Introduction
At present, esophageal cancer has one of the highest
morbidity and mortality rates, as well as one of the poorest
prognosis rates, of all cancers worldwide, with >480,000 new
cases and 400,000 mortalities annually (1). Only 10% of patients with esophageal
cancer survive for >5 years (2). Chemotherapy is the standard regimen
for patients with advanced esophageal cancer who are unable to
undergo curative surgery (3).
Although chemotherapy is able to improve the outcome of esophageal
cancer (4,5), drug resistance and the side-effects
of chemotherapy are the main reasons for therapeutic failure and
the high mortality rate of esophageal cancer.
Cisplatin (DDP) is a drug that is commonly used for
treatment of a number of cancers, including esophageal cancer
(6). Although DDP has been used as
an important agent in the treatment of esophageal cancer patients,
its clinical application and efficacy have been limited due to
side-effects, including neurotoxicity, hearing loss and
nephrotoxicity, and the emergence of drug resistance (7).
A previous study separated and identified esophageal
cancer related gene 2 (ECRG2) in normal and cancerous esophageal
tissue, and hypothesized that is a tumor-suppressor gene. The
results of the study revealed that the expression level of ECRG2
mRNA was lower in esophageal cancer tissue compared with the levels
in esophageal tissue and a variety of other normal tissues
(8). Further studies have
demonstrated that ECRG2 is able to inhibit tumor cell growth and
proliferation, and induce cell apoptosis in vivo and in
vitro (9,10). However, to the best of our
knowledge, only one study (11)
has been published to date on the combined effect of DDP and ECRG2
in the treatment of esophageal cancer. Therefore, in the present
study, the effects of ECRG2 in combination with DDP on EC9706
esophageal cell proliferation and apoptosis were investigated and
compared with those of ECRG2 alone. The effects of the combination
treatment on the expression levels of Bcl-2-associated X protein
(Bax) mRNA and proteins in the EC9706 cells were also
investigated.
Materials and methods
Materials
Human esophageal cancer cell line EC9706 was donated
by the tumor cell library of the Academy of Chinese Medical
Sciences (Beijing, China). The ECRG2 protein was synthesized by
Shanghai Sangon Biological Engineering Technology & Services
Co., Ltd (Shanghai, China). DDP was purchased from Qilu
Pharmaceutical Co., Ltd (Jinan, China). The rabbit anti-human Bax
and goat anti-rabbit IgG antibodies were purchased from Abcam
(Cambridge, UK). Hoechst 33258 was provided by the Beyotime
Institute of Biotechnology (Shanghai, China). HyClone™ RPMI-1640
medium and fetal calf serum (FCS) were obtained from GE Healthcare
Life Science (Pittsburgh, PA, USA). The
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT)
assay, dimethyl sulfoxide (DMSO) and TRIzol reagent were purchased
from Sigma Co. (St. Louis, MO, USA). A reverse transcription and
amplification kit was purchased from Promega Corporation (Madison,
WI, USA).
Cell culture
EC9706 cells were cultured in RPMI-1640 medium,
containing 10% FCS (100 u/ml penicillin and 100 mg/l streptomycin),
in a humidified incubator at 37°C with 5% CO2. Cells
were in the logarithmic phase in all experiments.
Cell viability and proliferation
In order to investigate the combined effects of DDP
and ECRG2 on cell proliferation and viability, EC9706 cells
(4×107/l) were seeded into 96-well plates and incubated
in RPMI-1640 medium supplemented with 10% FCS. Cells were randomly
divided into three groups with 10 repeated wells used for each
group. For the ECRG2 group, ECRG2 proteins at different
concentrations (5.5, 6.5, 7.5, 8.5 μg/l) were added to the EC9706
cells. For the ECRG2 protein + DDP group, 3 mg/l DPP was added to
the final concentration of ECRG2 protein, on the basis of each of
the ECRG2 protein concentrations above. EC9706 cells in the control
group were not treated with any drugs. Cell proliferation and
viability were detected by MTT assay at 24, 48 and 72 h following
treatment. To do this, 20 μl of a 5 g/l MTT solution was added to
each well and incubation was continued for 4 h at 37°C. The medium
was discarded and 150 μl DMSO was added to each well and agitated
to fully dissolve the blue-purple MTT precipitate. A microplate
reader (Bio-Rad 680; Bio-Rad, Hercules, CA, USA) was used to
measure the absorbance (A) of each well at 490 nm and average
values were obtained. Experiments were repeated ≥3 times and data
are expressed as the mean ± standard error of the mean.
Analysis of cell apoptosis
Hoechst 33258 staining was performed in order to
investigate the rate of cell apoptosis. EC9706 cells were randomly
divided into the three groups: control, ECRG2 and ECRG2 protein +
DDP. For the ECRG2 group, ECRG2 proteins at different
concentrations (5.5, 6.5, 7.5 and 8.5 μg/l) were respectively added
to the EC9706 cells. For the ECRG2 protein + DDP groups, 3 mg/l
cisplatin was added to the final concentration of ECRG2 protein, on
the basis of each of the ECRG2 protein concentrations above. After
24 h, Hoechst 33258 staining was conducted for the detection of
apoptosis. The apoptotic cells were observed and quantified under a
fluorescence microscope (Eclipse 80i; Nikon, Tokyo, Japan) and the
apoptotic rate was calculated as the ratio of the number of
apoptotic cells to the total number of cells.
Reverse transcription polymerase chain
reaction (RT-PCR)
The total RNA was isolated from cells using TRIzol
reagent according to the manufacturers’ instructions. The
expression levels of Bax mRNA were detected by RT-PCR. The primers
for Bax were 5′-TTCATCCAGGATCGAGCAGAG-3′ (forward) and
5′-TGAGGACTCCAGCCACAAAGAT-3′ (reverse). The product size was ~498
bp. The primers for glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were 5′-TCATGGGTGTGAACCATGAGAA-3′ (forward) and
5′-GGCATGGACTGTGGTCATGAG-3′ (reverse). The product size was ~206
bp. Each reaction system contained 12.5 μl GoTaq®
GreenMaster mix (Promega Corporation, Madison, WI, USA), 2.5 μl of
each primer and 5 μl cDNA. Following the activation of Taq
polymerase for 5 min at 95°C, cDNA was amplified for 40 sec at
95°C, 40 sec at 55°C, and 1 min at 72°C, for 35 cycles, ending with
a final extension for 5 min at 72°C. To verify the accuracy of the
amplification, the RT-PCR products were electrophoresed through a
1.5% agarose gel stained with ethidium bromide. The images were
collected under UV light. Data were analyzed using Light
Cycler® 480 software (Roche Diagnostics GmbH, Mannheim,
Germany). The expression levels of mRNA were measured by
densitometry. The target expressions were normalized using the
expression levels of β-actin as a reference.
Western blot analysis
The total cell proteins were extracted from the
cells of the three groups. Following quantification of the total
proteins, the proteins were separated through 8% polyacrylamide gel
electrophoresis (PAGE) and transferred to polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA). The membranes
were blocked with 5% skimmed dried milk in phosphate-buffered
saline (PBS)-Tween 20. Following incubation with a polyclonal
rabbit anti-human Bax antibody (diluted 1:1,000) overnight at 4°C,
the membranes were incubated with a secondary horseradish
peroxidase-conjugated anti-rabbit antibody (1:1,000) for 2 h. Blots
were visualized by chemiluminescence (Tanon 6200; Tanon, Shanghai,
China).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Statistical analysis was performed using one-way analysis of
variance on SPSS software, version 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory effects of ECRG2 and ECRG2 in
combination with DDP on the proliferation of EC9706 cells at
different time points
To explore the effects of ECRG2 and ECRG2 in
combination with DDP on the proliferation of EC9706 cells, an MTT
assay was performed. As shown in Table
I, EC9706 cell growth was inhibited by different concentrations
of the ECRG2 protein in a time- and concentration-dependent manner
within a certain range of concentrations. The inhibitory effect of
the ECRG2 protein on EC9706 cell proliferation at different
concentrations was enhanced following the addition of 3 mg/l DDP.
The proliferation rate of EC9706 cells exhibited a time-and
concentration-dependent reduction as the concentration of ECRG2
protein increased. The inhibition rate for the combination reached
its peak (33.61%) at 72 h.
 | Table IEffect of the ECRG2 protein and ECRG2
in combination with DDP on EC9706 proliferation at different time
points (%). |
Table I
Effect of the ECRG2 protein and ECRG2
in combination with DDP on EC9706 proliferation at different time
points (%).
| Group | 24 h | 48 h | 72 h |
|---|
| Control | 100 | 100 | 100 |
| ECRG2 protein |
| 5.5 μg/l | 96.24±0.62a | 91.33±0.60a | 87.42±0.56a |
| 6.5 μg/l | 92.10±0.56a | 88.74±0.54a | 85.40±0.60a |
| 7.5 μg/l | 87.39±0.52a | 84.87±0.63a | 80.35±0.64a |
| 8.5 μg/l | 81.31±0.66a | 79.41±0.50a | 76.10±0.57a |
| ECRG2 + DDP |
| 5.5 μg/l + 3
mg/l | 92.48±0.40a,b | 89.17±0.50a,b | 85.60±0.49a,b |
| 6.5 μg/l + 3
mg/l | 88.40±0.56a,b | 85.71±0.48a,b | 82.15±0.60a,b |
| 7.5 μg/l + 3
mg/l | 80.84±0.54a,b | 76.38±0.55a,b | 73.20±0.55a,b |
| 8.5 μg/l + 3
mg/l | 74.46±0.53a,b | 70.56±0.49a,b | 66.39±0.50a,b |
Effect of the ECRG2 protein and DDP on
the apoptosis rate of EC9706 cells
As shown in Table
II, the ECRG2 protein alone significantly increased the rate of
EC9706 cell apoptosis compared with that of the control group in a
concentration-dependent manner. When ECRG2 was used in combination
with DDP, the number of apoptotic cells was significantly increased
compared with that for the same concentration of ECRG2 used alone.
The apoptotic effect of the combination also increased in a
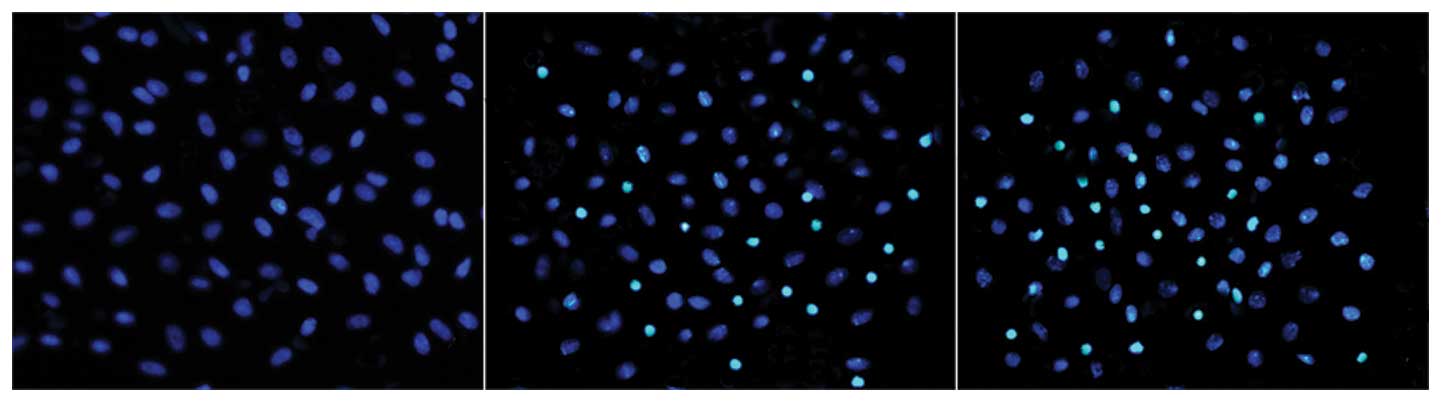
concentration-dependent manner. As shown in Fig. 1, the apoptotic cell bodies
decreased in volume and became rounder, whilst the cell nuclei
became more concentrated. Following Hoechst 33258 staining, the
apoptotic cells appeared white under a fluorescence microscope.
 | Table IIEffect of the ECRG2 protein and ECRG2
in combination with DDP on EC9706 cell apoptosis after 24 h
(%). |
Table II
Effect of the ECRG2 protein and ECRG2
in combination with DDP on EC9706 cell apoptosis after 24 h
(%).
| ECRG2 (μg/l) |
|---|
|
|
|---|
| Group | 5.5 | 6.5 | 7.5 | 8.5 |
|---|
| Control | 0 | 0 | 0 | 0 |
| ECRG2 | 4.10±0.26a | 7.20±0.25a | 12.45±0.22a | 18.22±0.19a |
| ECRG2 + DDP | 6.40±0.21a,b | 10.33±0.30a,b | 16.38±0.31a,b | 24.60±0.24a,b |
Effect of the ECRG2 protein and DDP on
the expression levels of Bax mRNA
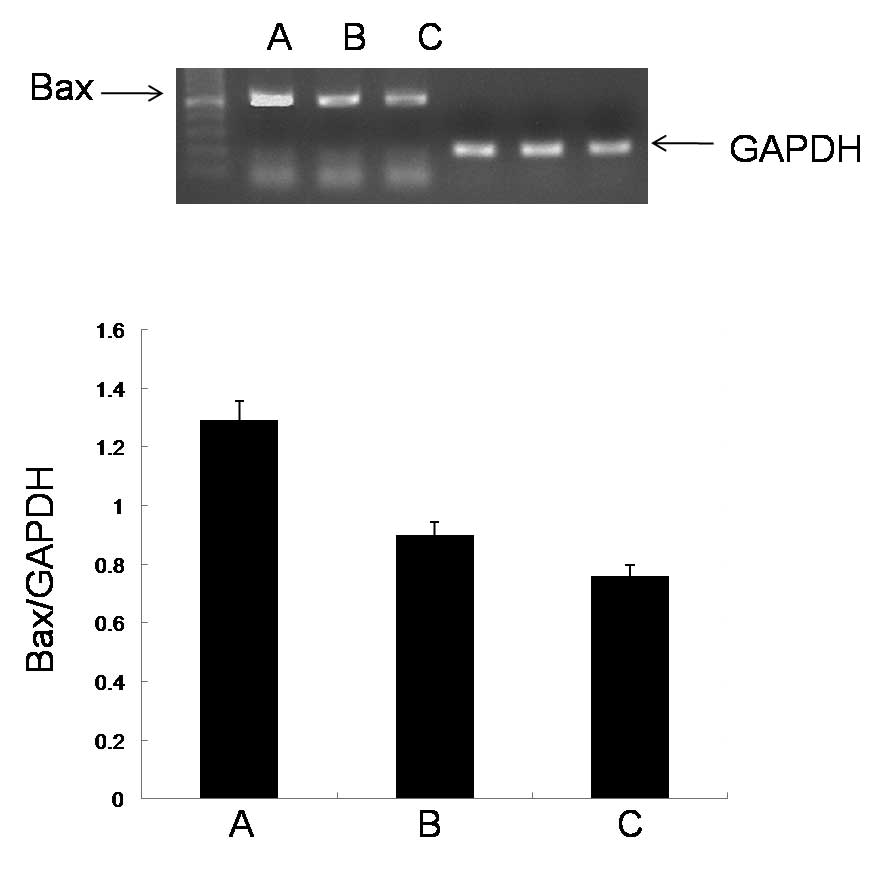
To investigate the mechanisms underlying the cell
apoptosis induced by ECRG2 and ECRG2 in combination with DDP, the
expression levels of Bax mRNA in the esophageal cancer cells were
studied by RT-PCR analysis. The ECRG2 protein significantly
upregulated the expression levels of Bax mRNA compared with those
in the control group. When the ECRG2 protein was combined with DDP,
the expression levels of Bax mRNA were significantly increased
compared with those observed when the ECRG2 protein was used alone
(Fig. 2).
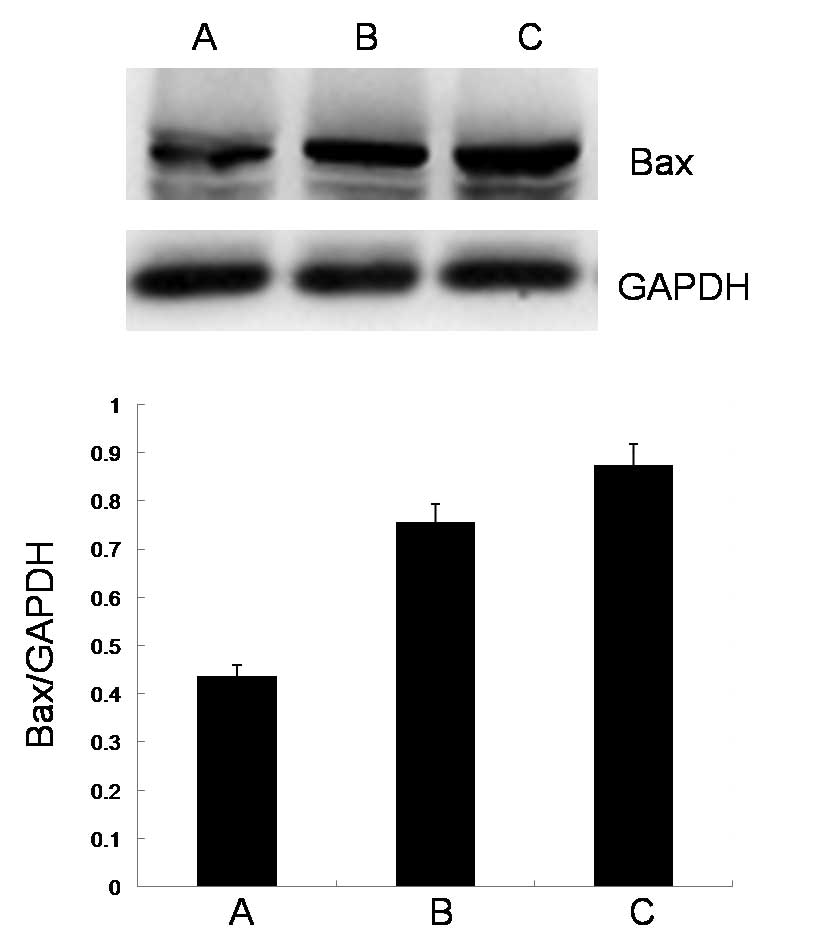
Effect of the ECRG2 protein and DDP on
the expression levels of Bax protein
As shown in Fig. 3,
the expression level of Bax protein was significantly upregulated
by a high concentration of the ECRG2 protein compared with the
level in the control group. When ECRG2 protein was combined with
DDP, the expression level of Bax protein was significantly
increased compared with that observed when the ECRG2 protein was
used alone.
Discussion
Esophageal cancer is one of the most common types of
cancer, with one of the highest levels of morbidity and mortality,
and it is a major cause of death worldwide (12). For patients with local esophageal
cancer, surgical resection is the preferred treatment. However, in
almost 50% of patients with esophageal cancer in clinical
diagnosis, the cancer cells have already metastasized. As advanced
cancer patients, these individuals mainly rely on chemotherapy for
treatment. Chemotherapy is regarded as an important treatment in
comprehensive therapies. DDP, a chemotherapy drug commonly used in
antitumor treatments since the 1960s, destroys tumor cells mainly
through the suppression of DNA replication (13); this activates apoptosis-related
signaling pathways and results in cell apoptosis. However, the
clinical application of DDP has been limited due to serious toxic
side-effects, including neurotoxicity and loss of hearing. A method
of reducing these side-effects is to decrease the dosage but leave
a sufficient concentration of DDP in the blood to destroy the
cancer cells. Thus, combined drug treatments have been recommended
for clinical application, as they not only ensure that the
concentration of platinum in the tumor tissue is sufficient to
provide improved therapeutic effects, but also enable the clinical
dose of the platinum-based chemotherapy drug to be reduced, which
alleviates the toxic side-effects.
Human ECRG2 is located on the human chromosome
5q32–33. It comprises four exons and three introns with a total
length of 3,540 bp. The ECRG2 cDNA that encodes a polypeptide with
85 amino acid residues is 569 bp in length. ECRG2 mRNA is expressed
in esophageal tissue and a number of normal tissues; however, it is
significantly downregulated in esophageal cancer tissue and
adjacent tissues (8). ECRG2 has
been revealed to inhibit tumor cell growth and proliferation, and
promote cell apoptosis in vivo and in vitro (9,10,14–15).
The incidence and progression of tumors results from the abnormal
proliferation, differentiation and apoptosis of cells. The majority
of anticancer drugs act via the induction of apoptosis in sensitive
tumor cells and this drug-induced apoptotic activity of tumor cells
is associated with the antitumor efficacy that is observed. Thus,
the induction of apoptosis in tumor cells is a particular focus of
cancer therapy studies, and apoptosis is often used as an index to
evaluate the efficacy of treatments (16). In the present study, the results of
Hoechst 33258 staining demonstrate that ECRG2 is able to promote
the apoptosis of EC9706 esophageal cancer cells.
The Bcl-2 family are the main regulatory factors in
the process of cell apoptosis through mitochondrial mediation.
Bcl-2 plays an important anti-apoptotic role by preventing the
release of cytochromes in the mitochondria. However, Bax has mainly
pro-apoptotic effects by increasing the permeability of the
mitochondrial membrane, causing the release of cytochrome c
and subsequent cell apoptosis. A previous study revealed that
cancer cells with upregulated levels of Bax exhibited a decreased
tolerance to DDP (17). In another
study, apoptosis of HeLa cells was induced by the upregulation of
p53, which subsequently increased the expression levels of p53 and
Bax (18). The apoptosis of EC9706
xenografts in mice treated with paclitaxel in a folate-mediated
micelle formulation, indicated that apoptosis may be mediated via
the upregulation of the expression levels of Bax (19).
EC9706 cells are a useful model in which to study
the inhibition of esophageal squamous cell growth by chemical,
physical and physiological agents. In the present study, using
EC9706 cells as a model, when ECRG2 was combined with DDP, the
inhibitory effect on cell proliferation, the inductive effect on
apoptosis and the increase in the expression levels of the
apoptosis-related protein Bax were significantly higher compared
with those observed when the ECRG2 protein was used alone. The
current results suggest that ECRG2 is not toxic and that, when used
in combination with DDP, it may alleviate the toxic side-effects of
DDP by allowing the clinical dosage of DDP to be reduced while
still providing the desired therapeutic effect.
Acknowledgements
This study was financially supported by grants from
the Social Public Welfare Project Preparatory Special Foundation in
Scientific Research Units of Henan province (20130010, XF Hou); the
Focus Research Field Tendering of Xinxiang Medical University
(ZD2011–14) and the Tumor and Signal Transduction Laboratory of
Xinxiang Medical University (Xinxiang, China).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Kollarova H, Machova L, Horakova D,
Janoutova G and Janout V: Epidemiology of esophageal cancer - an
overview article. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 151:17–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilson DH: Esophageal cancer chemotherapy:
recent advances. Gastrointest Cancer Res. 2:85–92. 2008.
|
|
4
|
Sjoquist KM, Burmeister BH, Smithers BM,
et al: Survival after neoadjuvant chemotherapy or chemoradiotherapy
for resectable oesophageal carcinoma: an updated meta-analysis.
Lancet Oncol. 12:681–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Hagen P, Hulshof MC, van Lanschot JJ,
et al: Preoperative chemoradiotherapy for esophageal or junctional
cancer. N Engl J Med. 366:2074–2084. 2012.
|
|
6
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
8
|
Su T, Liu H and Lu S: Cloning and
identification of cDNA fragments related to human esophageal
cancer. Zhonghua Zhong Liu Za Zhi. 20:254–257. 1998.(In
Chinese).
|
|
9
|
Cui Y, Wang J, Zhang X, et al: ECRG2, a
novel candidate of tumor suppressor gene in the esophageal
carcinoma, interacts directly with metallothionein 2A and links to
apoptosis. Biochem Biophys Res Commun. 302:904–915. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li MN, Huang G, Guo LP and Lu SH:
Inhibitory effects of esophageal cancer related gene 2 on
proliferation of human esophageal cancer cell EC9706. Zhonghua Yi
Xue Za Zhi. 85:2785–2788. 2005.(In Chinese).
|
|
11
|
Song HY, Deng XH, Yuan GY, et al:
Expression of bcl-2 and p53 in induction of esophageal cancer cell
apoptosis by ECRG2 in combination with cisplatin. Asian Pac J
Cancer Prev. 15:1397–1401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka T, Ishiguro H, Kuwabara Y, et al:
Vascular endothelial growth factor C (VEGF-C) in esophageal cancer
correlates with lymph node metastasis and poor patient prognosis. J
Exp Clin Cancer Res. 29:832010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasetto LM, D’Andrea MR, Brandes AA, Rossi
E and Monfardini S: The development of platinum compounds and their
possible combination. Crit Rev Oncol Hematol. 60:59–75. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue CM, Bi MX, Tan W, et al: Short tandem
repeat polymorphism in a novel esophageal cancer-related gene
(ECRG2) implicates susceptibility to esophageal cancer in Chinese
population. Int J Cancer. 108:232–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song H, Song C, Wang H, et al: Suppression
of hepatocarcinoma model in vitro and in vivo by
ECRG2 delivery using adenoviral vector. Cancer Gene Ther.
19:875–879. 2012.PubMed/NCBI
|
|
16
|
Tan TT and White E: Therapeutic targeting
of death pathways in cancer: mechanisms for activating cell death
in cancer cells. Adv Exp Med Biol. 615:81–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu Y, Zou YB, Li K, et al: Effect of
altered WIG-1 expression on DDP sensitivity in a DDP-resistant
esophageal squamous cancer cell line. Curr Cancer Drug Targets.
12:950–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ismail N, Pihie AH and Nallapan M:
Xanthorrhizol induces apoptosis via the up-regulation of bax and
p53 in HeLa cells. Anticancer Res. 25:2221–2227. 2005.PubMed/NCBI
|
|
19
|
Wu W, Zheng Y, Wang R, et al: Antitumor
activity of folate-targeted, paclitaxel-loaded polymeric micelles
on a human esophageal EC9706 cancer cell line. Int J Nanomedicine.
7:3487–3502. 2012. View Article : Google Scholar : PubMed/NCBI
|