Introduction
Heart failure (HF) following myocardial infarction
is the leading cause of morbidity and mortality in the world.
Coronary artery disease (CAD) is the most risk important factor for
HF, particularly among the older populations (1). Myocardial ischemia occurs following
CAD (2). The ischemic injury
causes cardiomyocyte death and myocardial fibrosis (3). The loss of cardiomyocytes and
accumulation of extracellular matrix (ECM) lead to myocardial
stiffness, dysfunction and eventually failure (2,3).
Ischemia is a process of insufficient blood flow in
tissues that results in hypoxia (low oxygen supply). Hypoxia is the
most important stimulus that leads to cardiomyocyte death in the
process of heart ischemia. Both in vitro and in vivo
studies have demonstrated that hypoxia can induce apoptosis and
inhibit proliferation in cardiomyocytes (4–6).
Previous studies have demonstrated that hypoxia-inducible factor 1α
(HIF-1α) and heat shock factor 60 (HSF60) are two important
molecular determinants of cardiomyocyte apoptosis in response to
myocardial ischemia/reperfusion and hypoxia (1,7,8).
Both molecules are acutely and chronically expressed in myocardial
cells in response to hypoxia and ischemia, and have diverse targets
that affect cell survival (1).
HIF-1α expression promotes cardiomyocyte apoptosis in response to
hypoxia via regulating the transcription of B-cell lymphoma 2 and
Bcl-associated X protein (Bax) (7). HSF60 forms a complex with Bax
following the translocation of cytosolic HSF60 to the membrane and
Bax to the mitochondria, which triggers cell apoptosis (8).
CyclinA2 is a highly conserved protein that is
encoded by the CCNA2 gene (9).
CyclinA2 combined with cyclin-dependent kinase (CDK) 1 and CDK2
controls the transition of the cell cycle from the G1/S
phase to the G2/M phase and promotes cell mitosis. In
general, CyclinA2 is silenced in postnatal hearts (10). CyclinA2 plays a crucial role in
cardiomyocyte growth in fetal and neonatal hearts, and artificially
continued expression of CyclinA2 in adult hearts induces
cardiomyocyte proliferation and/or hyperplasia (10). Therapeutic delivery of CyclinA2
into adult rat hearts has also been observed to induce
cardiomyocyte regeneration following myocardial ischemia (11). To date, the effect of CyclinA2 on
cardiomyocyte growth in in vitro hypoxic conditions has not
been examined; this is therefore the focus of the present
study.
Materials and methods
Materials
Sprague Dawley neonatal rats were obtained from the
Animal Center of Xinxiang Medical University (Xinxiang, China). The
study and animal use were approved by the Ethics Committee of
Xinxiang Medical University. Dulbecco’s modified Eagle’s medium
(DMEM) was purchased from Gibco-BRL (Grand Island, NY, USA). Fetal
bovine serum (FBS) and a cell counting kit-8 (CCK-8) were purchased
from Hangzhou Sijiqing Bioengineering Material Co., Ltd. (Hangzhou,
China). Enhanced green fluorescent protein (EGFP)-adenovirus
capsids with and without CyclinA2 cDNA were obtained from Shanghai
Genechem Co., Ltd. (Shanghai, China). Rabbit anti-rat CyclinA2 and
mouse anti-rat β-actin primary antibodies and horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Total
protein extraction and bicinchoninic acid (BCA) protein analysis
kits were purchased from Pierce (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Polyvinylidene fluoride (PVDF) membranes,
trypsin, 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid,
tetramethylethylenediamine and EDTA were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Centrifugal filter units were
purchased from Millipore Corporation (Billerica, MA, USA).
Cell culture and treatments
Cardiomyocytes were isolated from neonatal rat
hearts as previously described and cultured in the DMEM
supplemented with 20% FBS (12).
The cells were randomly separated into six groups: Control,
hypoxia, EGFP-Adv, EGFP-Ccna2, EGFP-Adv + hypoxia, and EGFP-Ccna2 +
hypoxia. The cells in the control group were cultured in a general
cell incubator; those in the EGFP-Adv group were transfected with
EGFP-adenovirus capsids for 18 h, and then placed in a cell
incubator for an additional 12 h; those in the EGFP-Ccna2 group
were transfected with EGFP-adenovirus capsids with CyclinA2 cDNA
for 18 h, and then placed in a cell incubator for an additional 12
h; those in the EGFP-Adv + hypoxia group were transfected with
EGFP-adenovirus capsids for 18 h, and then placed in a hypoxia
chamber for an additional 12 h; and those in the EGFP-Ccna2 +
hypoxia group were transfected with EGFP-adenovirus capsids with
CyclinA2 cDNA for 18 h, and then placed in a hypoxia chamber for an
additional 12 h.
Adenovirus transfection
Cardiomyocytes were plated into 24- and six-well
plates and cultured with complete growth medium for 48 h. The
complete medium was then replaced with fresh DMEM without serum and
antibiotics, and the cells were cultured for an additional 12 h.
The cells were subsequently transfected with EGFP-adenovirus
capsids [1×109 plaque-forming units (PFU)] or
EGFP-adenovirus capsids with CyclinA2 cDNA (1×109 PFU)
for 18 h. GFP fluorescence indicated the transfection
efficiency.
Exposure to hypoxia
The cardiomyocytes in the hypoxia, EGFP-Adv +
hypoxia and EGFP-Ccna2 + hypoxia groups were placed into a special
hypoxia chamber. The chamber was tightly closed and the air was
fully replaced with N2 three times. The chamber was then
placed in a 37°C incubator for 12 h.
Immunochemical staining
Following the appropriate treatment, the
cardiomyocytes grown on the coverslips were fixed with buffered
paraformaldehyde for 15 min, treated with 0.1% Triton X-100 in
phosphate-buffered saline (PBS) for 10 min, and then incubated with
2% H2O2 for 1 h at room temperature.
Following incubation, the cells were further incubated with 10%
goat serum/1% bovine serum albumin (BSA) in PBS at room temperature
for 30 min, and then incubated with CyclinA2 primary antibody
(1:200 in 1% BSA) at 4°C overnight. Subsequent to washing with PBS,
the cells were incubated with HRP-conjugated secondary antibody
(1:500 in 1% BSA) at room temperature for 1 h. The cells were then
incubated with 3,3′-diaminobenzidine substrate at room temperature
for 5 min. The images were viewed and captured using a BH-2 Olympus
microscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
Proteins were extracted from the treated cells using
a total protein extraction kit and the protein concentrations were
measured using a BCA protein analysis kit. Proteins (20 μg/sample)
were subsequently separated by SDS-PAGE, and then transferred to
the PVDF membranes. The membranes were incubated with 5% non-fat
milk in Tris buffered saline with Tween-20 (TBST) for 1 h, and then
incubated with CyclinA2 or β-actin primary antibodies (1:1,000) at
4°C overnight. The membranes were then incubated with
HRP-conjugated second antibody (1:10,000) for 1 h at room
temperature. The immunoreactive bands were visualized by enhanced
chemiluminescence.
Cell viability assay
Following an 18-h transfection, cardiomyocytes
(5×103/well) were plated in 96-well plates. In parallel,
another portion of cells (non-transfected) were directly plated in
the 96-well plates, serving as samples in the control and hypoxia
groups. The cells in the hypoxia, EGFP-Adv + hypoxia and EGFP-Ccna2
+ hypoxia groups were kept in a hypoxia chamber for 12 h at 37°C,
and the cells in the control, EGFP-Adv, EGFP-Ccna2 groups were
cultured in a general cell culture incubator for 12 h. Cell
viability was then measured using a CCK-8 in accordance with the
manufacturer’s instructions (cat. no. C0037; Hangzhou Sijiqing
Bioengineering Material Co., Ltd.). The absorbance was read using a
plate reader at 540 nm.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard error. Univariate comparisons of means were
evaluated using one-way analysis of variance with Tukey’s post hoc
adjustment for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
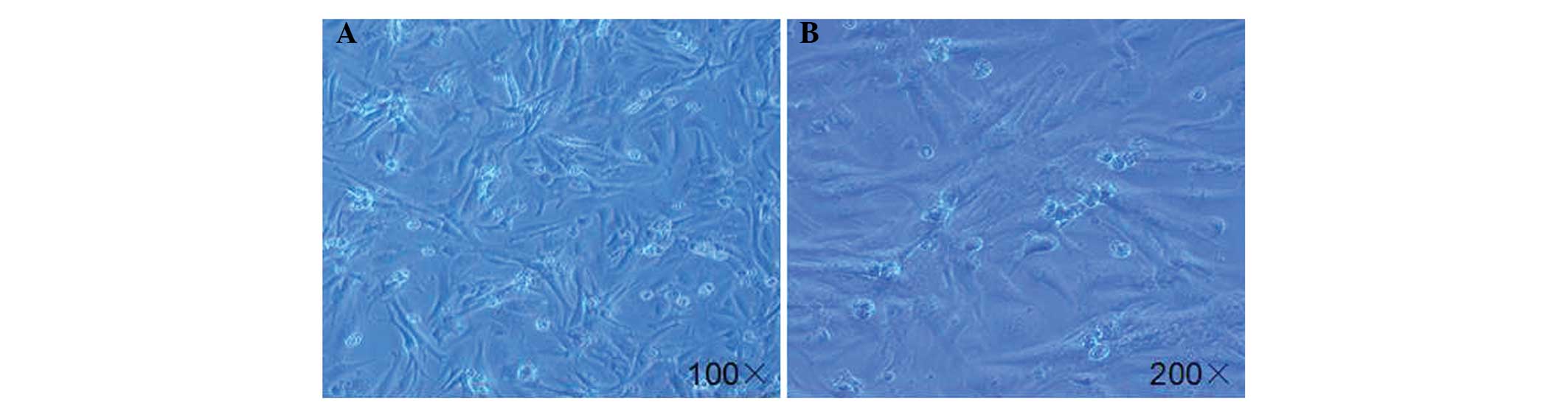
Morphology of primary cardiomyocytes
As shown in Fig. 1,
the primary cardiomyocytes from the neonatal rat hearts reached 80%
confluence subsequent to plating for five days. The majority of the
cells (>90%) were rod-shaped myocytes, while a few cells
(<10%) were round-shaped non-myocytes. These data are consistent
with the results from a previous study (13).
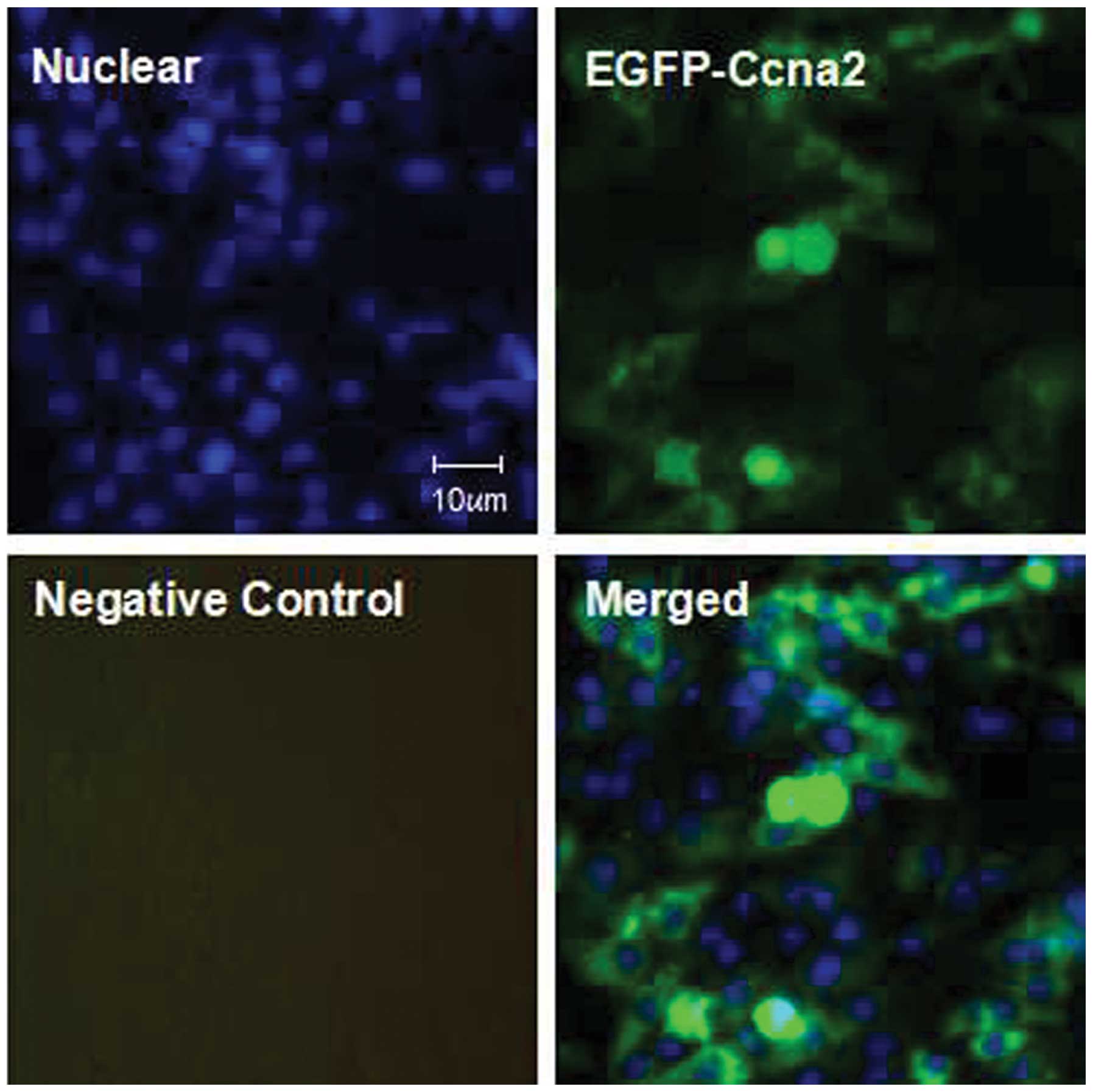
Efficiency of adenovirus
transfection
In this study, the transfection efficiency was
indicated by the positive rates of GFP fluorescence (green). As
shown in Fig. 2, following the
transfection of the EGFP-adenovirus capsids with CyclinA2 cDNA for
18 h, >80% of the cardiomyocytes were positive for green
fluorescence (GFP), which suggested that the CyclinA2 gene was also
overexpressed in the majority of the cells following the
transfection.
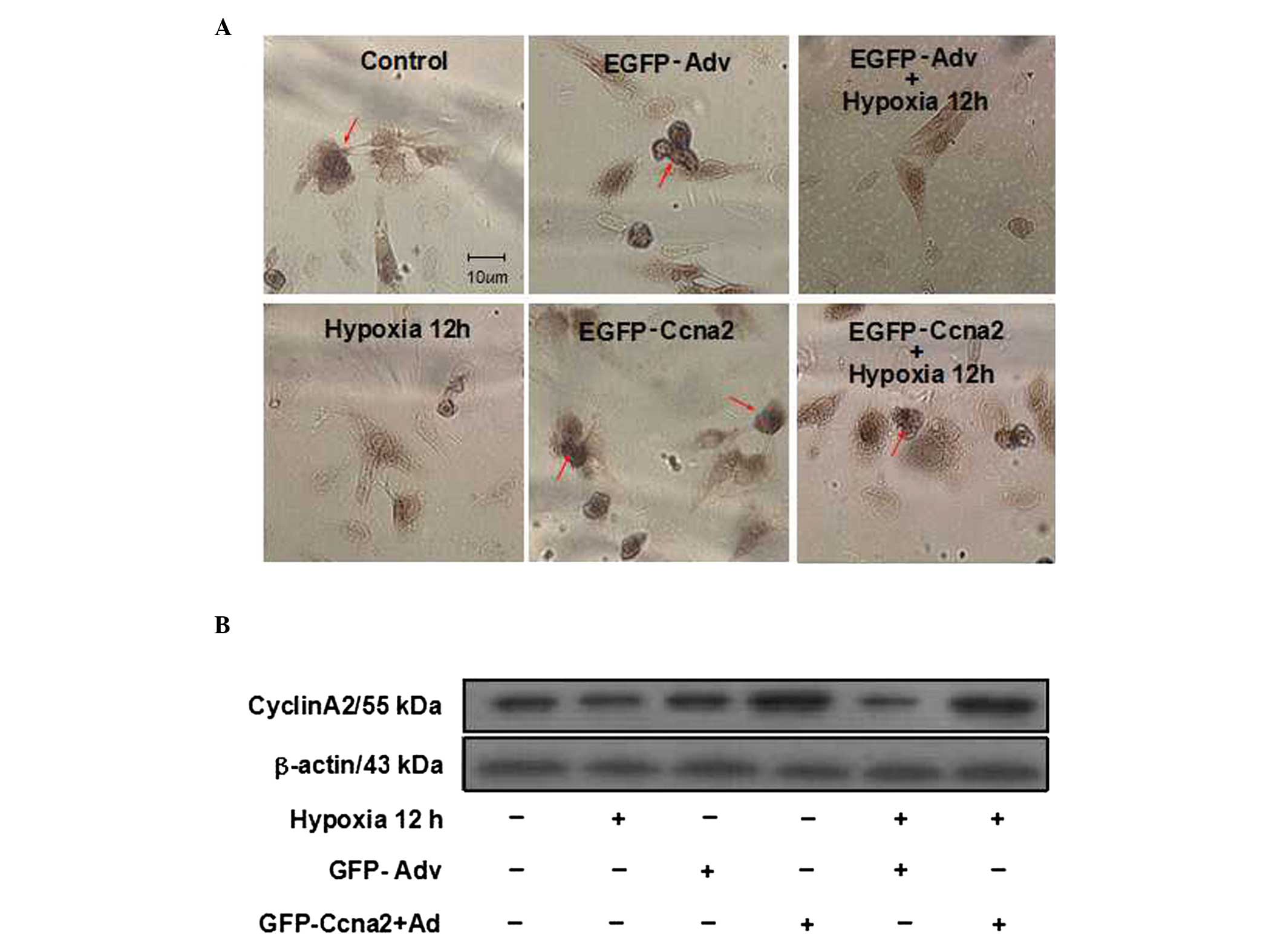
Expression levels of CyclinA2 following
treatments
In this study, protein expression of CyclinA2
following the different treatments was measured by immumochemical
staining and western blot analysis. Consistent with a previous
study (14), the results showed
that CyclinA2 was mainly distributed in the nucleus (Fig. 3A). Immunochemical staining and
western blot analysis showed that CyclinA2 expression was markedly
downregulated in the hypoxia group as compared with that in the
control group (Fig. 3A and B).
Compared with the transfection of EGFP-adenovirus capsids (the
EGFP-Adv group), CyclinA2 expression was markedly increased
following the transfection of EGFP-adenovirus capsids with CyclinA2
cDNA (the EGFP-Ccna2 group). Furthermore, CyclinA2 expression in
the EGFP-Ccna2 + hypoxia group was also much higher than that in
the EGFP-Adv + hypoxia group (Fig. 3A
and B).
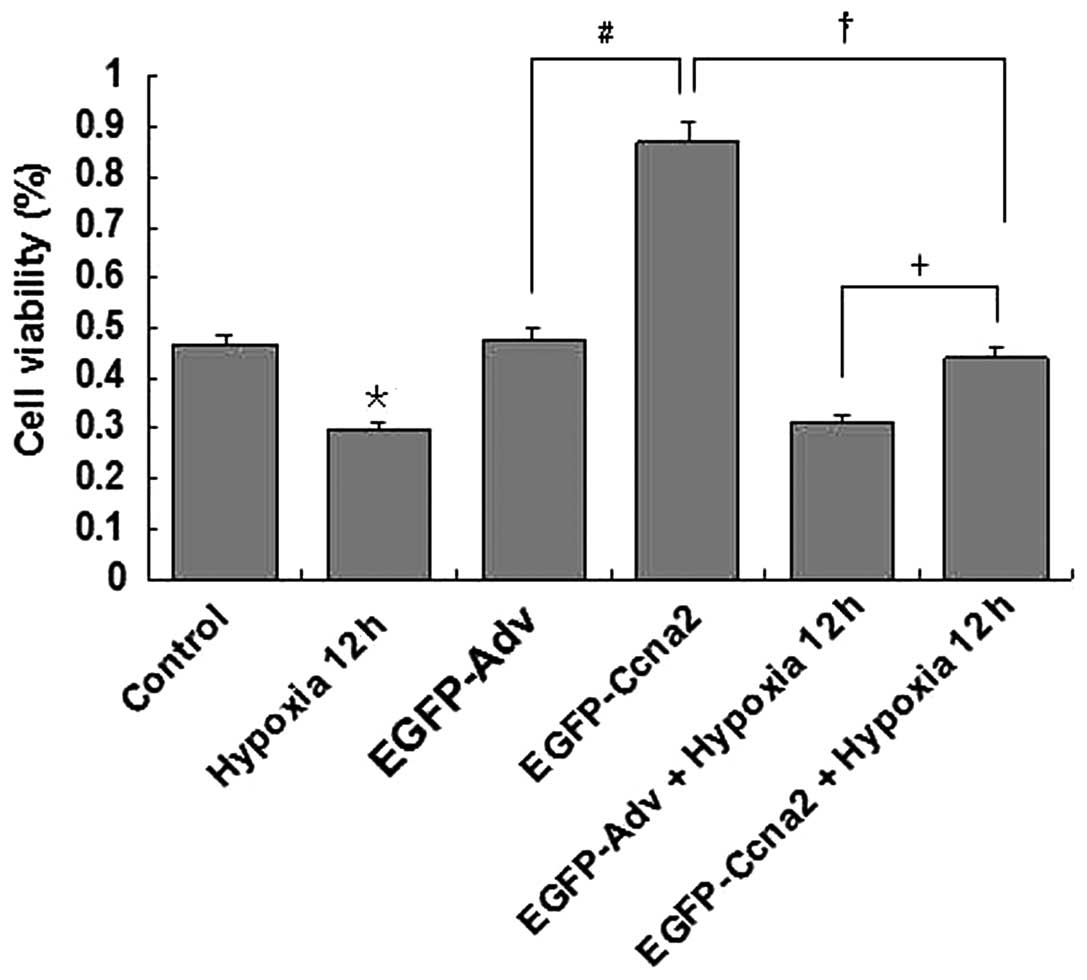
Effect of CyclinA2 overexpression on
cardiomyocyte proliferation
In this study, cardiomyocyte proliferation was
measured using a CCK-8 kit. As shown in Fig. 4, hypoxic treatment (12 h) markedly
decreased the proliferation of cardiomyocytes (P<0.05, hypoxia
group versus control group). Overexpression of CyclinA2
significantly increased cardiomyocyte proliferation (P<0.05,
EGFP-Ccna2 group vs. EGFP-Adv group). Hypoxic treatment also
markedly decreased the proliferation of cardiomyocytes
overexpressing CyclinA2 (P<0.05, EGFP-Ccna2 + hypoxia group
versus EGFP-Ccna2 group); however, CyclinA2 overexpression
partially recovered the hypoxia-impaired proliferation of
cardiomyocytes (P<0.05, EGFP-Ccna2 + hypoxia group versus
EGFP-Adv + hypoxia group).
Discussion
The results of the present study demonstrate that
hypoxia inhibits the proliferation of cardiomyocytes isolated from
neonatal rat hearts, and overexpression of CyclinA2 partially
recovers cardiomyocyte proliferation in hypoxic conditions.
Furthermore, CyclinA2 overexpression increases cardiomyocyte
proliferation in normal culture conditions. These findings suggest
that CyclinA2 plays a key role in the regulation of cardiomyocyte
growth, and its upregulation protects cardiomyocytes from the loss
of their proliferative ability in hypoxic conditions.
HF has become a leading cause of mortality and
morbidity in the world. HF is usually caused by acute myocardial
infarction following heart ischemia. While prolonged ischemia
without reperfusion causes the direct death of cardiomyocytes,
ischemia with reperfusion also leads to myocardial loss via free
radical-induced cardiomyocyte apoptosis (3,15).
It is known that mammalian cardiomyocytes are terminal and
non-proliferative cells. The myocardial injury is generally
repaired by fibroblast proliferation and ECM accumulation in adults
(16). This massive ECM
accumulation leads to cardiac fibrosis, dysfunction and even
failure (17).
CyclinA2 is a highly conserved protein that is
involved in control of the transition of the cell cycle from the
G1/S phase to the G2/M phase, and cell
mitosis (18). In mammals,
cardiomyocytes generally lose their proliferative ability a few
days after birth, which is associated with a sharp downregulation
of CyclinA2 in the hearts following the birth (10). Previous studies have shown that
CyclinA2 is nearly silenced in adult hearts, and delivery of
CyclinA2 to adult animal hearts can partially recover cardiomyocyte
growth (10,11). In the present study, it was
observed that overexpression of CyclinA2 increased the
proliferation of primary neonatal rat cardiomyocytes. Furthermore,
it was shown that CyclinA2 was markedly downregulated in the
neonatal rat cardiomyocytes following exposure to hypoxia for 12 h,
and cardiomyocyte proliferation was also markedly decreased
following the hypoxic treatment. Of note, overexpression of
CyclinA2 was able to partially recover the hypoxia-impaired
cardiomyocyte growth.
In conclusion, this study clearly demonstrates that
overexpression of CyclinA2 in neonatal cardiomyocytes cultured
in vitro can promote their proliferation in physiological
(general culture) and pathophysiological (hypoxic exposure)
conditions. The data obtained from this study will provide further
support for the application of CyclinA2 for the induction of
myocardial regeneration.
Acknowledgements
This study was supported by grants from the
Technology Research and Development Project of Henan Province (no.
082102310016) and from the National Natural Science Foundation of
China (no. 81370428).
References
|
1
|
Chi NC and Karliner JS: Molecular
determinants of responses to myocardial ischemia/reperfusion
injury: focus on hypoxia-inducible and heat shock factors.
Cardiovasc Res. 61:437–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gheorghiade M, Sopko G, De Luca L, et al:
Navigating the crossroads of coronary artery disease and heart
failure. Circulation. 114:1202–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okada H, Lai NC, Kawaraguchi Y, et al:
Integrins protect cardiomyocytes from ischemia/reperfusion injury.
J Clin Invest. 123:4294–4308. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tong W, Xiong F, Li Y and Zhang L: Hypoxia
inhibits cardiomyocyte proliferation in fetal rat hearts via
upregulating TIMP-4. Am J Physiol Regul Integr Comp Physiol.
304:R613–R620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Dai Y, Ding Z, et al: Regulation
of autophagy and apoptosis in response to angiotensin II in HL-1
cardiomyocytes. Biochem Biophys Res Commun. 440:696–700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu CL, Li X, Hu GL, et al: Salubrinal
protects against tunicamycin and hypoxia induced cardiomyocyte
apoptosis via the PERK-eIF2α signaling pathway. J Geriatr Cardiol.
9:258–268. 2012.PubMed/NCBI
|
|
7
|
Zhou YF, Zheng XW, Zhang GH, et al: The
effect of hypoxia-inducible factor 1-alpha on hypoxia-induced
apoptosis in primary neonatal rat ventricular myocytes. Cardiovasc
J Afr. 21:37–41. 2010.PubMed/NCBI
|
|
8
|
Gupta S and Knowlton AA: Cytosolic heart
shock protein 60, hypoxia, and apoptosis. Circulation.
106:2727–2733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paterlini P, De Mitri MS, Martin C, et al:
A Taql polymorphism in the human cyclin A gene. Nucleic Acids Res.
19:25161991. View Article : Google Scholar
|
|
10
|
Chaudhry HW, Dashoush NH, Tang H, et al:
Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic
myocardium. J Biol Chem. 279:35858–35866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo YJ, Panlilio CM, Cheng RK, et al:
Therapeutic delivery of cyclin A2 induces myocardial regeneration
and enhances cardiac function in ischemic heart failure.
Circulation. 114:I206–I213. 2006.PubMed/NCBI
|
|
12
|
Liao XH, Wang N, Liu QX, et al:
Myocardin-related transcription factor-A induces cardiomyocyte
hypertrophy. IUBMB Life. 63:54–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang PM, Haunstetter A, Aoki H, et al:
Morphological and molecular characterization of adult cardiomyocyte
apoptosis during hypoxia and reoxygenation. Circ Res. 87:118–125.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bendris N, Lemmers B, Blanchard JM and
Arsic N: Cyclin A2 mutagenesis analysis: a new insight into CDK
activation and cellular localization requirements. PLoS One.
6:e228792011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao ZQ and Vinten-Johansen J: Myocardial
apoptosis and ischemic preconditioning. Cardiovasc Res. 55:438–455.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang ZF, Wang NP, Harmouche S, et al:
Postconditioning promotes the cardiac repair through balancing
collagen degradation and synthesis after myocardial infarction in
rats. Basic Res Cardiol. 108:3182013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan D, Takawale A, Lee J and Kassiri Z:
Cardiac fibroblasts, fibrosis and extracellular matrix remodeling
in the heart disease. Fibrogenesis Tissue Repair. 5:152012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schulze A, Zerfass K, Spitkovsky D, et al:
Cell cycle regulation of the cyclin A gene promoter is mediated by
a variant E2F site. Proc Natl Acad Sci USA. 92:11264–11268. 1995.
View Article : Google Scholar : PubMed/NCBI
|