Introduction
Breast cancer has emerged as the most common
malignancy observed in females worldwide. It is the leading cause
of cancer mortalities among females, accounting for 23% of total
cancer cases and 14% of cancer mortalities (1). Chemotherapy is one of the main
treatments for patients diagnosed with breast cancer. However,
resistance to chemotherapeutic drugs has gradually emerged
(2). Resistance to
chemotherapeutics, in particular, multidrug resistance (MDR),
remains the leading cause of chemotherapy failure. The MDR
mechanism is an extremely complicated process, involving drug
metabolic biotransformation, drug efflux increase and alteration of
the repair ability for anticancer drug-induced DNA damage (3,4).
Therefore, it is imperative to find novel and effective strategies
to reverse drug resistance.
At present, the main strategy for overcoming MDR is
to use sensitizing or reversal agents, combined with
chemotherapeutic drugs (5).
Traditional Chinese herbs are a significant source of drugs that
serve as potential therapeutic compounds for cancer treatment
(6). Numerous studies have
identified reversal agents from natural products. Rhizoma Curcuma
is a widely used traditional herb for antitumor therapy in China
and other Asian countries (7).
Germacrone, the main component of Rhizoma Curcuma, has been shown
to possess antitumor, anti-inflammatory and immunomodulatory
properties. A recent study demonstrated that treatment of the
hepatoma cell lines HepG2 and Bel7402 with germacrone promoted cell
apoptosis, associated with the upregulation of bax and the
downregulation of bcl-2, indicating that germacrone may have a
potential role in the treatment of hepatocellular carcinoma
(8). Germacrone has also been
found to inhibit the proliferation of the breast cancer cell lines
MCF-7 and MDA-MB-231 by inducing G0/G1 and G2/M cell cycle arrest
and apoptosis through the mitochondria-mediated caspase pathway
(9). However, the function of
germacrone on MDR in human breast cancer has not yet been
investigated. Therefore, the present study aimed to investigate the
effect of germacrone on MCF-7/Adriamycin (ADR) multidrug-resistant
human breast cancer cells.
Materials and methods
Reagents
Germacrone and ADR were purchased from Sigma (St.
Louis, MO, USA). RPMI-1640 culture medium, fetal bovine serum
(FBS), phosphate-buffered saline (PBS), penicillin-streptomycin and
0.25% (w/v) trypsin/1 mM EDTA were purchased from Gibco (Grand
Island, NY, USA).
Cell culture
MCF-7 and MCF-7/ADR human breast cancer cells were
purchased from the Chinese Academy of Sciences (Shanghai, China)
and were maintained in RPMI-1640 medium containing 10% (v/v) FBS,
100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified 5% CO2 incubator. MCF-7/ADR cells were
cultured in the medium containing 1 μg/ml ADR in order to maintain
the MDR phenotype, and were then maintained in drug-free medium for
at least two days prior to use.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
In brief, MCF-7 and MCF-7/ADR cells were independently seeded at a
density of 3×104 cells/well into 96-well plates and left
to adhere overnight. The cells were then incubated with 0–125
μmol/l ADR, 0–250 μmol/l germacrone or a combination of 0–250
μmol/l germacrone and 1 μmol/l ADR for 48 h. A total of 10 ml 5
mg/ml MTT was added and the cells were incubated in the dark at
37°C for 2 h. The absorbance was then determined at a wavelength of
492 nm (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis assay
MCF-7/ADR cells were seeded in 12-well plates and
treated with different concentrations (0, 50, 150 and 250 μmol/l)
of germacrone and/or 1.0 μmol/l ADR for 48 h. The apoptotic
morphology of the cells was evaluated using hematoxylin and eosin
staining for visualization under a light microscope (Leica
Microsystems, Wetzlar, Germany; magnification, ×200). Cells
undergoing apoptosis were assessed using an Annexin V-FITC/PI
Apoptosis Detection kit, in accordance with the manufacturer’s
instructions (BD Biosciences, Franklin Lakes, NJ, USA). The number
of apoptotic cells was quantified using a flow cytometer
(FACSCalibur™; BD Biosciences) and analyzed using CellQuest
software (BD Biosciences).
Western blot analysis
For the western blot analysis of total cell lysates,
cells were harvested and washed with ice-cold PBS. The protein
concentration in the lysates was measured using a BCA Protein assay
kit (Thermo Fisher Scientific, Rockford, IL, USA) in accordance
with the manufacturer’s instructions. Cell lysate samples (50 μg
per lane) were separated using 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Membranes were incubated overnight at 4°C with antibodies
against p53, bax, bcl-2, P-gp and GAPDH (Cell Signaling Technology,
Inc., Danvers, MA, USA). Membranes were washed three times with
Tris-buffered saline with Tween® 20 and incubated for 1
h at room temperature with the appropriate secondary antibody (Cell
Signaling Technology, Inc.). Immunoreactive bands were detected
using the Enhanced Chemiluminescence kit for Western blotting
detection and using a ChemiGenius bioimaging system (Syngene,
Frederick, MD, USA).
Quantitative PCR (qPCR)
The mRNA expression of MDR1 was analyzed by qPCR.
Total RNA was extracted from the treated MCF-7/ADR cells using the
RNeasy kit with the DNase set (Qiagen GmbH, Hilden, Germany). For
cDNA synthesis, the template was reverse transcribed using
SuperScript II RNase H-reverse transcriptase and
oligo(dT)25 as a primer (Invitrogen Life Technologies,
Carlsbad, CA, USA). PCR was carried out under the following
conditions: an initial stage of 95°C for 30 sec, then a two step
program of 95°C for 5 sec and 60°C for 31 sec over 40 cycles and
was performed in triplicate. The relative target mRNA levels were
analyzed with ABI Prism 7300 software (Applied Biosystems, CA, USA)
and normalized against that of the internal control, GAPDH.
Dual luciferase assay
MCF-7/ADR cells were seeded in 96-well plates for 24
h until they reached 90–95% confluence at the time of transfection.
The cells were co-transfected with the MDR1 promoter recombinant
vector pGL3-basic-MDR1, and a control vector according to the
manufacturer’s instructions (Promega Corporation, Madison, WI,
USA). The cells were collected 48 h after transfection and analyzed
using a Dual-Luciferase Reporter assay system (Promega
Corporation).
Statistical analysis
For statistical analysis, all data were analyzed
using SPSS statistical software, version 13.0 (SPPS, Inc., Chicago,
IL, USA), and are presented as the mean ± standard deviation.
Comparisons between groups were performed using an analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of germacrone on ADR resistance in
breast cancer cells
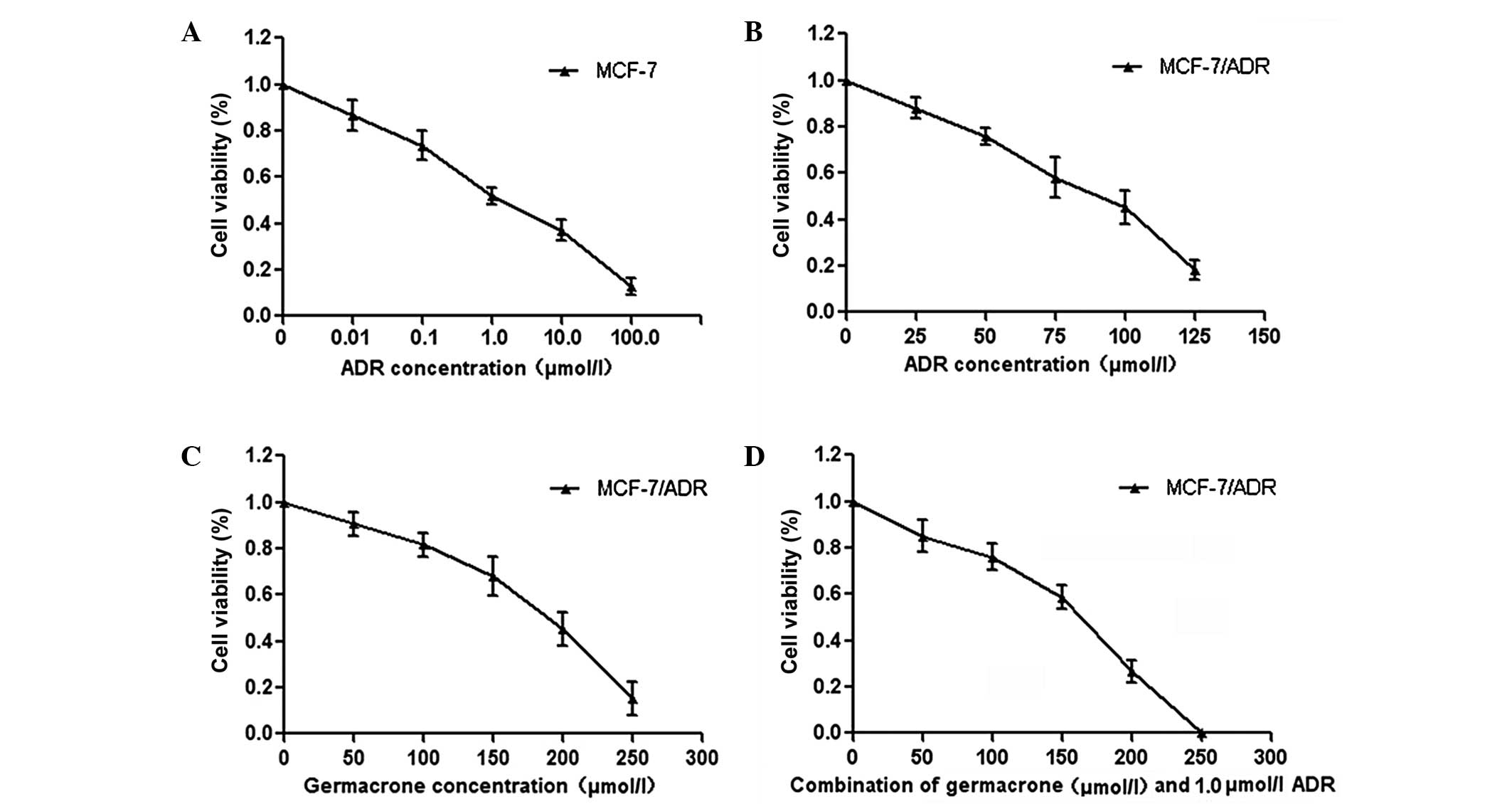
The cytotoxicity of germacrone and ADR in the
MCF-7/ADR human breast cancer cell line was analyzed using the MTT
assay. The results showed that the IC50 of ADR at 48 h
was 1.27±0.12 μmol/l in MCF-7 cells and 87.40±5.24 μmol/l in
MCF-7/ADR cells (Fig. 1A and B).
In addition, the IC50 of germacrone was 180.41±12.45
μmol/l in MCF-7/ADR cells following 48 h of treatment. The MTT
assay demonstrated that germacrone treatment inhibited cell
viability in a concentration-dependent manner (Fig. 1C). Furthermore, treatment with a
combination of germacrone and ADR inhibited cell viability
synergistically (Fig. 1D). In
combination, these results demonstrate that the treatment of
MCF-7/ADR cells with a combination of germacrone and ADR results in
an increase in cytotoxicity.
Germacrone promotes the rate of apoptosis
induced by ADR in MCF-7/ADR cells
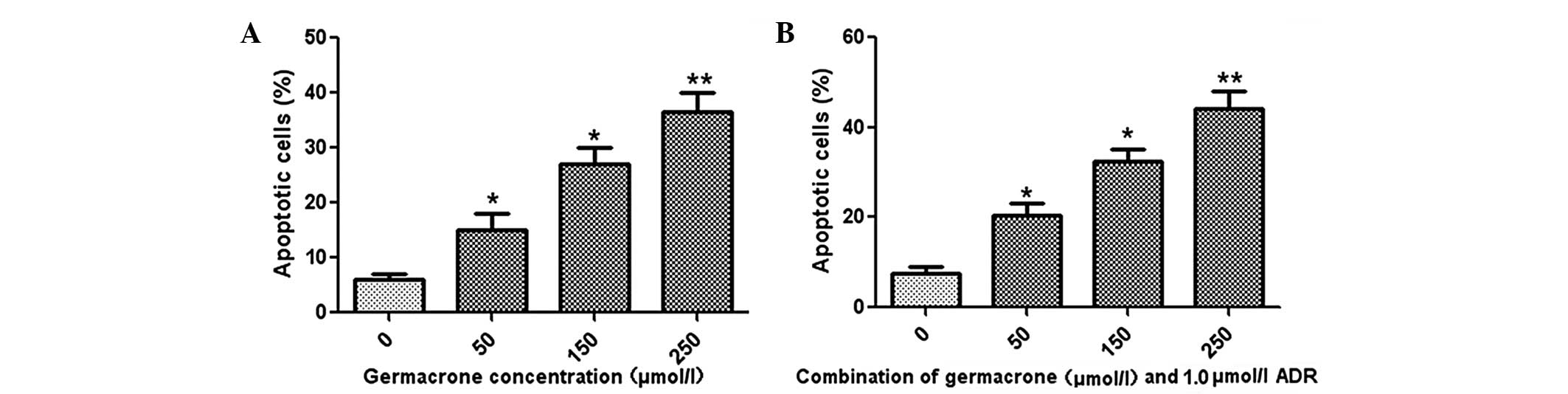
Flow cytometry was used to measure the effects of
germacrone and ADR on the apoptosis rate in MCF-7/ADR cells. The
results revealed that germacrone treatment promoted cell apoptosis
in a concentration-dependent manner in MCF-7/ADR cells (Fig. 2A). Furthermore, treatment with a
combination of ADR (1.0 μmol/l) and germacrone at different
concentrations (50, 150 or 250 μmol/l) caused a significant
increase in the apoptotic rate in the MCF-7/ADR cells (Fig. 2B).
Effect of germacrone and ADR on apoptotic
proteins in MCF-7/ADR cells
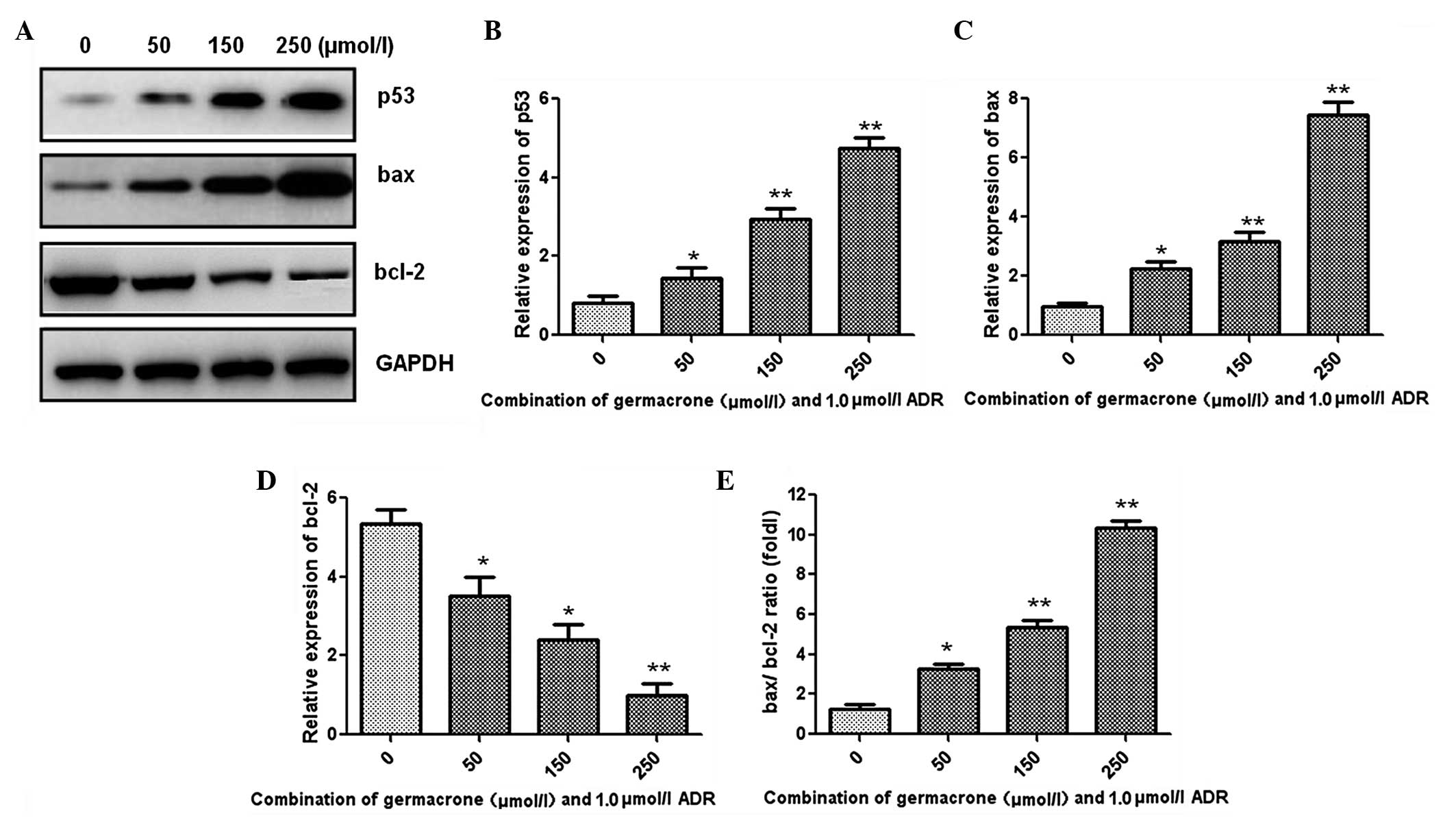
Western blot analysis was performed to detect the
changes in the levels of apoptosis-associated proteins, and the
results are shown in Fig. 3A.
Following treatment with a combination of germacrone (50, 150 or
250 μmol/l) and ADR (1.0 μmol/l), the expression of p53 and bax was
significantly increased (Fig. 3B and
C). Furthermore, the expression level of the anti-apoptotic
protein bcl-2 was markedly decreased by treatment with the
combination compared with that in the control group treated with
ADR (1.0 μmol/l) alone (Fig. 3D).
In addition, the bax/bcl-2 ratio was significantly increased in a
concentration-dependent manner following treatment with germacrone
and ADR together (Fig. 3E).
Germacrone treatment decreased the
expression of MDR1
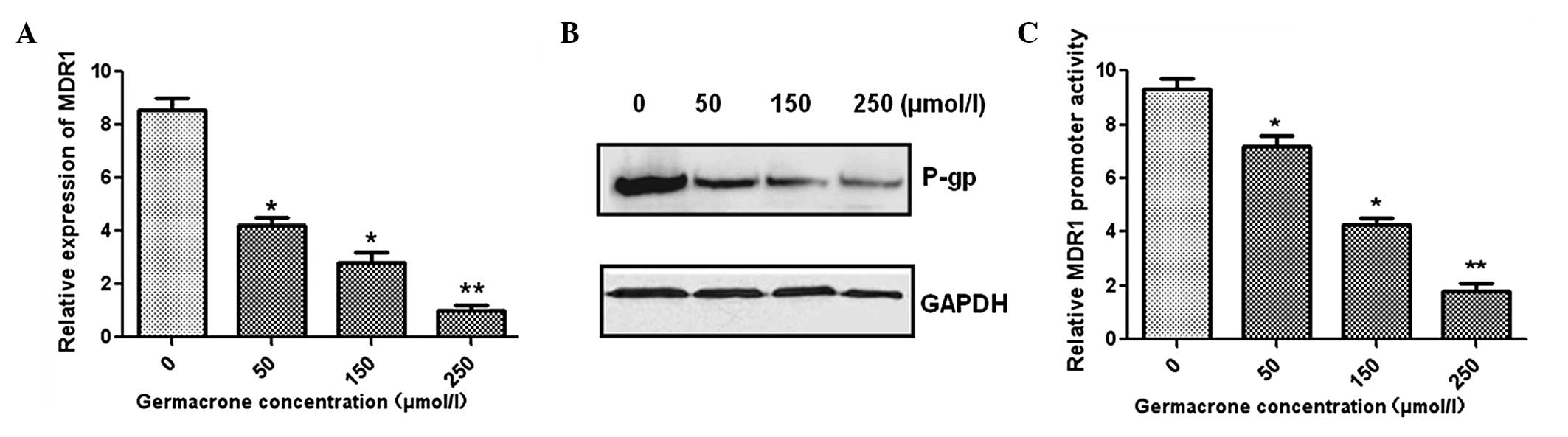
In order to investigate the reversal mechanism of
germacrone, MCF-7/ADR cells were treated with various
concentrations of germacrone. qPCR demonstrated that MDR1 gene
expression was significantly inhibited by germacrone administration
in a concentration-dependent manner (Fig. 4A). In addition, the expression of
P-gp was also downregulated, as shown by western blot analysis
(Fig. 4B). Furthermore, the dual
luciferase assay was used to measure the MDR1 promoter activity.
The results demonstrated that MDR1 promoter expression levels were
markedly decreased following treatment with germacrone at different
concentrations compared with the levels in the control group
(Fig. 4C). In combination, these
results indicate that germacrone may decrease the P-gp expression
levels via the inhibition of the activity of the MDR1 gene
promoter.
Discussion
Germacrone is a sesquiterpene and has been
previously demonstrated to be a promising therapeutic agent against
several types of cancer. A previous study showed that germacrone is
able to inhibit breast cancer cell proliferation (9). However, the function of germacrone on
MDR in human breast cancer has yet to be elucidated. Therefore, in
the present study, the effect of germacrone on MCF-7/ADR human
breast cancer multidrug-resistant cells was investigated.
The MTT assay results revealed that germacrone
significantly inhibited the proliferation of MCF-7/ADR cells in a
concentration-dependent manner. Treatment with a combination of
germacrone and ADR effectively decreased the viability of the
MCF-7/ADR cells in vitro. These results indicate that
germacrone reversed the ADR resistance of MCF-7/ADR cells.
ADR is a widely used chemotherapy drug that induces
tumor cell apoptosis; however, resistance against ADR has occurred
in numerous types of tumor cells (10,11).
Apoptosis is a complicated and precise process of programmed cell
death, characterized by cell shrinkage, phosphatidylserine
externalization and chromatin condensation (12). In the present study, flow
cytometric analysis indicated that germacrone dose-dependently
promoted MCF-7/ADR cell apoptosis whereas ADR did not significantly
induce apoptosis in the MCF-7/ADR cells. However, treatment with
ADR and germacrone markedly enhanced the apoptosis rate in the
MCF-7/ADR cells.
Furthermore, the expression of apoptosis-associated
proteins was determined using western blot analysis. The protein
p53, encoded by the TP53 gene, has an important role in
multi-cellular organisms, where it regulates cell apoptosis and
cell proliferation (13,14). Bax is a p53 primary-response gene
and is involved in the apoptotic induction regulated by p53. p53
directly activates the proapoptotic protein bax to permeabilize
mitochondria and engage the apoptotic pathway (15,16).
The anti-apoptotic protein bcl-2, which prevents disruption of the
mitochondrial physiology, is a response gene of p53 and involved in
p53-regulated apoptosis (17). The
present study showed that treatment with germacrone and ADR
significantly elevated the expression levels of p53 and bax and
decreased the expression levels of the anti-apoptotic protein
bcl-2.
Drug resistance in breast cancer cells is associated
with increased expression of resistance proteins (18). These proteins decrease the
accumulation of ADR in the cells, thereby reducing the effects of
ADR (19). P-gp, encoded by the
MDR1 gene, is one of the multi-drug resistance-associated proteins
acting as an efflux pump (20,21).
The overexpression of P-gp may lower intracellular drug
accumulation and decrease the cellular toxicity of
chemo-therapeutics, including ADR, epirubicin, mitoxantrone and
paclitaxel (22,23). The present study demonstrated that
germacrone reduced the gene and protein expression levels of P-gp
in MCF-7/ADR cells, as shown by the results from the qPCR and
western blot analysis. Furthermore, the activity of the MDR1 gene
promoter, which mediates P-gp expression, was significantly
downregulated following germacrone treatment, thereby promoting
ADR-induced MCF-7/ADR cell apoptosis.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that germacrone
reverses ADR resistance through cell apoptosis in MDR breast cancer
cells. Therefore, germacrone is of important clinical significance
for MDR during tumor therapy and may be a novel MDR reversal agent
for breast cancer chemotherapy.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Zhejiang Province (no. LY12H16008).
References
|
1
|
Serrano MJ, Rovira PS, Martinez-Zubiaurre
I, et al: Dynamics of circulating tumor cells in early breast
cancer under neoadjuvant therapy. Exp Ther Med. 4:43–48.
2012.PubMed/NCBI
|
|
2
|
Sakata S, Fujiwara M, Ohtsuka K, et al:
ATP-binding cassette transporters in primary central nervous system
lymphoma: decreased expression of MDR1 P-glycoprotein and breast
cancer resistance protein in tumor capillary endothelial cells.
Oncol Rep. 25:333–339. 2011.
|
|
3
|
Li RJ, Zhang GS, Chen YH, et al:
Down-regulation of mitochondrial ATPase by hypermethylation
mechanism in chronic myeloid leukemia is associated with multidrug
resistance. Ann Oncol. 21:1506–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Shuhendler A, Cheng J, et al:
Cytotoxicity and mechanism of action of a new ROS-generating
microsphere formulation for circumventing multidrug resistance in
breast cancer cells. Breast Cancer Res Treat. 121:323–333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuang YH, Shen T, Chen X, et al: Lapatinib
and erlotinib are potent reversal agents for MRP7 (ABCC10)-mediated
multidrug resistance. Biochem Pharmacol. 79:154–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X and Zhu X: Stellera
chamaejasme L. extract induces apoptosis of human lung cancer
cells via activation of the death receptor-dependent pathway. Exp
Ther Med. 4:605–610. 2012.
|
|
7
|
Bamba Y, Yun YS, Kunugi A and Inoue H:
Compounds isolated from Curcuma aromatica Salisb. inhibit
human P450 enzymes. J Nat Med. 65:583–587. 2011.
|
|
8
|
Liu Y, Wang W, Fang B, et al: Anti-tumor
effect of germacrone on human hepatoma cell lines through inducing
G2/M cell cycle arrest and promoting apoptosis. Eur J Pharmacol.
698:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Z, Chen X, Tan W, et al: Germacrone
inhibits the proliferation of breast cancer cell lines by inducing
cell cycle arrest and promoting apoptosis. Eur J Pharmacol.
667:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiozuka M, Nonomura Y and Matsuda R:
Transdermal delivery of adriamycin to transplanted Ehrlich ascites
tumor in mice. Pharmaceutics. 5:385–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QQ, Xu JD, Wang WJ, et al:
Twist1-mediated Adriamycin-induced epithelial-mesenchymal
transition relates to multidrug resistance and invasive potential
in breast cancer cells. Clin Cancer Res. 15:2657–2665. 2009.
View Article : Google Scholar
|
|
12
|
Peter ME: Programmed cell death: Apoptosis
meets necrosis. Nature. 471:310–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu W, Ge Y, Ojcius DM, et al: p53
signalling controls cell cycle arrest and caspase-independent
apoptosis in macrophages infected with pathogenic Leptospira
species. Cell Microbiol. 15:1642–1659. 2013.PubMed/NCBI
|
|
14
|
Mellert HS, Stanek TJ, Sykes SM, et al:
Deacetylation of the DNA-binding domain regulates p53-mediated
apoptosis. J Biol Chem. 286:4264–4270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng Y and Wu X: Peg3/Pw1 promotes
p53-mediated apoptosis by inducing Bax translocation from cytosol
to mitochondria. Proc Natl Acad Sci USA. 97:12050–12055. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gogada R, Prabhu V, Amadori M, et al:
Resveratrol induces p53-independent, X-linked inhibitor of
apoptosis protein (XIAP)-mediated Bax protein oligomerization on
mitochondria to initiate cytochrome c release and caspase
activation. J Biol Chem. 286:28749–28760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsutsui S, Yasuda K, Suzuki K, et al:
Bcl-2 protein expression is associated with p27 and p53 protein
expressions and MIB-1 counts in breast cancer. BMC Cancer.
6:1872006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roundhill EA and Burchill SA: Detection
and characterisation of multi-drug resistance protein 1 (MRP-1) in
human mitochondria. Br J Cancer. 106:1224–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diouf B, Cheng Q, Krynetskaia NF, et al:
Somatic deletions of genes regulating MSH2 protein stability cause
DNA mismatch repair deficiency and drug resistance in human
leukemia cells. Nat Med. 17:1298–1303. 2011. View Article : Google Scholar
|
|
20
|
Coles LD, Lee IJ, Voulalas PJ, et al:
Estradiol and progesterone-mediated regulation of P-gp in P-gp
overexpressing cells (NCI-ADR-RES) and placental cells (JAR). Mol
Pharm. 6:1816–1825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su CC: Tanshinone IIA potentiates the
efficacy of 5-FU in Colo205 colon cancer cells in vivo
through downregulation of P-gp and LC3-II. Exp Ther Med. 3:555–559.
2012.PubMed/NCBI
|
|
22
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
23
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: from genomics to mechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar : PubMed/NCBI
|