Introduction
The incidence of diabetes is increasing, with the
disease affecting ~347 million adults worldwide (1), which is projected to increase to 552
million by 2030 (2). Type 2
diabetes (T2D) is the most common type of diabetes. Increasing
evidence indicates that inflammation is involved in the
pathogenesis of T2D, with levels of C-reactive protein (CRP), a
biomarker of inflammation, increased in patients that are obese and
diabetic (3–5). Levels of proinflammatory cytokines,
including interleukin (IL)-1β, IL-6, tumor necrosis factor-α
(TNF-α) and plasminogen activator inhibitor (PAI-1), are also
increased in patients who are obese and diabetic (3–7).
Prospective studies have demonstrated that higher plasma levels of
CRP, fibrinogen, IL-6 and PAI may be used to predict the risk of
developing T2D (3,6–9). In
addition, alterations in the leukocyte count are involved in T2D.
Investigations by Nakanishi et al (10) indicated that a higher white blood
cell count may predict the development of impaired fasting glucose
and T2D. Additionally, an impaired T-cell balance has been observed
in patients with T2D, characterized by CD4+CD28 null
T-cell expansion and
CD4+CD25+Foxp3+ regulatory T-cell
reduction (11). Furthermore,
results from clinical trials have shown that the administration of
anti-inflammatory agents, such as IL-1 antagonists, in patients
with T2D significantly lowered blood glucose levels, as well as
CRP, IL-6 and other inflammatory biomarkers (12,13).
TNF-α and IL-6 have been shown to impair insulin
signaling pathways (14), blunting
the response of the liver, adipose tissue and skeletal muscle to
insulin. Increasing evidence has demonstrated that inflammation is
also involved in islet β-cell dysfunction (15). Therefore, the hypothesis that T2D
is a chronic low-grade inflammatory disease has arisen (4,5).
Nuclear receptor subfamily 4 group A member 1
(NR4A1), also known as Nur77, nerve growth factor I-B (NGFI-B) and
TR3, is encoded by the Nr4a1 gene and is a member of the
NR4A nuclear receptor superfamily (16,17).
The receptor also belongs to the orphan nuclear family (16,17).
The domain structure of NR4A1 is similar to other nuclear
receptors, containing an N-terminal activating function-1 domain, a
zinc finger DNA-binding domain and a C-terminal ligand-binding
domain (16,17). NR4A1 is reported to have multiple
biological functions, regulating cell proliferation,
differentiation, apoptosis, development, metabolism and immunity
(16,18–20).
The receptor exerts these physiological functions through
expression regulation, post-translational modification and
subcellular localization (21).
Previous studies have indicated that NR4A1 exerts
effects on inflammatory processes (17,22–26).
Macrophages stimulated by oxidized low-density lipoprotein (oxLDL),
lipopolysaccharide (LPS) and TNF-α result in a higher transcription
of Nr4a1 (23,24,26).
Overexpression of Nr4a1 in RAW macrophages induces several
inflammatory cytokines (23),
including IκB kinase (IKK)i/IKKɛ. Furthermore, previous studies
have indicated that NR4A1 is expressed by macrophages in human
atherosclerotic lesions (24,25).
You et al (22)
demonstrated that NR4A1 suppressed proinflammatory activation in
endothelial cells (ECs).
However, the association between NR4A1 expression
and the chronic low-grade inflammatory state in patients with T2D
remains unknown. Therefore, the aim of the present study was to
investigate the expression levels of NR4A1 in human peripheral
blood mononuclear cells (PBMCs), which are partly derived from the
circulatory system and can be regarded as an insight into
inflammation. In addition, the association between NR4A1 levels and
inflammation-related parameters was analyzed, as well as the
alteration in NR4A1 expression in palmitic acid (PA)-treated
PBMCs.
Materials and methods
Patients with T2D and healthy
subjects
The study was performed in accordance with and with
approval from the Ethics Committee of Zhongnan Hospital of Wuhan
University (approval no. 2012012; Wuhan, China). According to
medical history and clinical examination, 64 participants,
including 34 healthy subjects and 30 patients with newly diagnosed
T2D, were recruited from the Zhongnan Hospital of Wuhan University.
Written informed consent was obtained from the patient. All the
participants underwent a complete physical examination and
laboratory tests.
Exclusion criteria were as follows: i) Aged <20
or >65 years; ii) body mass index (BMI) of <15 or >32
kg/m2; iii) smoked; iv) evidence of infectious diseases;
v) prior history of cancer and/or other chronic diseases; vi)
treatment with anti-inflammatory drugs; vii) pregnant or
breast-feeding females; viii) diagnosed with type 1 diabetes; ix)
active liver diseases and/or significant liver dysfunction; x)
renal disease; xi) autoimmune disorder; or xii) experience of
severe complications, including diabetic ketoacidosis and
hyperglycemic hyperosmolar status.
Biochemical measurements
Whole blood samples were collected in K3 EDTA
Vacutainer tubes after ≥8 h fasting. The samples were centrifuged
for 5 min at 400 × g at room temperature (20 ± 2°C) and the plasma
was collected for further assessment of biochemical parameters,
including fasting blood glucose (FBG), fasting plasma insulin
(FIN), total cholesterol (TC), triglycerides (TG), high-density
lipoprotein cholesterol (HDL-C) and low-density lipoprotein
cholesterol (LDL-C). The concentration of free fatty acids (FFAs)
was determined by improved copper reagent colorimetry (Applygen
Technologies Inc., Beijing, China), according to the manufacturer’s
instructions. The insulin resistance (IR) was evaluated with the
homeostasis model assessment (HOMA) as follows: HOMA-IR = FIN
(μU/ml) × FBG (mM)/22.5 (27).
Collection of PBMCs
PBMCs were isolated from the heparinized peripheral
blood of 64 participants over Ficoll-Hypaque density gradient
centrifugation (Pharmacia Biotech, Piscataway, NJ, USA), following
the manufacturer’s instructions. The resultant PBMCs were used for
quantitative polymerase chain reaction (qPCR) analysis.
Culture and treatment of PBMCs
PBMCs, isolated from the healthy subjects, were
cultivated in RPMI 1640 complete culture medium (Gibco Life
Technologies, Grand Island, NY, USA), supplemented with 10% fetal
bovine serum (Gibco Life Technologies), 1% penicillin-streptomycin
(Gibco Life Technologies) and 5.6 mM glucose at 37.0°C in a
humidified atmosphere (5% CO2, 95% air). Following
overnight culture in six-well plates, the PBMCs were incubated with
and without 250 μM PA (Sigma-Aldrich, St. Louis, MO, USA) for 2 h.
The cells were harvested for RNA and protein expression analysis,
and the supernatant was collected for TNF-α and IL-6 measurement by
ELISA.
RNA isolation and reverse transcription
qPCR
Total RNA from the PBMCs was extracted with TRIzol
reagent (Takara Bio, Inc., Shiga, Japan) and cDNA was generated by
Moloney Murine Leukemia Virus reverse transcriptase (Promega
Corporation, Madison, WI, USA), according to the manufacturer’s
instructions. qPCR was performed using a SYBR Green PCR mix kit
(Takara Bio, Inc.), following the manufacturer’s instructions. The
primers used were as follows: NR4A1, 5′-CCAGCACTGCCAAACTGGACTA-3′
(forward) and 5′-CTCAGCAAAGCCAGGGATCTTC-3′ (reverse) (28); and β-actin, TCTACAATGAGCTGCGTGTG
(forward) and GGTGAGGATCTTCATGAGGT (reverse). Following an initial
denaturation step at 95°C for 30 sec, 40 PCR cycles consisting of 5
sec at 95°C, 30 sec at 58°C and 30 sec at 72°C were conducted. The
qPCR data were normalized against the levels of β-actin mRNA and
analyzed using ABI StepOne™ Data Analysis software (Applied
Biosystems, Foster City, CA, USA).
Analysis of NR4A1 protein using Western
blotting
Total protein from the PBMCs and cultured PBMCs of
the participants was extracted with radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China),
supplemented with 1% phosphatase and protease inhibitor cocktails
(Thermo Fisher Scientific, Waltham, MA, USA). The protein
concentration was measured using a bicinchoninic acid kit (Thermo
Fisher Scientific), according to the manufacturer’s instructions.
The lysates with equal amounts of protein were electrophoresed by
SDS-PAGE, and subsequent transblotting and immunodetection were
conducted, as described previously (29). Primary antibodies against NR4A1
(Bioworld Technology, Inc., St. Louis Park, MN, USA) and
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were used. The intensity
of the bands was determined by Image J 2× software (National
Institutes of Health, Bethesda, MD, USA) and normalized with
GAPDH.
Measurement of inflammatory
cytokines
The concentrations of TNF-α and IL-6 in the plasma
or cell supernatant were measured with human TNF-α and IL-6 ELISA
kits (Boster Biological Technology, Ltd., Wuhan, China.), according
to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS 20
software (IBM, Armonk, NY, USA), and the data are expressed as the
mean ± standard deviation. The χ2 test and Student’s
t-test of independent samples were used to compare the differences
between two groups. Pearson’s correlation analysis was used to
identify linear correlations between variables. P<0.05 was
considered to indicate a statistically significant difference
(*P<0.05 and **P<0.01). The graphs were
performed using Graphpad prism 6.0 (GraphPad Software, San Diego,
CA, USA) and Sigmaplot (Systat Software, Inc. San Jose, CA, USA)
(*P<0.05 and **P<0.01).
Results
Clinical parameters of the subjects
A summary of the clinical parameters of the subjects
enrolled in the study is shown in Table I. No statistically significant
differences were observed between the T2D and control groups with
regard to age, gender, blood pressure or liver or kidney function.
As expected, statistically significant differences were identified
in the BMI, FBG, FIN, TG, TC, LDL-C and HDL-C in patients with T2D
compared with the control group. A statistically significant
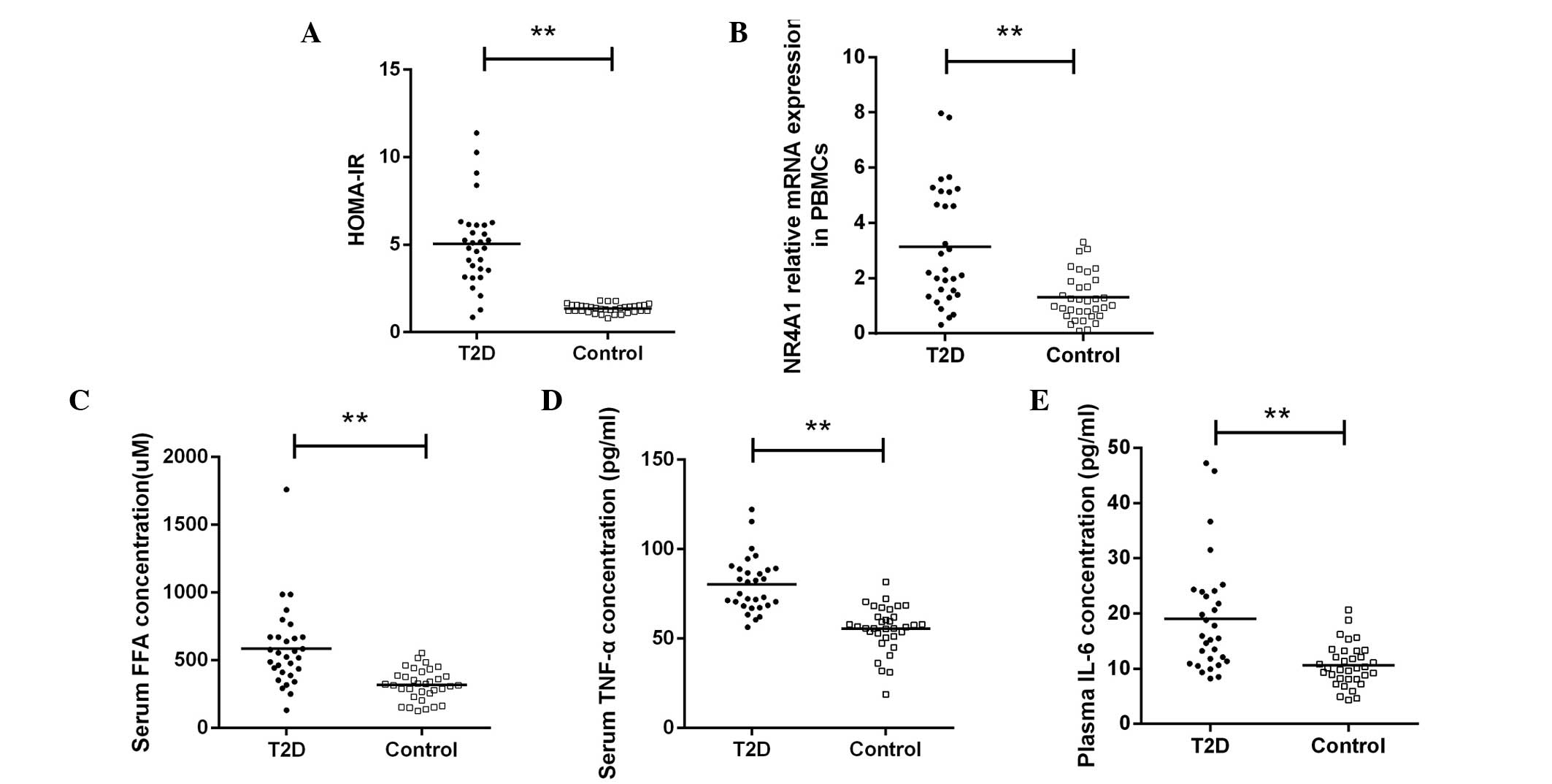
increase was also observed in the HOMA-IR for T2D patients
(5.06±2.41 vs. 1.35±0.25, P<0.01; Fig. 1A). Although an increase in the
leukocyte count was observed in T2D patients in previous trials, as
aforementioned (10,11), in the current study, the peripheral
total leukocyte and differential counts did not exhibit a
statistically significant difference between the two groups.
 | Table IClinical parameters of the
subjects. |
Table I
Clinical parameters of the
subjects.
| Variables | Type 2 diabetes
group | Control group | P-value |
|---|
| Age (years) | 49.00±7.68 | 44.94±10.94 | 0.088 |
| Gender, male/female
(n) | 21/13 | 17/13 | 0.681 |
| BMI
(kg/m2) | 26.20±2.67 | 23.66±3.11 | <0.01 |
| Systolic pressure
(mmHg) | 128.60±11.08 | 122.94±12.17 | 0.057 |
| Alanine
aminotransferase (U/l) | 33.47±15.06 | 28.06±11.26 | 0.106 |
| Blood urea nitrogen
(mM) | 5.30±1.45 | 4.81±1.17 | 0.141 |
| Serum creatinine
(μM) | 71.38±13.73 | 77.74±15.11 | 0.084 |
| FBG (mM) | 8.50±2.50 | 5.26±0.29 | <0.01 |
| FIN (μU/ml) | 13.69±5.73 | 5.81±1.05 | <0.01 |
| TG (mM) | 1.91±0.81 | 1.44±0.97 | <0.05 |
| TC (mM) | 5.41±1.08 | 4.81±0.81 | <0.05 |
| HDL-C (mM) | 1.06±0.16 | 1.21±0.17 | <0.01 |
| LDL-C (mM) | 3.65±0.92 | 3.10±0.76 | <0.05 |
| White blood cell
count (×109) | 6.80±1.82 | 6.38±1.57 | 0.326 |
| Neutrophil
(%) | 56.20±8.41 | 56.95±8.41 | 0.724 |
| Lymphocyte
(%) | 33.48±8.57 | 32.47±8.10 | 0.630 |
| Monocyte (%) | 7.64±1.87 | 7.25±1.50 | 0.358 |
Transcriptional increase in NR4A1
expression in PBMCs from patients with T2D
Compared with the controls, the relative mRNA
expression levels of NR4A1 in the PBMCs from the patients with T2D
increased (3.13±2.14 vs. 1.30±0.85, P<0.01; Fig. 1B).
Levels of FFAs, TNF-α and IL-6 increase
in the plasma of T2D patients
As expected, statistically significant differences
were observed in the levels of FFAs when comparing the patients
with T2D with the control group (586.58±301.93 vs. 319.07±113.41
μM, P<0.01; Fig. 1C). In
addition, the plasma concentrations of TNF-α (80.12±15.51 vs.
53.62±11.14 pg/ml, P<0.01; Fig.
1D) and IL-6 (19.08±10.19 vs. 9.82±3.05 pg/ml,
P<0.01; Fig. 1E) were increased
in the patients with T2D, as compared with the control
subjects.
TNF-α and IL-6 levels increase in the
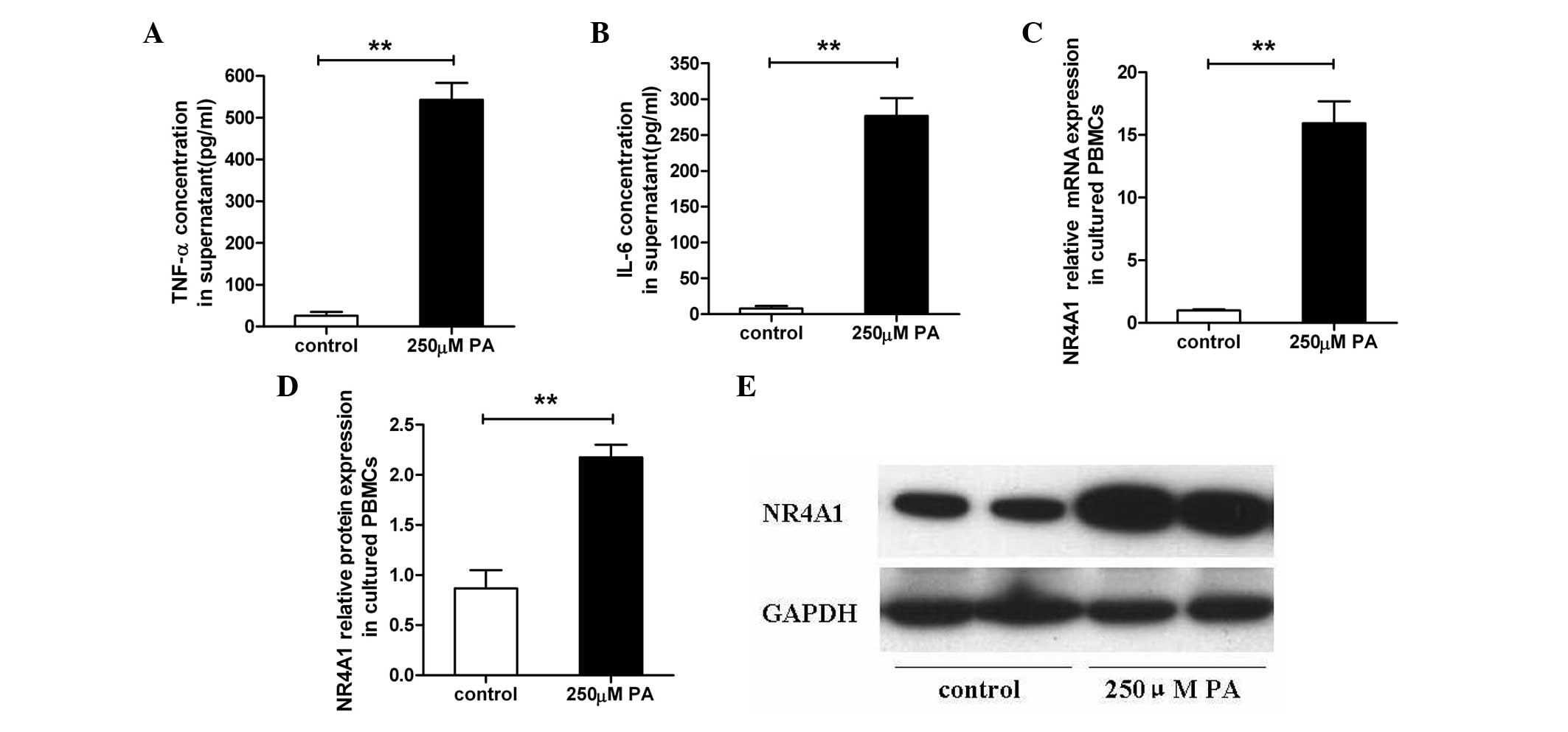
supernatant of PA-stimulated PBMCs from healthy participants
In the PBMCs from the healthy subjects, the
supernatants were collected following treatment with or without 250
μM PA for 2 h. Subsequently, the protein concentrations of TNF-α
(543.31±40.08 vs. 26.09±9.35 pg/ml, P<0.01; Fig. 2A) and IL-6 (276.82±24.88 vs.
7.80±3.45 pg/ml, P<0.01; Fig.
2B) were analyzed and were found to be significantly induced by
250 μM PA stimulation.
PA induces NR4A1 expression in cultured
PBMCs from healthy participants
In the cultured PBMCs from the healthy subjects,
NR4A1 mRNA relative expression increased in the 250 μM PA-induced
PBMCs when compared with the controls (15.92±1.75 vs. 1.00±0.09,
P<0.01, Fig. 2C), as well as
the NR4A1 relative protein expression level (2.18±0.12 vs.
0.87±0.18, P<0.01, Fig. 2D and
E), as determined by qPCR and western blot analysis.
Correlation between NR4A1 mRNA expression
and other parameters
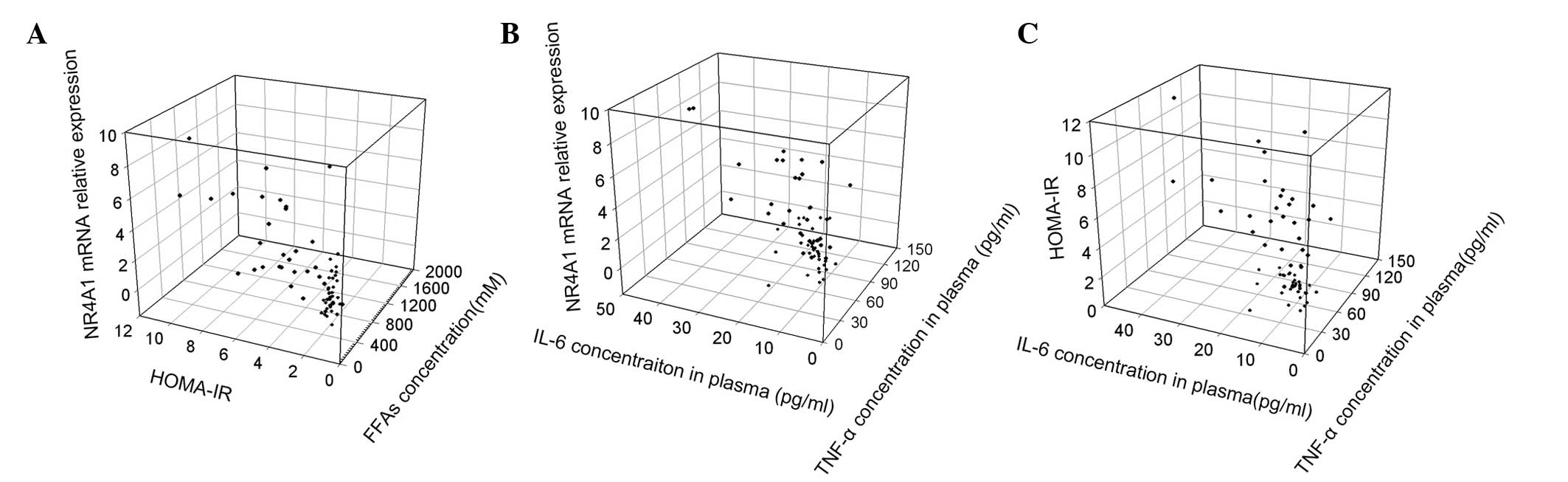
Using Pearson’s correlation analysis, several
positive correlations were identified among the parameters. The
NR4A1 mRNA relative expression level was found to exhibit a
positive correlation with several diabetes-related parameters,
including the HOMA-IR (r=0.761, P<0.01; Fig. 3A), FFAs (r=0.560, P<0.01;
Fig. 3A), TNF-α (r=0.697,
P<0.01; Fig. 3B) and IL-6
levels (r=0.796 P<0.01, Fig.
3B). The HOMA-IR was also shown to positively correlate with
the level of FFAs (r=0.513, P<0.01; Fig. 3A), TNF-α (r=0.728, P<0.01;
Fig. 3C) and IL-6 (r=0.590,
P<0.01; Fig. 3C). In addition,
statistically significant correlations were observed between the
levels of FFAs and the protein expression levels of TNF-α (r=0.475,
P<0.001) and IL-6 (r=0.402, P=0.001). However, there were no
evident correlations between the total leukocyte/differential count
and other variables, including NR4A1 mRNA expression, HOMA-IR,
FFAs, TNF-α and IL-6.
Discussion
T2D is widely recognized as not only a metabolic
disorder, but is also characterized by a chronic inflammatory
state. Similar to previous studies reporting alterations in
inflammatory biomarkers in patients diagnosed with T2D (3–7,10,11),
similar findings were obtained in the present study on PBMCs. The
concentration of TNF-α and IL-6 in the plasma was significantly
higher in patients with T2D compared with the healthy controls,
which further indicated the association between T2D and
inflammation. However, no statistically significant difference was
identified in the total leukocyte or differential count between
patients with T2D and healthy subjects in the current study.
NR4A1, a member of the NR4A orphan nuclear receptor
family, has received increasing attention due to its effects on
metabolic regulation, with outcomes affecting glucose metabolism,
lipolysis and energy expenditure (30–32).
The insulin response of the liver and skeletal muscle was
demonstrated to be blunted in NR4A1-deficient mice fed with a
high-fat diet (31). In the liver,
NR4A1 induced the expression of gluconeogenesis-related genes and
increased glucose production (32). In addition, other studies have
shown that NR4A1 is involved in the impairment of islet β-cells. A
study by Briand et al (33)
on murine pancreatic β-cells demonstrated that fatty acids and
cytokine treatment induced NR4A1 expression, and NR4A1
overexpression decreased insulin secretion. Therefore, NRF4A1
exhibits effects on insulin function and insulin secretion, which
are commonly recognized as two major pathophysiological bases of
T2D.
NR4A1 has been reported to be involved in the
inflammatory disease, atherosclerosis. For instance, higher
expression levels of NR4A1 were detected in macrophages in human
atherosclerotic lesions (24,25).
Similarly, the present study demonstrated that NR4A1 mRNA
expression is increased in the PBMCs of patients with T2D, as
compared with the healthy participants. Furthermore, mRNA
expression levels of NR4A1 were shown to positively correlate with
the levels of FFAs, TNF-α and IL-6, as well as the HOMA-IR. In
vitro, the expression of NR4A1 is highly inducible in
macrophages using diverse inflammatory stimuli, including oxLDL,
LPS and TNF-α (23,24,26).
You et al (22) found that
NR4A1 protein expression was upregulated in human ECs by TNF-α in a
time- and concentration-dependent manner. In accordance with these
studies, the present study demonstrated that NR4A1 expression in
cultured PBMCs was strongly upregulated by 250 μM PA stimulation,
as determined by qPCR and western blot analysis.
Therefore, NR4A1 may be involved in the inflammatory
process and may be associated with inflammatory disease. With
regard to the potential mechanism of NR4A1 induction, certain
studies have provided insights. Shao et al (34) reported that the mitogen-activated
protein kinase (MAPK) signaling pathway mediated the induction of
NR4A1 in Raw264.7 cells in response to an oxLDL stimulus. Treatment
with a p38 MAPK-specific inhibitor on oxLDL-induced Raw264.7 cells
was shown to attenuate NR4A1 expression. Further study indicated
that NR4A1 suppressed macrophages to uptake oxLDL and inhibit
proinflammatory activation, which subsequently decreased macrophage
activation.
The aforementioned studies (23,24,26)
demonstrated that NR4A1 was induced by multiple inflammatory
cytokines and processed the function to suppress inflammation.
However, the potential mechanism through which NR4A1 inhibits
inflammatory activity is not completely clear. In a further study
(22) that investigated the
detailed mechanisms underlying the suppression of inflammatory
activity by NR4A1, a role in the nuclear factor (NF)-κB pathway was
revealed. The NF-κB signaling pathway is known to play an important
role in inflammation. In stimulated cells, IKK degrades IκB
molecules, including IκBα, which inhibits the NF-κB pathway by
forming inactivated complexes with NF-κB. NF-κB is subsequently
released from the cytoplasm to the nucleus, where the molecule
activates target genes (35).
An in vitro experiment by Hong et al
(36) suggested that NR4A1
directly interacts with the p65 subunit of NF-κB through its
C-terminal region. Pei et al (23,26)
also identified NR4A1 as an NF-κB-responsive gene in macrophages.
Furthermore, You et al (22) found that adenovirus-mediated
overexpression of NR4A1 markedly attenuated basal, TNF-α- and
IL-1β-stimulated NF-κB promoter activity in a dose-dependent
manner. NR4A1 also exhibited dose-dependent upregulation of IκBα
expression, which subsequently inhibited the translocation of
NF-κB.
In early studies, NR4A1 was shown to regulate gene
transcription by binding to the NGFI-B response element (NBRE;
AAAGGTCA) (37), which has been
shown to exist in the human IκBα promoter. Mutation of the NBRE
site eliminated the responsiveness of the human IκBα promoter to
NR4A1, indicating that this site mediates IκBα transcriptional
induction by NR4A1 (22). The
induction of NR4A1 by proinflammatory signals was hypothesized to
generate an additional negative feedback loop in the NF-κB
signaling pathway (38).
Therefore, the orphan nuclear receptor, NR4A1, is
induced in response to inflammatory stimuli, including TNF-α, LPS,
oxLDL and FFAs, and may be induced via the p38 MAPK signaling
pathway. The receptor subsequently exerts an anti-inflammatory
function by suppressing NF-κB activity via the induction of IκBα
expression. NR4A1 functions as a negative regulator by inhibiting
NF-κB activation. The regulation, expression and activity of NR4A1
are hypothesized to represent a potential target for the prevention
and treatment of inflammatory diseases.
In conclusion, the present study demonstrated that
the expression of NR4A1 is increased in PBMCs from patients that
have been newly diagnosed with T2D. In addition, NR4A1 expression
was shown to significantly correlate with the levels of the
inflammatory cytokines, TNF-α and IL-6, as well as the
diabetes-related parameters, FIN, FBG, HOMA-IR and FFA. Therefore,
NR4A1 is associated with the inflammatory state in T2D. However,
understanding the specific role of NR4A1 in the regulation of
inflammation in diabetes requires further study.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (nos. 81170769 and 81370872).
The authors thank Joshua W. Knowles and Hope Lancero (Stanford
University, School of Medicine, CA, USA) for providing assistance
in writing this study.
References
|
1
|
Danaei G, Finucane MM, Lu Y, et al; Global
Burden of Metabolic Risk Factors of Chronic Diseases Collaborating
Group (Blood Glucose). National, regional, and global trends in
fasting plasma glucose and diabetes prevalence since 1980:
systematic analysis of health examination surveys and
epidemiological studies with 370 country-years and 2.7 million
participants. Lancet. 378:31–40. 2011.
|
|
2
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldberg RB: Cytokine and cytokine-like
inflammation markers, endothelial dysfunction, and imbalanced
coagulation in development of diabetes and its complications. J
Clin Endocrinol Metab. 94:3171–3182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolb H and Mandrup-Poulsen T: An immune
origin of type 2 diabetes? Diabetologia. 48:1038–1050. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalupahana NS, Moustaid-Moussa N and
Claycombe KJ: Immunity as a link between obesity and insulin
resistance. Mol Aspects Med. 33:26–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Festa A, D’Agostino R Jr, Tracy RP and
Haffner SM; Insulin Resistance Atherosclerosis Study. Elevated
levels of acute-phase proteins and plasminogen activator
inhibitor-1 predict the development of type 2 diabetes: the insulin
resistance atherosclerosis study. Diabetes. 51:1131–1137. 2002.
View Article : Google Scholar
|
|
7
|
Barzilay JI, Abraham L, Heckbert SR, et
al: The relation of markers of inflammation to the development of
glucose disorders in the elderly: the Cardiovascular Health Study.
Diabetes. 50:2384–2389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spranger J, Kroke A, Möhlig M, et al:
Inflammatory cytokines and the risk to develop type 2 diabetes:
results of the prospective population-based European Prospective
Investigation into Cancer and Nutrition (EPIC)-Potsdam Study.
Diabetes. 52:812–817. 2003. View Article : Google Scholar
|
|
9
|
Wang X, Bao W, Liu J, et al: Inflammatory
markers and risk of type 2 diabetes: a systematic review and
meta-analysis. Diabetes Care. 36:166–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakanishi N, Yoshida H, Matsuo Y, Suzuki K
and Tatara K: White blood-cell count and the risk of impaired
fasting glucose or type II diabetes in middle-aged Japanese men.
Diabetologia. 45:42–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pedicino D, Liuzzo G, Trotta F, et al:
Adaptive immunity, inflammation, and cardiovascular complications
in type 1 and type 2 diabetes mellitus. J Diabetes Res.
2013:1842582013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ridker PM, Howard CP, Walter V, et al;
CANTOS Pilot Investigative Group. Effects of interleukin-1β
inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive
protein, interleukin-6, and fibrinogen: a phase IIb randomized,
placebo-controlled trial. Circulation. 126:2739–2748. 2012.
|
|
13
|
Sauter NS, Schulthess FT, Galasso R,
Castellani LW and Maedler K: The antiinflammatory cytokine
interleukin-1 receptor antagonist protects from high-fat
diet-induced hyperglycemia. Endocrinology. 149:2208–2218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emanuelli B, Peraldi P, Filloux C, et al:
SOCS-3 inhibits insulin signaling and is up-regulated in response
to tumor necrosis factor-alpha in the adipose tissue of obese mice.
J Biol Chem. 276:47944–47949. 2001.PubMed/NCBI
|
|
15
|
Donath MY, Böni-Schnetzler M, Ellingsgaard
H and Ehses JA: Islet inflammation impairs the pancreatic beta-cell
in type 2 diabetes. Physiology (Bethesda). 24:325–331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMorrow JP and Murphy EP: Inflammation: a
role for NR4A orphan nuclear receptors? Biochem Soc Trans.
39:688–693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamers AA, Hanna RN, Nowyhed H, Hedrick CC
and de Vries CJ: NR4A nuclear receptors in immunity and
atherosclerosis. Curr Opin Lipidol. 24:381–385. 2013.PubMed/NCBI
|
|
18
|
Hanna RN, Shaked I, Hubbeling HG, et al:
NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory
phenotype and increases atherosclerosis. Circ Res. 110:416–427.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlin LM, Stamatiades EG, Auffray C, et
al: Nr4a1-dependent Ly6C (low) monocytes monitor endothelial cells
and orchestrate their disposal. Cell. 153:362–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SO, Ono K, Tobias PS and Han J: Orphan
nuclear receptor Nur77 is involved in caspase-independent
macrophage cell death. J Exp Med. 197:1441–1452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fahrner TJ, Carroll SL and Milbrandt J:
The NGFI-B protein, an inducible member of the thyroid/steroid
receptor family, is rapidly modified posttranslationally. Mol Cell
Biol. 10:6454–6459. 1990.PubMed/NCBI
|
|
22
|
You B, Jiang YY, Chen S, Yan G and Sun J:
The orphan nuclear receptor Nur77 suppresses endothelial cell
activation through induction of IkappaBalpha expression. Circ Res.
104:742–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pei L, Castrillo A and Tontonoz P:
Regulation of macrophage inflammatory gene expression by the orphan
nuclear receptor Nur77. Mol Endocrinol. 20:786–794. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arkenbout EK, de Waard V, van Bragt M, et
al: Protective function of transcription factor TR3 orphan receptor
in atherogenesis: decreased lesion formation in carotid artery
ligation model in TR3 transgenic mice. Circulation. 106:1530–1535.
2002. View Article : Google Scholar
|
|
25
|
Bonta PI, van Tiel CM, Vos M, et al:
Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in
atherosclerotic lesion macrophages reduce lipid loading and
inflammatory responses. Arterioscler Thromb Vasc Biol.
26:2288–2294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pei L, Castrillo A, Chen M, Hoffmann A and
Tontonoz P: Induction of NR4A orphan nuclear receptor expression in
macrophages in response to inflammatory stimuli. J Biol Chem.
280:29256–29262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haffner SM, Miettinen H and Stern MP: The
homeostasis model in the San Antonio Heart Study. Diabetes Care.
20:1087–1092. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Hu Y, Yang J, et al: The orphan
nuclear receptor Nur77 regulates hepatic cholesterol metabolism
through the suppression of LDLR and HMGCR expression. Mol Med Rep.
5:1541–1547. 2012.PubMed/NCBI
|
|
29
|
Catalán V, Gómez-Ambrosi J, Lizanzu A, et
al: RIP140 gene and protein expression levels are downregulated in
visceral adipose tissue in human morbid obesity. Obes Surg.
19:771–776. 2009.PubMed/NCBI
|
|
30
|
Perez-Sieira S, Martinez G, Porteiro B, et
al: Female Nur77-deficient mice show increased susceptibility to
diet-induced obesity. PLoS One. 8:e538362013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao LC, Wroblewski K, Zhang Z, et al:
Insulin resistance and altered systemic glucose metabolism in mice
lacking Nur77. Diabetes. 58:2788–2796. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pei L, Waki H, Vaitheesvaran B, Wilpitz
DC, Kurland IJ and Tontonoz P: NR4A orphan nuclear receptors are
transcriptional regulators of hepatic glucose metabolism. Nat Med.
12:1048–1055. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Briand O, Helleboid-Chapman A, Ploton M,
et al: The nuclear orphan receptor Nur77 is a lipotoxicity sensor
regulating glucose-induced insulin secretion in pancreatic β-cells.
Mol Endocrinol. 26:399–413. 2012.PubMed/NCBI
|
|
34
|
Shao Q, Shen LH, Hu LH, et al: Nuclear
receptor Nur77 suppresses inflammatory response dependent on COX-2
in macrophages induced by oxLDL. J Mol Cell Cardiol. 49:304–311.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: key elements of proinflammatory
signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong CY, Park JH, Ahn RS, et al: Molecular
mechanism of suppression of testicular steroidogenesis by
proinflammatory cytokine tumor necrosis factor alpha. Mol Cell
Biol. 24:2593–2604. 2004. View Article : Google Scholar
|
|
37
|
Wilson TE, Fahrner TJ, Johnston M and
Milbrandt J: Identification of the DNA binding site for NGFI-B by
genetic selection in yeast. Science. 252:1296–1300. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans PC: Nur77: orphaned at birth but
adopted by the nuclear factor kappaB signaling pathway. Circ Res.
104:707–709. 2009. View Article : Google Scholar : PubMed/NCBI
|