa cells. |
Table III
Effect of VEGF-C and GA on the
proliferation and apoptosis of HeLa cells.
| Proliferation | Early apoptosis
index |
|---|
|
|
|
|---|
| Treatment | Absorbancea | Proliferation
index | P-value | Apoptosis
indexa | Relative
ratiob | P-value |
|---|
| Control | 0.62±0.02 | 1 | | 7.44±0.54 | 1 | |
| VEGF-C | 1.32±0.09 | 2.13 | <0.05c | 3.29±0.35 | 0.44 | <0.05c |
| VEGF-C+GA | 0.54±0.02 | 0.87 | <0.05c | 6.06±0.78 | 0.81 | <0.05d |
| GA | 0.46±0.18 | 0.74 | <0.05c,d | 10.46±1.12 | 1.41 | <0.05c |
Effect of Hsp90 on the apoptosis
inhibition of HeLa cells induced by VEGF-C
The early apoptosis index was determined using
annexin V-FITC/PI double staining, and the results are shown in
Table III. There was a
significant difference in early apoptosis index between the HeLa
cells treated with VEGF-C and those treated with VEGF-C + GA
(3.29±0.35 versus 6.06±0.78, respectively; P<0.05), and the
early apoptosis index of HeLa cells treated with GA alone was
significantly higher compared with that of the control cells
(10.46±1.12 versus 7.44±0.54, respectively; P<0.05), indicating
that GA induced apoptosis of HeLa cells. The results indicate that
Hsp90 works in association with VEGF-C in regulating the apoptosis
of HeLa cells.
Role of Hsp90 in the Bcl-2/Bax protein
expression induced by VEGF-C
HeLa cells were incubated without serum for 24 h,
and then treated with VEGF-C (50 ng/μl) and VEGF-C (50 ng/μl) + GA
(0.02 μmol/l) for 24 h. Bcl-2 and Bax protein expression levels
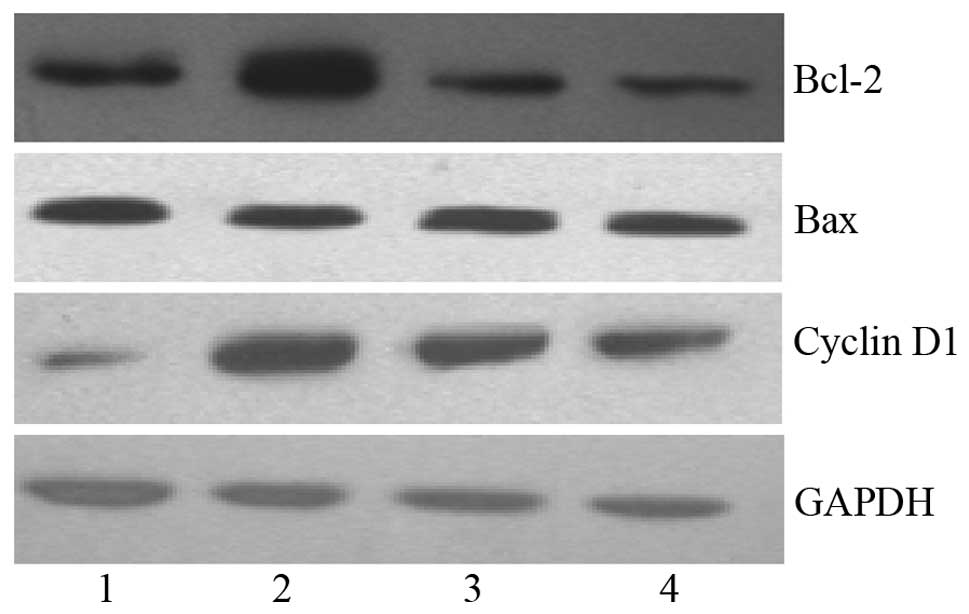
were then determined by western blot analysis. Bcl-2 protein
expression was found to be significantly lower in VEGF-C + GA
treated HeLa cells than in VEGF-C treated HeLa cells (0.87±0.23
versus 1.90±0.15, respectively; P<0.05), and Bcl-2 protein
expression following treatment with GA was significantly lower
compared with that in the control cells (0.67±0.16 versus
0.97±0.07, respectively; P<0.05), indicating that even a low
concentration of GA (0.02 μmol/l) is able to inhibit the Bcl-2
protein expression induced by VEGF-C. However, there were no
significant differences in the levels of Bax protein expression
induced by GA, VEGF-C and VEGF-C + GA (P>0.05). In addition, the
Bcl-2/Bax expression ratio was lowest in the GA group, followed by
the VEGF-C + GA and VEGF-C groups (P<0.05), indicating that even
a low concentration of GA had a marked inhibitory effect on Bcl-2
expression. All these results suggest that Hsp90 works in
association with VEGF-C in regulating the apoptosis of HeLa cells.
The Bcl-2 and Bax protein bands in HeLa cells are shown in Fig. 3, with GAPDH as the internal
reference.
Role of Hsp90 in cyclin D1 protein
expression induced by VEGF-C
GA was found to significantly inhibit the cyclin D1
protein expression induced by VEGF-C (Table IV). Cyclin D1 protein expression
was significantly lower in VEGF-C + GA-treated HeLa cells than in
VEGF-C-treated HeLa cells (0.64±0.21 versus 1.30±0.13,
respectively; P<0.05), and cyclin D1 protein expression was
significantly lower in GA-treated HeLa cells than in control cells
(0.42±0.11 versus 0.57±0.07; P<0.05). These results indicate
that even a low concentration of GA had a marked inhibitory effect
on cyclin D1 expression, and this suggests that Hsp90 has a role in
the induction of cyclin D1 expression by VEGF-C. Cyclin D1 protein
bands in HeLa cells are shown in Fig.
3, with GAPDH as the internal reference.
 | Table IVEffect of GA on Bcl-2, Bax and cyclin
D1 protein expression induced by VEGF-C. |
Table IV
Effect of GA on Bcl-2, Bax and cyclin
D1 protein expression induced by VEGF-C.
| Bcl-2 | Bax | Bcl-2/Bax | Cyclin D1 |
|---|
|
|
|
|
|
|---|
| Treatment | Expressiona | Ratio | P-value | Expressiona | Ratio | P-value | Expressiona | Ratio | P-value | Expressiona | Ratio | P-value |
|---|
| Control | 0.97±0.07 | 1 | | 0.70±0.09 | 1 | | 1.38±0.09 | 1 | | 0.57±0.07 | 1 | |
| V | 1.90±0.15 | 2.73 | <0.05b,c | 0.79±0.14 | 1.13 | >0.05b | 2.40±0.15 | 1.74 | <0.05b | 1.30±0.13 | 2.29 | <0.05b |
| V+GA | 0.87±0.23 | 1.28 | <0.05c | 0.68±0.13 | 0.97 | >0.05b | 1.28±0.20 | 0.93 | <0.05c | 0.64±0.21 | 1.13 | <0.05c |
| GA | 0.67±0.16 | 0.74 | <0.05b,c | 0.90±0.16 | 1.30 | >0.05b | 0.74±0.16 | 0.54 | <0.05b,c | 0.42±0.11 | 0.76 | <0.05b,c |
Discussion
Molecular chaperone Hsp90 is not only of major
current interest in fundamental biological research, but is also a
target for the treatment of cancer and other diseases. Hsp90 guides
the normal folding, intracellular localization and proteolytic
turnover of a number of key regulators for cell growth,
differentiation and survival (9).
Inhibition of Hsp90 function has been shown to cause degradation of
target proteins via the ubiquitin proteasome pathway, resulting in
the simultaneous depletion of multiple oncoproteins, combinatorial
downregulation of signals being propagated via numerous signaling
pathways, and modulation of all aspects of the malignant phenotype
(10,11). The PI3K and MAPK pathways are
involved in the proliferation and apoptosis of HeLa cells induced
by VEGF-C, with the overexpression of several downstream genes,
including Bcl-2 and cyclin D1. The aim of the present study was to
investigate the effect of Hsp90-specific inhibitor GA and VEGF-C on
the expression of Hsp90 in HeLa cells.
The effect of Hsp90 and Hsp90-specific inhibitor GA
on the proliferation and apoptosis of HeLa cells was investigated.
Hsp90 binds to a number of signaling proteins, including ligand
dependent transcription factors (e.g., steroid receptor),
ligand-independent transcription factors (e.g., MyoD), tyrosine
kinases (e.g., v-Src) and serine/threonine kinases (e.g., Raf-1).
The role of Hsp90 is to promote the conformational maturation of
these receptors and signal-transducing kinases. It interacts with
proteins that have already attained a high degree of tertiary
structure, and appears to be involved in the maturation and
activation of these target proteins rather than their initial
folding. Hsp90 chaperone activity depends on its ability to bind
and hydrolyze ATP (12,13), which drives a molecular clamp via
transient dimerization of the N-terminal domains. HSP90 expression
has been shown to be increased in cancer cells (14). It interacts with the signaling
proteins to maintain the normal structure and functions of these
proteins, and has an important role in the development of tumors
(15).
The association between Hsp90 and the proliferation
and apoptosis of tumor cells has been investigated in numerous
studies. Hsp90 may be involved in the proliferation and apoptosis
of tumor cells via the PI3K-AKT/PKB and RAS-RAF-MEK-ERK1/2 pathways
(16). Inhibition of Hsp90
function may downregulate Akt kinase, dephosphorylate extracellular
signal-regulated kinase and induce cell cycle arrest and cell death
(17,18). At present, a number of Hsp90
molecular chaperones have been identified with possible
implications on the proliferation and apoptosis of tumor cells,
including Bcl-2, AKT/PKB, survivin, c-Raf, JNK, pp60 (v-src),
Bcr-Abl, mutant p53, ErbB2 (Her-2), Flt3, HIF-1α, B-Raf and CDK4
(19,20).
GA is a naturally occurring benzoquinone ansamycin,
which binds specifically to the N-terminal ATP binding domain of
Hsp90 (21), and causes the
destabilization and degradation of numerous Hsp90 target proteins.
GA specifically inhibits Hsp90 by binding to the ATP hydrolysis
site with an affinity >500-times greater than for ATP, thus
effectively displacing ATP and disrupting Hsp90-substrate
interactions. This makes GA an important candidate in the study of
Hsp90 function (22). In a
previous study, Duus et al (23) investigated Hsp90 expression in a
myeloma cell line (U266) using immunofluorescence and flow
cytometric analysis, and the results demonstrated that GA treatment
resulted in a significant increase in apoptosis and reduction in
Bcl-2 expression levels. The Bcl-2-binding protein BAG-1 binds to
Bcl-2, Raf-1 kinase and growth factor receptors to inhibit the
apoptosis of cells. BAG-1 also binds to steroid hormone receptors
associated with Hsp family members.
In the present study, whether Hsp90 is involved in
the proliferation and apoptosis of HeLa cells was investigated.
In vitro treatment of HeLa cells with GA leads to the
inhibition of cell proliferation, an exponential increase in
apoptosis and a reduction in Bcl-2 expression, indicating that
Hsp90 has an important role in the proliferation and apoptosis of
cervical carcinoma cells by regulating Bcl-2 expression. However,
treatment with GA does not affect Hsp90 expression, indicating that
GA downregulates Bcl-2 expression, not by inhibiting Hsp90 mRNA or
protein expression, but by inhibiting Hsp90 function. GA may
inhibit the binding of Hsp90 to Bcl-2, promoting apoptosis and
mediating the signaling pathways for the apoptosis of cervical
carcinoma cells. Consequently, it has an important role in the
proliferation and apoptosis escape of cervical carcinoma cells.
The association between VEGF-C and Hsp90 was also
investigated in the present study. Whether VEGF-C induces Hsp90
expression was investigated. The results of the western blot
analysis revealed that Hsp90 protein expression in HeLa cells was
induced by VEGF-C when treated for different periods of time. Hsp90
protein expression was increased 3.84-fold following 3 h of VEGF-C
stimulation, peaked at 12 h and decreased slightly after 24 h,
indicating that VEGF-C induced Hsp90 expression.
In order to investigate whether VEGF-C induced Hsp90
expression via VEGFR-2 (KDR), MAPK and PI3K pathways, HeLa cells
were treated with VEGF-C, VEGF-C + KDR-Ab, VEGF-C + LY294002,
VEGF-C + PD98059 and VEGF-C + GA. It was found that Hsp90
expression was increased 3.31-fold in VEGF-C treated HeLa cells,
and was attenuated in other treatment groups (2.17-, 1.69-,
1.82-fold in VEGF-C + KDR-Ab, VEGF-C + PD98059 and VEGF-C +
LY294002, respectively). However, there was no significant
difference between the GA-treated cells and control cells
(P>0.05). These results indicate that GA functions not by
inhibiting Hsp90 mRNA or protein expression, but by inhibiting
Hsp90 function. VEGF-C may induce Hsp90 expression via the PI3K and
MAPK pathways. In VEGFR-2 (KDR) positive HeLa cells, VEGF-C actives
PI3K/AKT and ERK/MAPK pathways via the KDR receptors, and
upregulates Hsp90 expression.
The role of Hsp90 in the proliferation and apoptosis
of HeLa cells induced by VEGF-C was also investigated in the
present study. The Hsp90-specific inhibitor GA was found to
completely inhibit the proliferation of HeLa cells induced by
VEGF-C. The proliferation of the VEGF-C treated HeLa cells was
increased ~2.13-fold, whereas that of the VEGF-C + GA treated HeLa
cells decreased 0.87-fold (P<0.05). The proliferation of
GA-treated HeLa cells was significantly lower compared with that of
control cells (P<0.05). These results indicate that Hsp90
participates in the VEGF-C induced proliferation and apoptosis of
HeLa cells. In addition, it was shown that even a low concentration
of GA (0.02 μmol/l) inhibits the Bcl-2 and cyclin D1 protein
expression induced by VEGF-C, but appears to have no effect on Bax
protein expression.
In the present study, VEGF-C was indicated to induce
Hsp90 expression via the PI3K and MAPK pathways. Hsp90 binds to a
number of specific signaling proteins that require this interaction
to execute their function, and upregulates the expression of
downstream genes, including Bcl-2 and cyclin D1. Therefore, Hsp90
has a critical role in the proliferation and apoptosis of HeLa
cells. Hsp90 modulates Bcl-2 expression, as shown by the complete
inhibition of VEGF-induced Bcl-2 expression and binding to Hsp90 by
Hsp90-specific inhibitor GA; VEGF has been shown to promote the
survival of leukemic cells by the Hsp90-mediated induction of Bcl-2
expression and apoptosis inhibition (23). Typical Hsp90 target proteins
include Bcl-2, AKT/PKB, c-Raf, B-Raf and CDK4 (19). Whether other target proteins are
involved requires further investigation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81303108 and 81201871).
References
|
1
|
Bishop SC, Burlison JA and Blagg BS:
Hsp90: a novel target for the disruption of multiple signaling
cascades. Curr Cancer Drug Targets. 7:369–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiosis G, Dickey CA and Johnson JL: A
global view of Hsp90 functions. Nat Struct Mol Biol. 20:1–4. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee WY, Chen YC, Shih CM, et al: The
induction of heme oxygenase-1 suppresses heat shock protein 90 and
the proliferation of human breast cancer cells through its
byproduct carbon monoxide. Toxicol Appl Pharmacol. 274:55–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwock J, Pham NA, Cao MP and Hedley DW:
Efficacy of Hsp90 inhibition for induction of apoptosis and
inhibition of growth in cervical carcinoma cells in vitro and in
vivo. Cancer Chemother Pharmacol. 61:669–681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neckers L: Chaperoning oncogenes: Hsp90 as
a target of geldanamycin. Handb Exp Pharmacol. 172:259–277. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehta PP, Kung PP, Yamazaki S, et al: A
novel class of specific Hsp90 small molecule inhibitors demonstrate
in vitro and in vivo anti-tumor activity in human melanoma cells.
Cancer Lett. 300:30–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du X and Mi R: Study on the cell signal
transductions of cell proliferation and apoptosis induced by VEGF-C
in cervical carcinoma. Xian Dai Fu Chan Ke Za Zhi. 11:877–880.
8852011.
|
|
9
|
Chinnaiyan P, Allen GW and Harari PM:
Radiation and new molecular agents, part II: targeting HDAC, HSP90,
IGF-1R, PI3K, and Ras. Semin Radiat Oncol. 16:59–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JW, Yeh MW, Wong MG, et al: The heat
shock protein 90-binding geldanamycin inhibits cancer cell
proliferation, down-regulates oncoproteins, and inhibits epidermal
growth factor-induced invasion in thyroid cancer cell lines. J Clin
Endocrinol Metab. 88:3346–3353. 2003. View Article : Google Scholar
|
|
11
|
Sreedhar AS, Kalmár E, Csermely P and Shen
YF: Hsp90 isoforms: functions, expression and clinical importance.
FEBS Lett. 562:11–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt E, Gehrmann M, Brunet M, Multhoff
G and Garrido C: Intracellular and extracellular functions of heat
shock proteins: repercussions in cancer therapy. J Leukoc Biol.
81:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polier S, Samant RS, Clarke PA, et al:
ATP-competitive inhibitors block protein kinase recruitment to the
Hsp90-Cdc37 system. Nat Chem Biol. 9:307–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richter K and Buchner J: Hsp90:
chaperoning signal transduction. J Cell Physiol. 188:281–290. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cappello F, David S, Rappa F, et al: The
expression of HSP60 and HSP10 in large bowel carcinomas with lymph
node metastase. BMC Cancer. 5:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powers MV and Workman P: Targeting of
multiple signalling pathways by heat shock protein 90 molecular
chaperone inhibitors. Endocr Relat Cancer. 13(Suppl 1): 125–135.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Georgakis GV, Li Y, Rassidakis GZ, et al:
Inhibition of heat shock protein 90 function by
17-allylamino-17-demethoxy-geldanamycin in Hodgkin’s lymphoma cells
down-regulates Akt kinase, dephosphorylates extracellular
signal-regulated kinase, and induces cell cycle arrest and cell
death. Clin Cancer Res. 12:584–590. 2006.PubMed/NCBI
|
|
18
|
Mitsiades CS, Mitsiades NS, McMullan CJ,
et al: Antimyeloma activity of heat shock protein-90 inhibition.
Blood. 107:1092–1100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrario A, Rucker N, Wong S, Luna M and
Gomer CJ: Survivin, a member of the inhibitor of apoptosis family,
is induced by photodynamic therapy and is a target for improving
treatment response. Cancer Res. 67:4989–4995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Röhl A, Rohrberg J and Buchner J: The
chaperone Hsp90: changing partners for demanding clients. Trends
Biochem Sci. 38:253–262. 2013.PubMed/NCBI
|
|
21
|
Sidera K and Patsavoudi E: HSP90
Inhibitors: current development and potential in cancer therapy.
Recent Pat Anticancer Drug Discov. 9:1–20. 2014.PubMed/NCBI
|
|
22
|
Gooljarsingh LT, Fernandes C, Yan K, et
al: A biochemical rationale for the anticancer effects of Hsp90
inhibitors: slow, tight binding inhibition by geldanamycin and its
analogues. Proc Natl Acad Sci USA. 103:7625–7630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duus J, Bahar HI, Venkataraman G, et al:
Analysis of expression of heat shock protein-90 (HSP90) and the
effects of HSP90 inhibitor (17-AAG) in multiple myeloma. Leuk
Lymphoma. 47:1369–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|