Introduction
Gout is a common form of arthritis. Elevated uric
acid levels are observed in the blood of gout patients
(hyperuricemia), resulting from the dysfunction of purine
metabolism, increased uric acid formation and deposition. Other
common gout characteristics are recurrent acute arthritis, tophus,
joint deformity and gouty nephropathy, accompanied by hypertension,
hyperlipemia, renal and cardiovascular diseases (1–3).
The worldwide prevalence of gout has doubled within
the past three decades (4). It was
reported that 6.1 million individuals were suffering from gout in
America in 2010, constituting 1–2% of the adult population
(5). In East China, the incidence
rate was found to be 1.14% (6);
thus, gout is becoming an increasingly important issue for public
health.
Hyperuricemia is the most important risk factor in
the development of gout, and epidemiological studies have
demonstrated that the increasing incidence of gout is closely
associated with diet, life style, medical care and lifetime
extension (1,7–10).
The Health Professionals Follow-up Study and National Health and
Nutrition Examination Survey III revealed that dietary patterns had
a significant influence on gout incidence (11,12).
In particular, a high intake of meat, fish, beer and soft drinks
was identified to be closely associated with increased rates of
gout incidence, while a high intake of coffee, vitamin C and
low-fat food was associated with reduced gout rates. Studies have
suggested that excessive consumption of fructose also contributes
towards an increased gout incidence. Although an unhealthy diet was
the main cause of gout, genetic factors, renal function and
medication, such as diuretics, also played a crucial role in
disease development (3,13,14).
Almost all regular treatments administered to gout
patients show side-effects and limitations. The use of
non-steroidal anti-inflammatory drugs has been found to aggravate
renal failure (15,16), hypertension and cardiovascular
diseases (17) in gout patients,
while glucocorticoid drugs have been shown to aggravate diabetes
and hyperlipemia (18). In spite
of the adverse effects of these drugs, long-term use remains very
common for the treatment of gout. An effective gout therapy to
replace the traditional treatment methods has yet to be developed,
and efficient clinical recommendations for gout treatment are also
unavailable (19).
Ozonated autohemotherapy (O3-AHT) is a
controversial, but successful method of treatment for a number of
diseases. To date, various studies have demonstrated that
O3-AHT exhibits beneficial effects as an adjuvant
therapy in patients with hepatitis B (20), diabetes, degenerative eye disease,
complex regional pain syndrome, ischemic peripheral vascular
disease (21) and osteonecrosis of
the jaw (22,23). However, studies reporting the role
of ozone in the therapy of gout are rare. In a preliminary study,
O3-AHT was demonstrated to alleviate the pain of cancer
patients, and during the treatment, a decline in the blood uric
acid levels was detected. Based on these observations, a pilot
study was designed to investigate the application of
O3-AHT in gout patients.
Materials and methods
Patient characteristics
In total, 10 patients that had been diagnosed with
gout were recruited for the study, including six males and four
females, with ages ranging between 24 and 59 years. Two of the
patients suffered from tophus, and three cases were diagnosed with
acute gout attack. The clinical data were obtained from patient
medical records. The study was approved by the Ethics and Academic
Committees of the Capital Medical University (Beijing, China), and
informed consent was obtained from all participants. The patients
received standard O3-AHT at the Department of Pain
Therapeutic Center of Xuanwu Hospital (Capital Medical
University).
Procedure
Screening criteria were based on the European League
Against Rheumatism diagnosis standard for gout (24). The exclusion criteria comprised the
main conditions known to change the concentration of uric acid in
the blood: i) Deficiency of glucose-6-phosphate dehydrogenase; ii)
severe allergic diseases; iii) use of angiotensin-converting enzyme
inhibitors; iv) hyperthyroidism; v) thrombopenia; vi) severe
cardiovascular diseases; vii) severe renal impairment (creatinine
clearance rate of <30 ml/min); and viii) mental diseases.
Patients who did not provide consent for participation in this
trial were also excluded.
After screening, the 10 patients were treated with
O3-AHT (1–4 weeks) and followed-up for 5–28 weeks. A
commercially available ozone generator (Hyper Medozon Comfort;
Herrmann Apparatebau GmbH, Kleinwallstadt, Germany) and Solar 8000M
patient monitor (GE Healthcare, Pittsburgh, PA, USA) were used.
During O3-AHT, 200-ml samples of the patient’s blood was
mixed with 20 ml sodium citrate (3.8%), and exposed to an
oxygen-ozone mixture with an ozone concentration of 50 μg/ml, for 5
min. Next, the blood was transfused back to the same patient.
O3-AHT was performed three times a week, for a total of
ten times for each patient.
Data collection
Pain visual analog scale (VAS) scores (range, 0–10;
a score of 0 indicated ‘no pain’ and higher scores indicate higher
pain intensity) were reported for all the patients. Routine blood
examinations (analyzing the count of leukocytes, erythrocytes,
hemoglobin, platelets, neutrophils, neutrophil ratio, lymphocytes,
lymphocyte ratio, mononuclear cells, eosinocytes and basophils)
were performed for all the patients, as well as a comprehensive
metabolic panel (analyzing the concentrations of serum creatinine,
uric acid, high-density lipoprotein, low-density lipoprotein, total
cholesterol, albumin, glucose and alanine aminotransferase).
Immunological parameters (IgA, IgG, IgM, complement component 3 and
complement component 4) were also examined. Data were collected
prior to (T0), during (after the fifth session of treatment; 1–4
weeks; T1) and following the full course of treatment (5–28 weeks;
T2).
Statistical analysis
Statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Experimental
data obtained for each patient are presented as the mean ± standard
deviation. The Student’s t-test (for paired samples) was used when
the variances of two normal distributions were assumed to be equal.
Under non-normal distribution or unequal variance conditions, the
Wilcoxon signed-rank test was performed. Due to the small sample
size of this pilot study, P<0.1 was considered to indicate a
statistically significant difference, in order to reduce the
probability of type II error.
Results
General patient characteristics
All 10 patients completed the treatment protocol.
Nine patients underwent 10 courses of O3-AHT, while one
patient received seven courses of O3-AHT.
Four cases were diagnosed with obesity (body mass
index, >28 kg/m2), and two cases were diagnosed with
type II diabetes mellitus and renal dysfunction (creatinine
clearance rate, <80 ml/min). Six patients had food or medicine
allergies, and one case was diagnosed with hyperlipemia. Patients
had not been routinely administered drugs that may have affected
the level of uric acid for at least six weeks prior to and during
the study. The average time from the first diagnosis of gout was
4.5 years (range, 1–7 years; Table
I).
 | Table IPatient demographics and baseline
disease characteristics. |
Table I
Patient demographics and baseline
disease characteristics.
| No. | Age (years) | BMI
(kg/m2) | Creatinine clearance
(ml/min) | Onset and region of
gout | Tophus | Pain VAS score | Combined
diseases |
|---|
| 1 | 43 | 26.57 | 69.91 | 2005, right meta-
tarsophalangeal joint | + | 8 | Noninfectious
periodic fever syndrome |
| 2 | 24 | 34.29 | 159.38 | 2010, right ankle
joint | − | 5 | Allergic to shrimp;
family history of gout |
| 3 | 39 | 24.62 | 98.88 | 2006, right ankle
joint | − | 8–9 | Allergic to seafood
and erythromycin; history of hepatitis A |
| 4 | 43 | 24.21 | 130.28 | 2007, general joint
pain | − | 0 | Isn-Ab positive |
| 5 | 43 | 31.74 | 84.42 | 2008, general joint
pain | − | 5 | History of left knee
surgery; sulfanilamide allergy |
| 6 | 35 | 29.92 | 118.21 | 2009, left ankle
joint | − | 3 | Rheumatoid
factor-negative |
| 7 | 33 | 32.72 | 158.23 | 2009, general joint
pain | − | 5 | HBsAb-, HBeAb- and
HBcAb-positive |
| 8 | 54 | 26.12 | 57.82 | 2006, right toe
joint | + | 9–10 | Type II diabetes;
allergic to cold air |
| 9 | 59 | 23.03 | 82.96 | 2005, left toe
joint | − | 5 | NK cell count of
28% |
| 10 | 40 | 27.64 | 91.02 | 2006, right toe
joint | − | 4–5 | Type II diabetes |
Efficacy on the creatinine clearance rate
and pain VAS scores
In general, a complete clinical response with an
increased creatinine clearance rate and decreased pain VAS scores
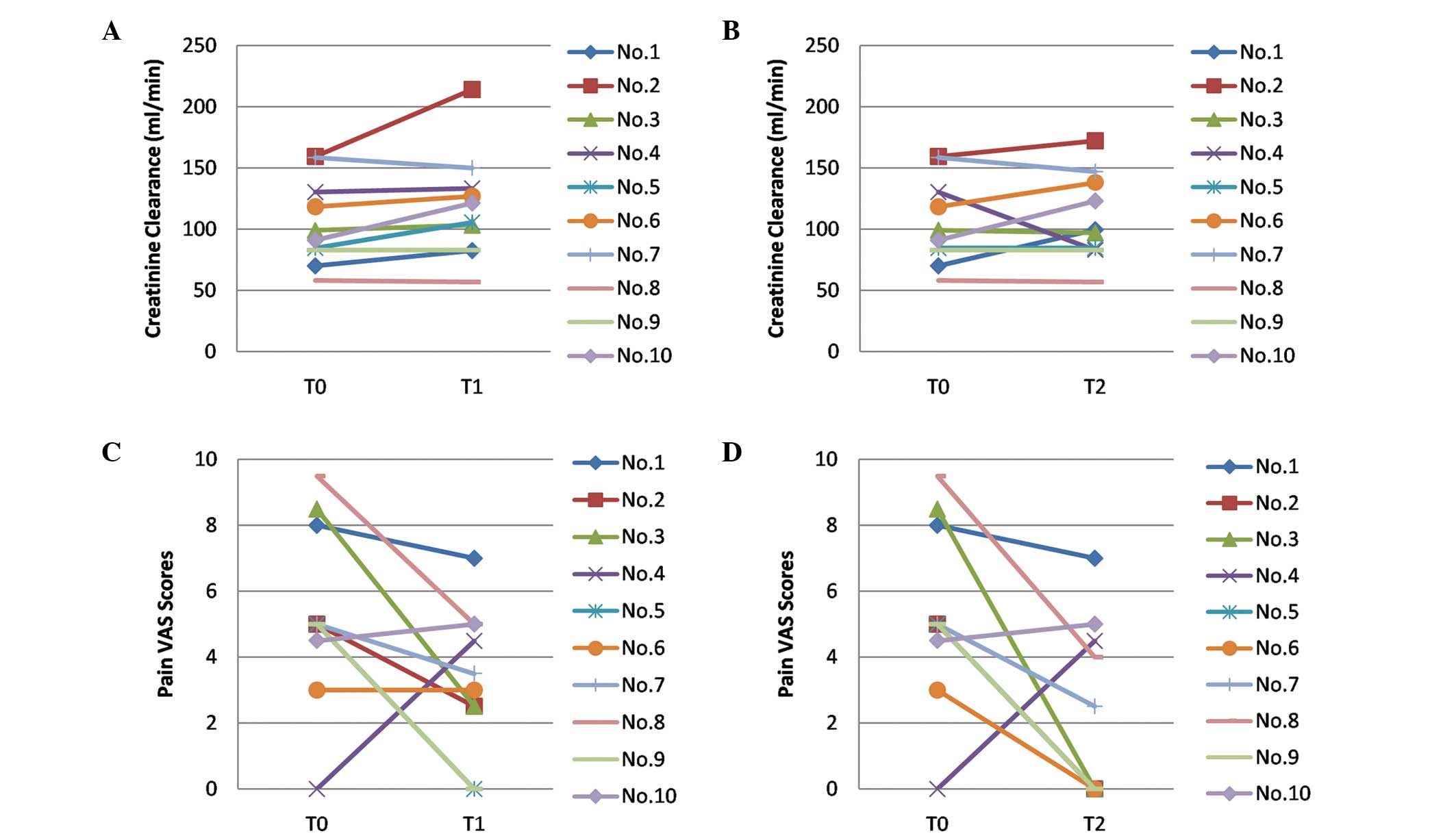
was achieved for all the patients (Table II; Fig. 1).
 | Table IIChanges in the creatinine clearance
rate and pain VAS scores in patients with hyperuricemia and gout
treated with O3-AHT. |
Table II
Changes in the creatinine clearance
rate and pain VAS scores in patients with hyperuricemia and gout
treated with O3-AHT.
| Time point | Creatinine clearance
(ml/min)a | Paired t-test (vs.
T0) | Pain VAS
scoresb | Paired t-test (vs.
T0) |
|---|
| T0 | 105.14±35.33 | - | 5.35±2.78 | - |
| T1 | 121.45±44.52 | t=2.165 | 3.30±2.21 | t=2.004 |
| | P=0.062 | | P=0.076 |
| T2 | 111.15±36.52 | t=1.723 | 2.30±2.66 | t=2.628 |
| | P=0.123 | | P=0.027 |
The creatinine clearance rate, which normally ranges
between 80 and 120 ml/min, was determined to be 105.14±35.33 ml/min
prior to O3-AHT (T0). After the fifth session of
O3-AHT (T1), the creatinine clearance rate increased to
121.45±44.52 ml/min, which was significantly higher compared with
the value at T0 (t=2.165, P=0.062). However, after the course of
O3-AHT (T2), the creatinine clearance rate decreased
slightly to 111.15±36.52 ml/min, and exhibited no statistically
significant difference with the creatinine clearance rate at T0
(t=1.723, P=0.123).
Pain VAS scores are one of the most commonly used
measures of pain intensity. At T0, the patients showed a mean pain
VAS score of 5.35±2.78. The mean pain VAS score decreased to
3.30±2.21 at T1, which was significantly lower than the score at T0
(t=2.004, P=0.076). The mean VAS score further decreased to
2.30±2.66 at T2, and a statistically significant difference was
observed when compared with the pain VAS score at T0 (t=2.628,
P=0.027).
Safety
Patients with hyperuricemia and gout showed good
tolerance to the O3-AHT. No serious adverse reactions,
including a rash, low blood pressure, abnormal liver function and
abdominal pain, or acute gout attacks were observed during the
treatment course. One patient developed mild dizziness and nausea
during the seventh treatment session; however, normality was
regained after 2 h.
Discussion
Ozone is a gas found naturally in the Earth’s
atmosphere, but can be produced from oxygen for medical use. Ozone
exhibits wound-healing and antimicrobial properties, which may
promote tissue repair and regeneration (25). Reactive oxygen species (ROS) and
lipid oxidation products (LOPs) generated from the acute oxidative
stress reaction of water, ozone and serum antioxidants have been
reported to promote red blood cells to provide oxygen to ischemic
tissues, resist viral infections by activating the immune system
and release protective factors, including nitric oxide, carbon
monoxide and platelet-derived growth factor (22,25,26).
O3-AHT is a new complementary therapeutic
technology, during which physicians inject medical grade ozone gas
into blood collected from a patient, and subsequently intravenously
transfuse the blood back to the same patient. O3-AHT has
recently developed into a modern medical approach that is widely
used in the treatment of hepatitis B, diabetes and numerous other
diseases (22). However, the
efficacy and safety of O3-AHT in hyperuricemia and gout
treatment remains unknown.
Low-dose ozone treatment has been universally
accepted as a treatment for lumbar disc herniation, arterial
atherosclerosis, ischemic cerebrovascular disease and a number of
other diseases. Accurate measurements of ozone using a
spectrophotometer have revealed that an ozone concentration of
20–80 μg/ml is not toxic. A preliminary clinical study identified
that O3-AHT using this dose appeared to have an effect
on pain alleviation in cancer patients. In addition, during this
treatment, a decrease in the level of uric acid in the blood was
observed, indicating a therapeutic potential for the application of
O3-AHT in cases of hyperuricemia and gout. To explore
the safety and efficacy of O3-AHT, a phase I clinical
trial was performed in patients with hyperuricemia and gout.
In the present study, the creatinine clearance rate
of patients receiving O3-AHT increased significantly,
reaching values even beyond the reference range. Self-reported pain
VAS scores were also significantly decreased after five sessions of
O3-AHT, and were further decreased by 50% following
completion of the treatment course. These results indicated that
O3-AHT may be a potential complementary medical approach
for patients with hyperuricemia and gout.
Neutrophils and macrophages are the main
inflammatory cells in the pathological process of gout. It has been
reported that monosodium urate causes the death of neutrophils and
the release of lysozyme, and its metabolism may be influenced by
the ROS and LOPs generated from the acute oxidative stress reaction
resulting from O3-AHT (27–29).
However, the exact mechanisms require further research.
However, limitations of the present study also
require addressing. Firstly, the study involved a small group of
patients; therefore, further studies involving more patients are
required in order to confirm the observations. Secondly, a
double-blind randomized controlled trial, which may provide further
evidence, was unable to be performed due to the small study size
population. Finally, in the current study, only the potential
therapeutic role of O3-AHT was addressed; thus, the
exact mechanisms and pathways require further elucidation. However,
as a pilot clinical study, the present study has provided the
basics for further research into the therapeutic potential and
molecular mechanisms of O3-AHT in patients with
hyperuricemia and gout.
In conclusion, 20 μg/ml ozone was identified to be
an effective biological dose for O3-AHT, achieving a
good curative effect and safety in patients with hyperuricemia and
gout. Thus, low-dose O3-AHT may be a potential effective
approach for hyperuricemia and gout patients. Further studies with
a larger sample size, as well as investigations into the underlying
mechanisms of O3-AHT, are required in the future.
References
|
1
|
Wallace KL, Riedel AA, Joseph-Ridge N and
Wortmann R: Increasing prevalence of gout and hyperuricemia over 10
years among older adults in a managed care population. J Rheumatol.
31:1582–1587. 2004.PubMed/NCBI
|
|
2
|
Choi HK and Ford ES: Prevalence of the
metabolic syndrome in individuals with hyperuricemia. Am J Med.
120:442–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kramer HM and Curhan G: The association
between gout and nephrolithiasis: the National Health and Nutrition
Examination on Survey III, 1988–1994. Am J Kidney Dis. 40:37–42.
2002.PubMed/NCBI
|
|
4
|
Smith E, Hoy D, Cross M, et al: The global
burden of gout: estimates from the Global Burden of Disease 2010
study. Ann Rheum Dis. 73:1470–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith EU, Díaz-Torné C, Perez-Ruiz F and
March LM: Epidemiology of gout: an update. Best Pract Res Clin
Rheumatol. 24:811–827. 2010. View Article : Google Scholar
|
|
6
|
Miao Z, Li C, Chen Y, et al: Dietary and
lifestyle changes associated with high prevalence of hyperuricemia
and gout in the Shandong coastal cities of Eastern China. J
Rheumatol. 35:1859–1864. 2008.PubMed/NCBI
|
|
7
|
Doherty M: New insights into the
epidemiology of gout. Rheumatology (Oxford). 48(Suppl 2): ii2–ii8.
2009. View Article : Google Scholar
|
|
8
|
Roddy E, Zhang W and Doherty M: The
changing epidemiology of gout. Nat Clin Pract Rheumatol. 3:443–449.
2007. View Article : Google Scholar
|
|
9
|
Lawrence RC, Helmick CG, Arnett FC, et al:
Estimates of the prevalence of arthritis and selected
musculoskeletal disorders in the United States. Arthritis Rheum.
41:778–799. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helmick CG, Felson DT, Lawrence RC, et al:
Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States. Part I. Arthritis Rheum. 58:15–25.
2008. View Article : Google Scholar
|
|
11
|
Kim SY, De Vera MA and Choi HK: Gout and
mortality. Clin Exp Rheumatol. 26(5 Suppl 51): S115–S119.
2008.PubMed/NCBI
|
|
12
|
Luk AJ and Simkin PA: Epidemiology of
hyperuricemia and gout. Am J Manag Care. 11(15 Suppl): S435–S442.
2005.PubMed/NCBI
|
|
13
|
Arromdee E, Michet CJ, Crowson CS,
O’Fallon WM and Gabriel SE: Epidemiology of gout: is the incidence
rising? J Rheumatol. 29:2403–2406. 2002.PubMed/NCBI
|
|
14
|
Lawrence RC, Felson DT, Helmick CG, et al:
Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States. Part II. Arthritis Rheum.
58:26–35. 2008. View Article : Google Scholar
|
|
15
|
Hoskison KT and Wortmann RL: Management of
gout in older adults: barriers to optimal control. Drug Aging.
24:21–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng HF and Harris RC: Renal effects of
non-steroidal anti-inflammatory drugs and selective
cyclooxygenase-2 inhibitors. Curr Pharm Des. 11:1795–1804. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White WB: Cardiovascular risk,
hypertension, and NSAIDs. Curr Rheumatol Rep. 9:36–43. 2007.
View Article : Google Scholar
|
|
18
|
Soleimani M: Dietary fructose, salt
absorption and hypertension in metabolic syndrome: towards a new
paradigm. Acta Physiol (Oxf). 201:55–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keenan RT, O’Brien WR, Lee KH, et al:
Prevalence of contraindications and prescription of pharmacologic
therapies for gout. Am J Med. 124:155–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chernyshev AL, Filimonov RM, Karasev AV,
et al: Combined treatment including ozonotherapy of patients with
viral hepatitis. Vopr Kurortol Fizioter Lech Fiz Kult. 3:19–22.
2008.(In Russian).
|
|
21
|
De Monte A, van der Zee H and Bocci V:
Major ozonated autohemotherapy in chronic limb ischemia with
ulcerations. J Altern Complement Med. 11:363–367. 2005.PubMed/NCBI
|
|
22
|
Bocci V, Borrelli E, Travagli V and
Zanardi I: The ozone paradox: ozone is a strong oxidant as well as
a medical drug. Med Res Rev. 29:646–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ripamonti CI, Cislaghi E, Mariani L and
Maniezzo M: Efficacy and safety of medical ozone (O(3)) delivered
in oil suspension applications for the treatment of osteonecrosis
of the jaw in patients with bone metastases treated with
bisphosphonates: Preliminary results of a phase I-II study. Oral
Oncol. 47:185–190. 2011. View Article : Google Scholar
|
|
24
|
Zhang W, Doherty M, Pascual E, Bardin T,
et al: EULAR evidence based recommendations for gout. Part I:
Diagnosis Report of a task force of the Standing Committee for
International Clinical Studies Including Therapeutics (ESCISIT).
Ann Rheum Dis. 65:1301–1311. 2006. View Article : Google Scholar
|
|
25
|
Bocci V: Ozone as Janus: this
controversial gas can be either toxic or medically useful.
Mediators Inflamm. 13:3–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bocci V, Valacchi G, Corradeschi F, et al:
Studies on the biological effects of ozone: 7. Generation of
reactive oxygen species (ROS) after exposure of human blood to
ozone. J Biol Regul Homeost Agents. 12:67–75. 1998.PubMed/NCBI
|
|
27
|
Hoffstein S and Weissmann G: Mechanisms of
lysosomal enzyme release from leukocytes. IV Interaction of
monosodium urate crystals with dogfish and human leukocytes.
Arthritis Rheum. 18:153–165. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kozin F, Ginsberg MH and Skosey JL:
Polymorphonuclear leukocyte responses to monosodium urate crystals:
modification by adsorbed serum proteins. J Rheumatol. 6:519–526.
1979.PubMed/NCBI
|
|
29
|
Nuki G: Colchicine: its mechanism of
action and efficacy in crystal-induced inflammation. Curr Rheumatol
Rep. 10:218–227. 2008. View Article : Google Scholar : PubMed/NCBI
|