Introduction
Tranilast was introduced as an anti-atopic agent
forty years ago (1). Several
studies and clinical reports have described anti-inflammatory and
antifibrotic effects in the following years and tranilast was used
for the treatment of dermatological disorders, such as scleroderma,
cheilitis granulomatosa, granuloma anulare or sarcoidosis (2,3). In
cell culture experiments, it has been shown that tranilast inhibits
collagen synthesis in fibroblasts, partially as a direct effect on
the protein expression and partially via inhibition of the
stimulating effect of transforming growth factor β1 (TGF-β1) on the
collagen synthesis of fibroblasts (4,5).
Tranilast prevented intraperitoneal adhesions in an animal model
(6) and it has been used for the
prevention of hypertrophic scar development following sternotomy in
children (7).
Tranilast inhibited the collagen synthesis and
proliferation of vascular smooth muscle cells in cell culture
experiments by interacting with the receptors for the
platelet-derived growth factor, TGF-β, and angiotensin II (8). These effects could be translated in a
significant reduction of the intimal hyperplasia subsequent to
treatment with tranilast in different animal models following
arterial injury (9), into reduced
restenosis rates subsequent to percutaneous coronary angioplasty of
de novo or restenotic lesions in smaller human studies
(10,11), but not in the large Prevention of
REStenosis with Tranilast and its Outcomes trial (11,12).
In addition, tranilast showed positive effects on intracardiac
inflammatory processes positively influencing cardiac remodeling in
several animal models (13,14):
Tranilast administered once daily for four weeks led to a decrease
of the left ventricular hypertrophy and ameliorated the
perivascular and interstitial intracardiac fibrosis in
spontaneously hypertensive rats. The left ventricular end diastolic
pressure or chamber stiffness constants were not affected (13). Similar results were obtained in the
animal model of uni-nephrectomized deoxycorticosterone acetate
(DOCA)/salt hypertensive rats. Tranilast administered for 28 days
attenuated the intracardiac perivascular and interstitial fibrosis,
in parallel with an inhibition of TGF-β1 expression and
a suppression of cardiac mRNA levels of different cytokines
(14).
In contrast to these animal models the processes
leading to the cardiac remodeling following myocardial infarction
(MI) do not involve the entire circumference of the left ventricle
(LV). Cardiac remodeling following large MI can be interpreted as a
response of the remote non-infarcted section of the LV to changes
in ventricular geometry. However, the key features of cardiac
remodeling are the same, involving myocyte hypertrophy, apoptosis
and interstitial fibrosis. Several data show that the inflammatory
processes in the ischemic area, as well as in the remote
non-infarcted area of the LV following MI, are involved in the
different phases. TGF-β is believed to play a central role in each
of these phases. TGF-β promotes extracellular matrix protein
expression by fibroblasts and inhibits matrix degradation via
several mechanisms (15).
In experimental models of MI the expression of
different isoforms of TGF-β was found to be upregulated in
different phases post-infarction. TGF-β1 and
-β2 are induced in the early phase, whereas
TGF-β3 shows delayed and prolonged upregulation
(16). The process of cardiac
remodeling outside the infarcted area of the LV starts within hours
following the infarction and continues to progress over weeks or
months (17). An animal study with
mice revealed that the blockade of TGF-β-signaling by
overexpression of the extracellular domain of the TGF-β type II
receptor during the early phase following MI resulted in left
ventricular dilatation and increased early mortality. By contrast,
blockade of the TGF-β signaling in the later phase following MI
prevented the LV dilatation and the reduction of the contractile
function, as well as myocyte hypertrophy and interstitial fibrosis
(18). Recently, the study by See
et al (19) reported on
early and late administration of tranilast following MI (early,
between 24 h and seven days post-MI; late, 7–28 days post-MI). The
study revealed that tranilast inhibited myocardial TGFβ1
expression, fibrosis in rat post-MI and collagen production in
cardiac fibroblasts. However, tranilast intervention from 24 h
post-MI exacerbated infarct expansion, delaying the commencement of
treatment to seven days post-MI impeded LV remodeling. Therefore,
the aim of the present study was to investigate extremely late
tranilast administration (starting at day 28) and its effect on
cardiac remodeling and the six-month mortality rate in an
experimental model of chronic ischemic heart failure following a
large MI in the rat.
Materials and methods
Animal model and study groups
Studies were performed on 268 female Lewis rats,
inbred and raised in the Institute for Animal Experiments of the
Friedrich Schiller University (Jena, Germany). The studies were
approved by the Ethics Committee of the Friedrich Schiller
University. The investigation conforms to the Guide for the Care
and Use of Laboratory Animals published by the National Institutes
of Health (NIH; publication no. 85-23, revised 1996) and to the
German law on the protection of animals.
The study was designed in an intention-to-treat
manner. During the randomization process the animals were
designated to the different surgical/treatment groups
(MI/tranilast, MI/placebo or sham-operation (ShO)/placebo), as well
as to the different analysis groups (collagen content via
high-performance liquid chromatography (HPLC) and resting
pressure-volume-curve/histological studies) prior to surgery. For
the induction of an MI, the proximal left anterior descending
coronary artery was ligated via left lateral thoracotomy following
tracheotomy for controlled ventilation (20). In the sham-operated animals, the
suture only was loosely tied. Ribs, muscles and skin were closed in
separate layers. The animals were housed for six months, four of
each in one polyethylene cage, with a maintained 12 h light/dark
cycle. Animals had free access to standard food and water ad
libitum.
Due to a high mortality rate of 39.3% (97 animals)
following ligation of the proximal left anterior descending
coronary artery and 4.8% (one animal) following the ShO in the
first 48 h after surgery, a total number of 268 animals were
randomized and the surgery performed according to the protocol
until the intended group sizes of n=75 in each group following MI
and n=20 following the ShO were reached. No difference was
identified between the groups concerning body weight or age
(Table I).
 | Table IAge and body weight of the rats at the
time of randomization. |
Table I
Age and body weight of the rats at the
time of randomization.
| Characteristic | Plac (n=75) | Tra (n=75) | ShO (n=20) | P-value |
|---|
| Age, weeks | 24.5±0.9 | 26.2±0.5 | 25.5±0.8 | 0.20 |
| Body weight, g | 219.8±2.5 | 215.1±3.1 | 223.8±3.6 | 0.26 |
Drug administration
The administration of the study drug or placebo was
performed at days 28–182 post-operation by gavage. The scar
formation following MI was completed, so that the treatment with
tranilast did not have an effect on the scar formation in the
infarcted area of the LV. Tranilast was administered at a daily
dose of 300 mg/kg in two doses, dispersed in a solution of Tylose H
300, which served as the placebo as well.
Preparation
The rats that succumbed during the study period were
autopsied and the hearts and lungs were excised. The rats surviving
the experimental period were sacrificed by decapitation after 182
days. Hearts were perfused in situ with ice-cold heparinized
physiological saline following cannulation of the proximal aorta
and its ligation distal. The hearts were stopped in diastole by
additional flushing with 7.45% potassium chloride. Transmural MI
was clearly visible as a thin scar tissue with aneurysmatic
bulging. Only rats with a transmural MI reaching from the base of
the LV to its apex and including more than one-third of its
circumference were included into further analysis. The resting
pressure-volume curve analyses were performed in all the LVs, and
the hearts were subsequently prepared either for chromatographical
or histological determination of the collagen content.
Resting pressure-volume curve
A pressure-volume-curve of the resting isolated LV
was performed in all the probes. The tip of a double-lumen catheter
(polyethylene tube, innerlumen 0.5 mm inside polyethylene tube,
innerlumen 2.0 mm) was placed in the LV and ligated in the
atrioventricular groove. The catheter was connected to a Statham
pressure transducer to record a pressure-volume curve (infusion of
0.9% saline, 15 ml/h, ≤30 mmHg) (21). For each heart, two measurements
were performed within 10 min after cardiac arrest. Pressure and
volume were recorded every second. These data were used for an
automated regression curve (best-fit curve) analysis. At pressure
ranges of 0–3 mmHg, curves were generated following a linear model
and were expressed as y = ax + b. The chamber stiffness is
described by the slope of the curve, ‘a’. At pressure ranges of
3–10 and 10–30 mmHg, exponential curves had to be constructed as
regression curves to fit the data. These curves can be described as
y = cxd. Subsequent to calculating the logarithm, log(y) can be
expressed as a linear function of ‘d’. In the exponential model the
chamber stiffness is described by ‘d’. To determine the ventricular
dilatation, the infused volumes at 5, 10 and 15 mmHg were measured
for each heart.
Collagen content
The collagen content in the non-infarcted area of
the LV was assessed using two different methods. In 30 animals, the
hydroxyproline content of the probes of the non-infarcted inferior
left ventricular wall was measured by HPLC. Two probes, each
weighing 100–150 mg, were taken from each heart. Hydroxyproline is
solely found in collagen, constituting a fraction of 13.4%.
Subsequent to homogenization in phosphate buffer and
lyophilisation, the probes were hydrolyzed for 24 h at 114°C in 6 M
HCl. Hydroxyproline bound to an ion-exchanger (Dowex W-X8,
Sigma-Aldrich, St. Louis, MO, USA) in phosphate-citric-acid-buffer
(pH 5.0), and was eluted from the ion-exchanger by heating up the
probes to 115°C for 16 h and washing them with
acetate-citrate-citric acid-buffer (pH 6.0). Aliquots were diluted
in 20% acetate-buffer in methanol. The content of hydroxyproline in
the probes was analyzed by HPLC with 7-chloro-4-nitrobenzofurazan
as the fluorescent-labeling reagent. The results were expressed as
nmol per probe and subsequently converted into microgram collagen
per milligram dry weight of cardiac tissue.
The collagen content of the non-infarcted section of
the LV was measured by planimetry of cryosections in 38 animals.
The LVs were cut into four transversal cross-sectional slices of
equal thickness and shock frozen in liquid nitrogen. Cryostat
sections (7-μm) were cut from each probe and stained with Masson’s
trichrome stain. Five optical fields (magnification, ×40) in the
non-infarcted section of each cryostat section were analyzed. These
five fields were dispersed across the slice circumferences, but no
field with tissue defects due to the cutting process or further
preparation, nor an optical field with large vessels respectively
perivascular collagen were analyzed. The blue-stained collagen
could be clearly distinguished from cardiac myocytes (with
red-stained myofibrils and brown nuclei) or defects. This
differentiation was performed automatically using an individualized
macro for the NIH-Scion Image® program (Scion Corp.,
Walkersville, MD, USA). The averaged gray scale values of all three
colour channels were subtracted from the inverted gray scale values
of the blue channel. A cut-off point was set to differentiate the
blue staining. Calculation was performed as percentage of area
using the pixel-based program. The hearts of these animals, which
succumbed during the study period, were analyzed via histology.
Infarct size and thickness of the
scar
The infarct size was measured as the percentage of
the inner and outer diameter in the cryostat sections of each of
the four transversal cross-sectional slices (22). The thickness of the scar was
measured three times in each of the cryostat sections.
Statistics
All the values are expressed as mean ± standard
error of the mean. Results were analyzed using a two-tailed
Student’s t-test for unpaired data. For multiple comparisons
analysis of variance was performed, followed by the Kruskal-Wallis
test and two-sided Mann-Whitney U Test, where appropriate.
Kaplan-Meier curves were constructed and statistical analysis of
the survival curves was performed using the log-rank test. For
histological analysis of the collagen content, scar thickness and
infarct size, the raw data were aggregated for every animal and
statistical analysis was performed as described above. P<0.05
was considered to indicate a statistically significant difference.
PAWS Statistics for Windows, version 18 (SPSS Inc., Chicago, IL,
USA) was used.
Results
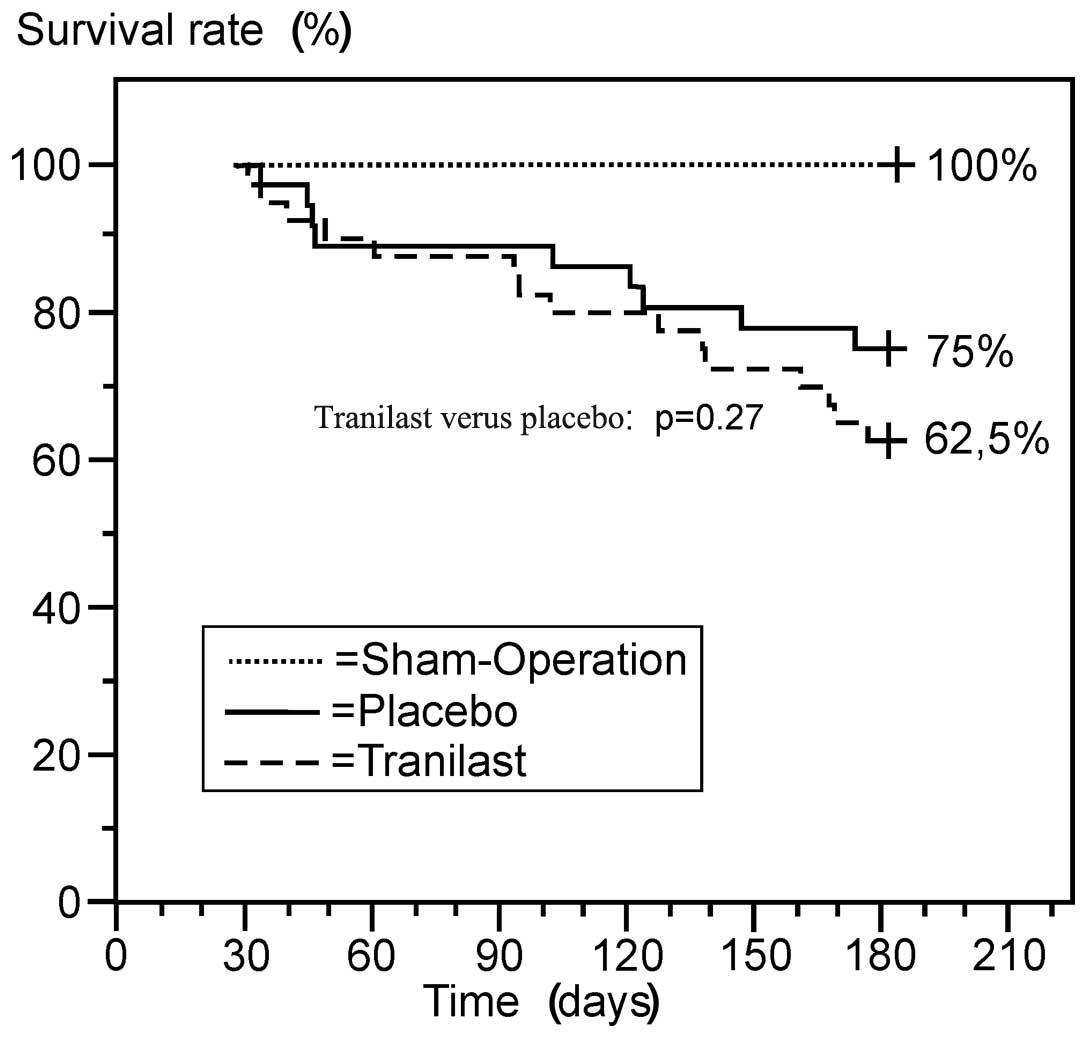
Survival rates
The number of animals that succumbed during the
different study periods and those included into the final analysis
are shown in Fig. 1. The majority
of the animals that succumbed up to day 28 had a large MI and signs
of severe heart failure in the autopsies (pneumonia, n=2 and
hematothorax, n=1). All the animals that succumbed within the
treatment period of days 28–182 had a large MI, reaching from the
base to the apex of the LV and comprising more than one-third of
its circumference. No animal succumbed during the treatment period
following the ShO.
At the end of the study period, 36 rats in the
tranilast group, 36 rats in the placebo group and 18 rats following
the ShO were sacrificed. Their organs and blood samples were
obtained for analysis. There was no infarction of the right
ventricle observed. None of the sham-operated rats had MI. In 11
rats of the tranilast group and in nine rats of the placebo group,
the sizes of the MIs were too small to fit the aforementioned
criteria. These animals were excluded from the Kaplan-Meier
analysis and the weights of these lungs and hearts were analyzed
separately. This led to a further reduction in the size of the
groups, but enabled a comparison between the survivors and
non-survivors. Finally, 40 animals that were treated with tranilast
(15 succumbed and 25 survived) and 36 animals that were treated
with placebo (nine succumbed and 27 survived) had a large MI,
resulting in six-month survival rates of 62.5 and 75%,
respectively. The curves in the Kaplan-Meier analysis were similar
(Fig. 2).
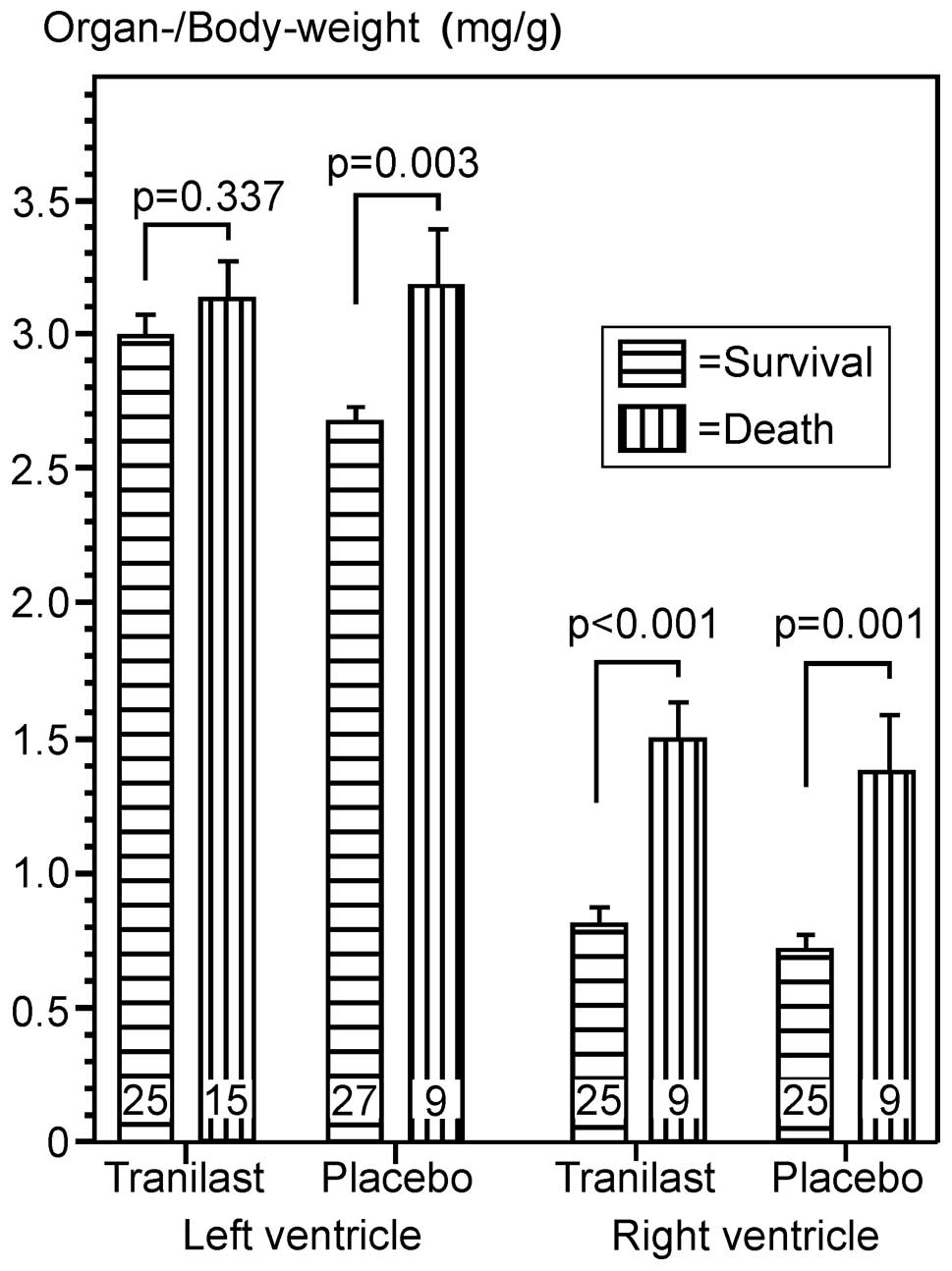
The body weights at day 182 tended to differ among
the three study groups, with the lowest body weight in the
tranilast group, without reaching statistical significance
(P=0.051). Induction of large MI led to a significant hypertrophy
of the LV and right ventricle. However, the weights of the LV in
the tranilast group were higher than those in the placebo group and
this combination of lower body weight and higher weight of the LV
led to a significant difference of the left-ventricular-index
[heart weight (mg)/body weight (g)], between the two groups in the
statistical analysis (Table
II).
 | Table IIBody and organ weights six months
after large myocardial infarction or sham-operation. |
Table II
Body and organ weights six months
after large myocardial infarction or sham-operation.
| Parameter | Plac (n=27) | Tra (n=25) | ShO (n=18) |
|---|
| BW182,
g | 282.00±5.79 | 268.32±6.91 | 293.06±6.48 |
| Weight gain,
ga | 62.21±7.60 | 51.15±6.10 | 67.07±5.73 |
| LV, mg | 742.2±12.3b | 796.6±23.5b | 614.1±14.2 |
| LVI, mg/g | 2.67±0.06b | 2.99±0.09b,d | 2.09±0.06 |
| RV, mg | 197.0±14.0c | 209.0±15.8b | 144.1±9.1 |
| RVI, mg/g | 0.70±0.05c | 0.80±0.07b | 0.49±0.03 |
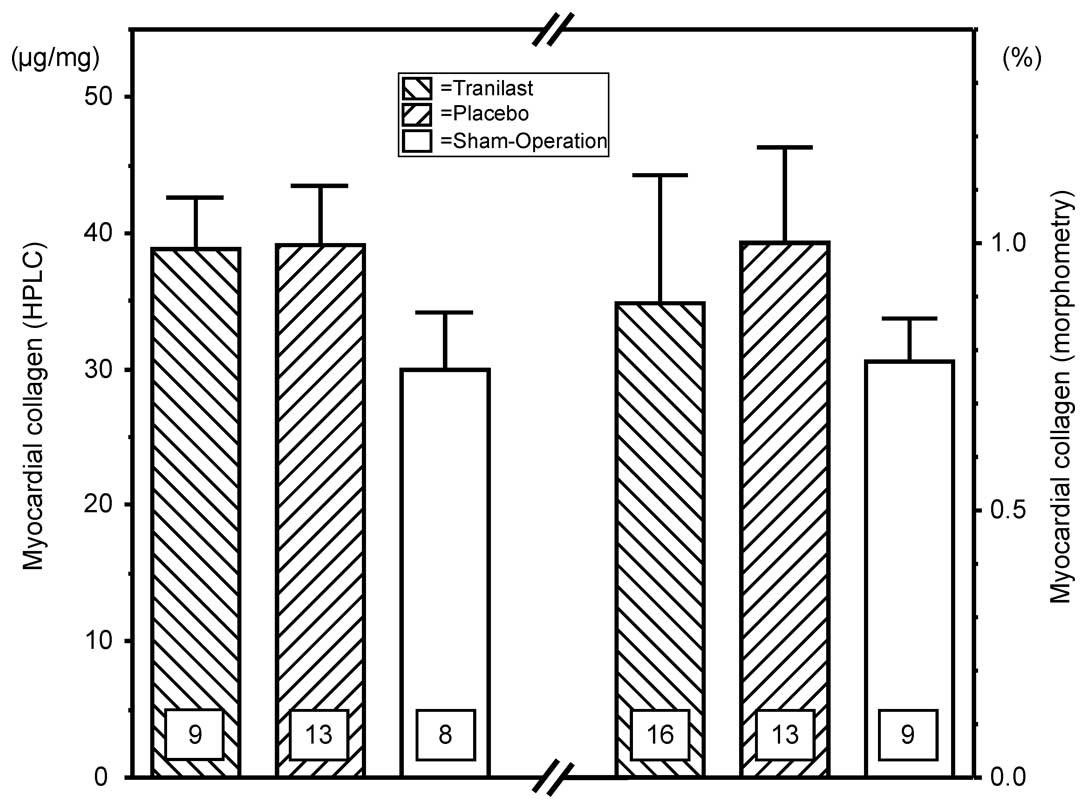
Cardiac collagen content following large
MI
The cardiac hypertrophy following large MI was
accompanied by an increase of intracardiac collagen content in the
remote non-infarcted part of the LV. This aspect was shown in the
histological analysis, as well as in the measurement of the
hydroxyproline by HPLC. However, the two methods revealed that the
difference compared with the hearts following the ShO was not
statistically significant. The levels of collagen content were not
significantly different between the treatment groups following MI
(Fig. 3).
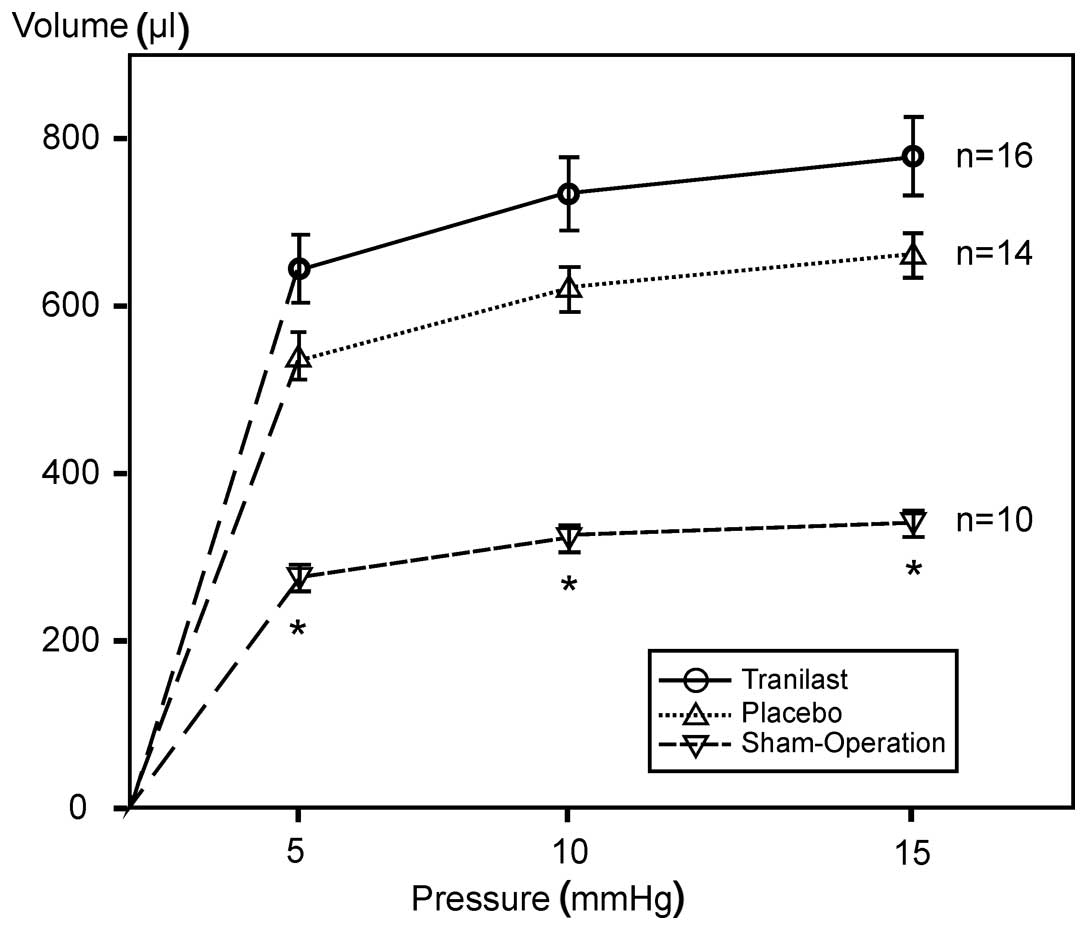
A severe dilatation of the LV following large MI
could be found in the analysis of the pressure volume curves of the
isolated LV (Fig. 4), as well as
by measuring the circumference of the LV in the histological
analysis. The infarct size was the same in the two treatment
groups. Treatment with tranilast did not lead to a difference in
the scar extension or thickness (Table III).
 | Table IIIHistological evaluation of the infarct
size, dilatation of the LV and scar thickness following large
myocardial infarction or sham-operation (pixel-based analysis of
stained cryosections). |
Table III
Histological evaluation of the infarct
size, dilatation of the LV and scar thickness following large
myocardial infarction or sham-operation (pixel-based analysis of
stained cryosections).
| Parameter | Tra (n=16) | Plac (n=13) | ShO (n=9) |
|---|
| Circumference LV,
mm | 26.67±0.85 | 28.32±0.96 | 14.77±0.64a |
| Scar length,
mm | 9.91±0.55 | 10.66±0.46 | |
| Scar, % of
circumference | 37.29±1.80 | 40.44±1.67 | |
| Scar thickness,
mm | 0.705±0.034 | 0.750±0.038 | |
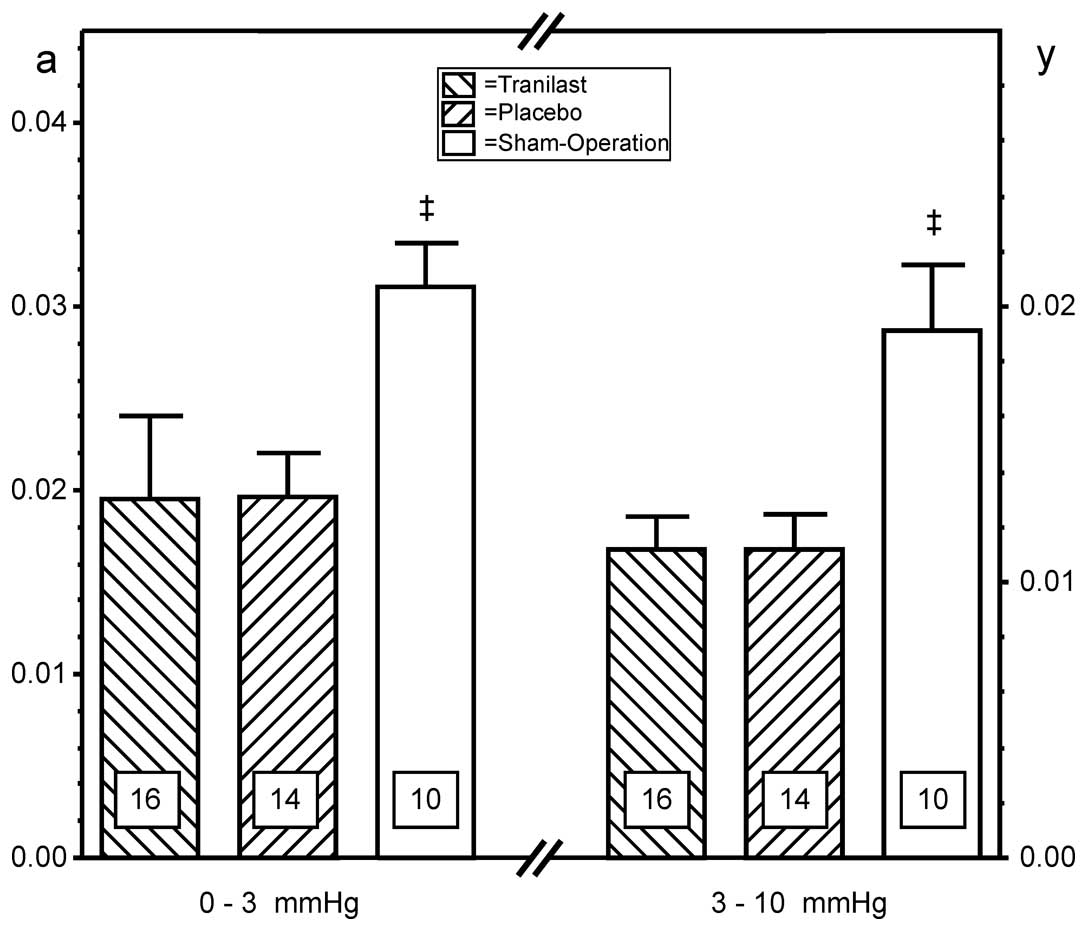
Corresponding to the morphological changes following
MI described above, a decrease of the chamber stiffness variables
‘a’ (pressure range, 0–3 mmHg) and ‘d’ (pressure range, 3–10 mmHg)
was measured in the two treatment groups, without any significant
difference between the treatment groups (Fig. 5).
Non-survivors
Following analysis of the organs from the animals
that succumbed during the treatment period with large MI, no
relevant difference between the treatment groups was found
regarding the weight gain of the animals or the ventricular weight
or the collagen content (histology) in the remote non-infarcted
part of the LV (Table IV). Of
note are the high weights of the ventricles compared with those of
the animals that survived over the study period. This finding can
be interpreted as a sign of an extensive cardiac hypertrophy, which
led to a progressive ventricular failure and thereby to the early
mortality of these animals (Fig.
6).
 | Table IVBody and organ weights and
intracardiac collagen content in the remote non-infarcted part of
the left ventricle obtained from the animals that succumbed during
the treatment period following large myocardial infarction. |
Table IV
Body and organ weights and
intracardiac collagen content in the remote non-infarcted part of
the left ventricle obtained from the animals that succumbed during
the treatment period following large myocardial infarction.
| Parameter | Plac (n=9) | Tra (n=15) |
|---|
| Survival time,
days | 170±26 | 163±18 |
| Collagen content,
%a | 0.98±0.13 | 1.00±0.44 |
| BWR | 1.15±0.13 | 1.11±0.05 |
| LGR | 5.72±0.37 | 5.37±0.24 |
| LVI, mg/g | 3.23±0.17 | 3.13±0.14 |
| RVI, mg/g | 1.36±0.21 | 1.48±0.15 |
Small MIs
Compared with the sham-operated hearts, the
induction of small MIs led to a moderate increase of the weight,
the intracardiac collagen content and a moderate dilatation of the
LV, without reaching statistical significance. The ratios of the
wet/dry weight of the lungs and the weights of the right ventricles
were found at similar levels in all the groups. Chronic left
ventricular failure induced following small MI was not severe
enough to determine pulmonary edema and secondary right ventricular
hypertrophy. Treatment with tranilast led to a lower weight gain
compared with placebo. Regarding the other parameters, no
statistically significant difference could be found between the
treatment groups (Table V).
 | Table VBody and organ weights, collagen
content and results of the pressure volume analysis of the isolated
left ventricle, six months after small myocardial infarction or
sham-operation. |
Table V
Body and organ weights, collagen
content and results of the pressure volume analysis of the isolated
left ventricle, six months after small myocardial infarction or
sham-operation.
| Parameter | Plac (n=9) | Tra (n=11) | ShO (n=18) |
|---|
| BWR | 1.44±0.03a | 1.24±0.05 | 1.30±0.03 |
| LGR | 5.51±0.27 | 5.78±0.22 | 5.51±0.24 |
| LVI, mg/g | 2.31±0.21 | 2.34±0.16 | 2.09±0.06 |
| RVI, mg/g | 0.50±0.05 | 0.54±0.04 | 0.49±0.03 |
| CSV at 0–3
mmHg | 0.022±0.003 | 0.115±0.049 | 0.031±0.002 |
| CSV at 3–10
mmHg | 0.008±0.002 | 0.018±0.003 | 0.019±0.002 |
| Volume 5 mmHg | 398.2±43.2 | 326.7±40.7 | 283.4±16.2 |
| Volume 10 mmHg | 483.7±35.9 | 377.5±33.1 | 323.2±15.3 |
| Volume 15 mmHg | 546.3±33.8 | 411.6±28.1 | 342.7±15.6 |
| Collagen HPLC
(μg/mg) | 41.31±4.83
(n=5) | 39.98±5.87
(n=8) | 29.98±4.18
(n=5) |
Discussion
Treatment with the anti-inflammatory and
antifibrotic drug tranilast, initiated four weeks after the
induction of a large MI in the rat, did not attenuate the cardiac
remodeling in chronic ischemic heart failure. There was no effect
on the six-month mortality rate. These findings indicate that
extremely late tranilast therapy does not positively influence
recovery and remodeling in this setting.
By contrast, animal models of salt hypertensive
rats, spontaneously hypertensive rats, renovascular hypertension
and experimental diabetic cardiomyopathy have been reported to
positively influence by tranilast therapy (13). Cardiac interstitial fibrosis and
remodeling have been shown to improve, however, similarly the
pathogenic mechanisms, as well as the therapeutic interventions,
involve the entire LV in their whole circumference of the per
se viable myocardium. When treatment is successful, the
recovery process also comprises the entire circumference of the LV.
The process of cardiac remodeling in the setting of definite
myocardial infarction is different and can be described as a
response of the remote non-infarcted section of the LV to cell
damage in another area of the heart. These processes involve
changes in ventricular geometry and shape during systolic
contraction. The pathological background consists of myocyte
hypertrophy, apoptosis and interstitial fibrosis in the remote
non-infarcted area. Several studies examined the effects of
revascularization procedures in acute myocardial ischemia.
Therapeutic interventions, such as revascularization procedures,
started in the early phase lead to the salvage of myocardial tissue
at risk and reverse local dysfunction in the ischemic area. When
effective, reverse geometric remodeling is observed and this is
associated with beneficial effects on the left ventricular
function, not only in the ischemic but also in the remote part of
the LV (23). In addition,
coronary revascularization of patients with viable, also known as
hibernating, myocardium in the infarcted zone positively influenced
cardiac remodeling and prevented further major cardiac events, but
these interventions were not effective, if the ventricles were
severely dilated and the ejection fraction was too low (24). In patients undergoing coronary
revascularization during or subsequent to an acute MI, the left
ventricular end-systolic volume could be identified as the most
important discriminator for the development of heart failure and
mortality in patients following MI, irrespective of the
revascularization status (25).
Any therapeutic approach other than revascularization during the
acute phase of the MI may prevent further damage in the remote
non-infarcted area, but the dilatation and change of geometry of
the LV due to necrotic tissue cannot be reversed. In the present
experimental setting, large MIs with prominent LV dilatation were
induced. Starting treatment with tranilast four weeks after the
induction of the large MI was too late to induce reverse cardiac
remodeling. At this time the severe dilatation of the LV that was
found after six months may already have been completed. Recently,
it has been described that tranilast intervention from 24 h post-MI
exacerbated infarct expansion, but delayed the commencement of
treatment to seven days post-MI impeded LV remodeling (19). Taken together with the present
data, starting on day 7 appears to be the optimal timing.
The extremely late time point of treatment
initiation had been chosen to prevent an interaction of tranilast
with the wound healing processes in the infarcted area of the LV
with the risk of the formation of left ventricular aneurysms.
Interactions of this type are described for other anti-inflammatory
drugs administered during the acute phase of MI (26,27).
During this maturation phase, the fibroblasts and vascular cells in
the infarcted area undergo apoptosis. Cross-links are formed
between the collagen bundles of the developing scar. After four
weeks an organized assembly of collagen fibers in terms of scar
tissue is found in the infarcted area of the LV (28). However, this appears not to be of
relevance regarding the study endpoints.
Limitations of the present study include that it was
performed in female Lewis rats. Compared with our previous results
in male Lewis rats, lower levels of interstitial collagen were
found measured in the two methods following ShO, as well as
subsequent to the induction of a large MI (29). Similar results were reported for
the animal model of uni-nephrectomized DOCA plus salt hypertensive
mice. While the ratio of the heart weight to the body weight after
four weeks was significantly increased in male animals, this was
not the case in female animals (30). Gender differences can also be found
concerning the mRNA levels for TGF-β1
(31). The different results in
male rats cannot be excluded. Care was taken during the procedure
of the present study to produce large MIs by ligating the left
anterior descending coronary artery as proximally as possible.
Every animal that succumbed during the study period was autopsied
to ensure that the mortality was caused by a large MI and
subsequent heart failure. At the end of the study period, every
heart was examined and only rats with comparable large MIs were
included in the analysis. The aim was to create comparable study
groups. The major drawbacks of this strategy were the high
mortality rate in the perioperative time (44%) and an additional
loss of animals at the end of the study period due to too small MIs
(18%). There was only a small number of hearts available for the
analysis of cardiac remodeling at the end of the study period.
Treatment with the anti-inflammatory and
antifibrotic drug, tranilast, started four weeks after the
induction of a large MI in the rat, does not attenuate or
positively influence remodeling in chronic ischemic heart failure
and survival rate. Further studies are required to explore the
effects of tranilast on cardiac myocytes post-MI in more
detail.
Acknowledgements
The authors would like to thank Mrs. Martina Voigt
for her excellent technical assistance.
References
|
1
|
Azuma H, Banno K and Yoshimura T:
Pharmacological properties of N-(3′,4′-dimethoxycinnamoyl)
anthranilic acid (N-5′), a new anti-atopic agent. Br J Pharmacol.
58:483–488. 1976.
|
|
2
|
Taniguchi S, Yorifuji T and Hamada T:
Treatment of linear localized scleroderma with the anti-allergic
drug, tranilast. Clin Exp Dermatol. 19:391–393. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamada H, Ide A, Sugiura M and Tajima S:
Treatment of cutaneous sarcoidosis with tranilast. J Dermatol.
22:149–152. 1995.PubMed/NCBI
|
|
4
|
Yamada H, Tajima S, Nishikawa T, Murad S
and Pinnell SR: Tranilast, a selective inhibitor of collagen
synthesis in human skin fibroblasts. J Biochem. 116:892–897.
1994.PubMed/NCBI
|
|
5
|
Suzawa H, Kikuchi S, Ichikawa K and Koda
A: Inhibitory action of tranilast, an anti-allergic drug, on the
release of cytokines and PGE2 from human monocytes-macrophages. Jpn
J Pharmacol. 60:85–90. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adachi S, Maruyama T, Kondo T, Todoroki T
and Fukao K: The prevention of postoperative intraperitoneal
adhesions by tranilast: N-(3′,4′-dimethoxycinnamoyl)anthranilic
acid. Surg Today. 29:51–54. 1999.PubMed/NCBI
|
|
7
|
Nakamura K, Irie H, Inoue M, Mitani H,
Sunami H and Sano S: Factors affecting hypertrophic scar
development in median sternotomy incisions for congenital cardiac
surgery. J Am Coll Surg. 185:218–223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe S, Matsuda A, Suzuki Y, et al:
Inhibitory mechanism of tranilast in human coronary artery smooth
muscle cells proliferation, due to blockade of PDGF-BB-receptors.
Br J Pharmacol. 130:307–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi A, Taniguchi T, Ishikawa Y and
Yokoyama M: Tranilast inhibits vascular smooth muscle cell growth
and intimal hyperplasia by induction of p21(waf1/cip1/sdi1) and
p53. Circ Res. 84:543–550. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamai H1, Katoh O, Suzuki S, et al: Impact
of tranilast on restenosis after coronary angioplasty: tranilast
restenosis following angioplasty trial (TREAT). Am Heart J.
138:968–75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamai H, Katoh K, Yamaguchi T, et al: The
impact of tranilast on restenosis after coronary angioplasty: the
Second Tranilast Restenosis Following Angioplasty Trial (TREAT-2).
Am Heart J. 143:506–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holmes DR Jr, Savage M, LaBlanche JM, et
al: Results of Prevention of REStenosis with Tranilast and its
Outcomes (PRESTO) trial. Circulation. 106:1243–1250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umemura K, Kikuchi S, Suzuki Y and
Nakashima M: Inhibitory effect of tranilast on hypertrophic
collagen production in the spontaneously hypertensive rat heart.
Jpn J Pharmacol. 78:161–167. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kagitani S, Ueno H, Hirade S, Takahashi T,
Takata M and Inoue H: Tranilast attenuates myocardial fibrosis in
association with suppression of monocyte/macrophage infiltration in
DOCA/salt hypertensive rats. J Hypertens. 22:1007–1015. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dewald O, Ren G, Duerr GD, et al: Of mice
and dogs: species-specific differences in the inflammatory response
following myocardial infarction. Am J Pathol. 164:665–677. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohn JN, Ferrari R and Sharpe N: Cardiac
remodeling - concepts and clinical implications: a consensus paper
from an international forum on cardiac remodeling. Behalf of an
International Forum on Cardiac Remodeling. J Am Coll Cardiol.
35:569–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikeuchi M, Tsutsui H, Shiomi T, et al:
Inhibition of TGF-beta signaling exacerbates early cardiac
dysfunction but prevents late remodeling after infarction.
Cardiovasc Res. 64:526–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
See F, Watanabe M, Kompa AR, et al: Early
and delayed tranilast treatment reduces pathological fibrosis
following myocardial infarction. Heart Lung Circ.
Sept.14–2012.(Epub ahead of print).
|
|
20
|
Jung C, Gonon AT, Sjöquist PO, Lundberg JO
and Pernow J: Arginase inhibition mediates cardioprotection during
ischaemia-reperfusion. Cardiovasc Res. 85:147–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeffer MA, Pfeffer JM, Fishbein MC,
Fletcher PJ, Spadaro J, Kloner RA and Braunwald E: Myocardial
infarct size and ventricular function in rats. Circ Res.
44:503–512. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fletcher PJ, Pfeffer JM, Pfeffer MA and
Braunwald E: Left ventricular diastolic pressure-volume relations
in rats with healed myocardial infarction. Effects on systolic
function. Circ Res. 49:618–626. 1981.PubMed/NCBI
|
|
23
|
Carluccio E, Biagioli P, Alunni G, et al:
Patients with hibernating myocardium show altered left ventricular
volumes and shape, which revert after revascularization: evidence
that dyssynergy might directly induce cardiac remodeling. J Am Coll
Cardiol. 47:969–977. 2006.
|
|
24
|
Bax JJ, Schinkel AF, Boersma E, et al:
Extensive left ventricular remodeling does not allow viable
myocardium to improve in left ventricular ejection fraction after
revascularization and is associated with worse long-term prognosis.
Circulation. 110:II18–II22. 2004.PubMed/NCBI
|
|
25
|
Senior R, Lahiri A and Kaul S: Effect of
revascularization on left ventricular remodeling in patients with
heart failure from severe chronic ischemic left ventricular
dysfunction. Am J Cardiol. 88:624–629. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown EJ Jr, Kloner RA, Schoen FJ,
Hammerman H, Hale S and Braunwald E: Scar thinning due to ibuprofen
administration after experimental myocardial infarction. Am J
Cardiol. 51:877–883. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bulkley BH and Roberts WC: Steroid therapy
during acute myocardial infarction. A cause of delayed healing and
of ventricular aneurysm. Am J Med. 56:244–250. 1974. View Article : Google Scholar
|
|
28
|
Weber KT, Sun Y and Cleutjens JP:
Structural remodeling of the infarcted rat heart. EXS. 76:489–499.
1996.PubMed/NCBI
|
|
29
|
Betge S, Lutz K, Roskos M and Figulla HR:
Oral treatment with probucol in a pharmacological dose has no
beneficial effects on mortality in chronic ischemic heart failure
after large myocardial infarction in rats. Eur J Pharmacol.
558:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karatas A, Hegner B, de Windt LJ, et al:
Deoxycorticosterone acetate-salt mice exhibit blood
pressure-independent sexual dimorphism. Hypertension. 51:1177–1183.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tyagi P, Tyagi V, Yoshimura N, et al:
Gender-based reciprocal expression of transforming growth
factor-beta1 and the inducible nitric oxide synthase in a rat model
of cyclophosphamide-induced cystitis. J Inflamm (Lond). 6:232009.
View Article : Google Scholar
|