Introduction
Chronic heart failure (CHF) is one of the most
frequent complications in patients with chronic kidney failure
receiving long-term hemodialysis. Despite the significant mortality
associated with heart failure, there are limited therapeutic
options proven to prevent and treat heart failure in patients on
dialysis. Digoxin is one of the most commonly prescribed drugs for
the treatment of CHF but, as a substantial fraction of the absorbed
dose is cleared by the kidneys, its toxicity is often the result of
an impaired renal function (1).
Digoxin has a small therapeutic-to-toxic margin in patients with
CHF (2), particularly in those
patients who often have renal dysfunction, and it is therefore
logical that, in recent years, numerous physicians have stopped
using digoxin for the treatment of patients with CHF on
hemodialysis.
Accumulative evidence indicates that digoxin
significantly reduces the primary combined end-point of all-cause
mortality or cardiovascular hospitalization in elderly patients
with heart failure at low doses and low serum digoxin
concentrations (SDCs) (3–5). The National Kidney Foundation Kidney
Disease Outcomes Quality Initiative also included digoxin in its
end-stage renal disease cardiovascular disease guidelines for the
treatment of cardiomyopathy and atrial fibrillation (6). On the basis of such evidence, several
studies have attempted to verify the safety of digoxin and how the
drug should be properly managed and monitored in patients with
chronic renal insufficiency (4,7).
Ahmed et al (4) reported
that a low dose (125 μg every other day) may be preferable in
frail, elderly heart-failure patients with impaired kidney
function; however, Chan et al (7) reported that digoxin use at a similar
dose among patients who were on hemodialysis was associated with an
increased mortality, particularly among those with low predialysis
K+ concentrations. There is, therefore, uncertainty
about the long-term efficacy and safety of digoxin in patients with
heart failure undergoing maintenance hemodialysis. Owing to the
discrepancy in the results of the above studies, the objective of
the present study was to determine the effect of digoxin at lower
doses (62.5 μg every other day) on heart function and safety in
heart-failure patients on maintenance hemodialysis.
Materials and methods
Study design
A retrospective cohort study was conducted to
evaluate the effectiveness and safety of lower doses of digoxin in
dialysis patients with symptomatic heart failure and normal sinus
rhythm. Patients received two different doses of digoxin (62.5 μg
every day and 62.5 μg every other day) or received no digoxin as a
matching disease control group. The study was in compliance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Xiangya Hospital of Central South University
(Changsha, China). Informed consent was obtained from each
patient.
Study patients
A total of 67 patients with CHF on maintenance
hemodialysis who were from the Renal Division, Xiangya Hospital of
Central South University and who underwent a 4-h hemodialysis
session five times every two weeks between September 2010 and
September 2013 were included in this study. The patients fulfilled
the 1928 New York Heart Association Functional Classification
(NYHA) revised criteria for CHF (8). Inclusion criteria for this study were
symptomatic heart failure with NYHA functional classification class
II–IV, left ventricular ejection fraction (LVEF) of ≤45%, normal
sinus rhythm and aged ≥50 years. Exclusion criteria were mainly
associated with different types of cardiac arrhythmias. The
patients returned for follow-up visits after 15, 30 and 60 days.
Clinical and laboratory data of the patients were collected prior
to digoxin therapy and at each follow-up visit. Clinical and
laboratory examination, including SDCs, brain natriuretic peptide
(BNP) levels, heart rates (HRs), blood pressure and
echocardiography were performed at baseline and at each follow-up
visit.
Drug therapy
Twenty-four patients received 125 μg digoxin per day
orally for three days and then 62.5 μg every other day
[intermittent low doses of digoxin (ILDD) group]. Twenty-three
patients received 125 μg digoxin per day orally for three days and
then 62.5 μg per day thereafter [continuous low doses of digoxin
(CLDD) group]. Twenty patients who were not using digoxin were
observed as a disease control (control group).
Several patients were taking angiotensin-converting
enzyme inhibitors or angiotensin-receptor antagonists, or
calcium-channel blockers and α- or β-blockers. Recombinant human
erythropoietin (EPO) and calcitriol were administered to those
patients. The EPO (3000U iH three times a week) was administered to
dialysis patients to improve anemia, and Calcitriol (0.25 μg po.
every day) increases blood calcium levels (Ca2+) of
dialysis patients with low calcium and acts in concert with
parathyroid hormone.
Observation of the symptoms of digoxin
toxicity
The symptoms of digoxin toxicity in the ILDD and
CLDD groups were recorded and analyzed in every follow-up visit.
These symptoms included loss of appetite, nausea, vomiting,
diarrhea, headaches, blurred or yellowish-green vision, confusion,
irregular heartbeat and fatigue.
Two-dimensional echocardiography
assessment and measurement of SDCs and BNP levels
Left ventricular end diastolic diameter (LVEDD),
LVEF, cardiac output (CO) and HR were assessed by two-dimensional
echocardiography. ELISA was used to determine SDCs were quantified
by the ELISA kit and this was performed according to the protocol
provided by the supplier (Yueyan Biological Technology, Shanghai,
China). The levels of plasma BNP were quantified by ELISA kits and
performed according to the manufacturer’s instructions (Kaibo
Biological Technology, Shanghai, China) in accordance with the
manufacturer’s instructions. Following baseline assessment and the
introduction of digoxin treatment, patients were monitored for 60
days, with follow-up visits and measurement of heart function, SDCs
and BNP levels after 15, 30 and 60 days.
Statistical analysis
Enumeration data were analyzed with the
χ2 test, and measurement data were analyzed by one-way
analysis of variance or repeated-measures analysis of variance,
followed by Fisher’s protected least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference. Analyses were conducted using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Characteristics of the study
population
The demographic and clinical features of the
patients with heart failure on maintenance hemodialysis are shown
in Table I. In the present study,
no significant differences were observed in the baseline
characteristics among the different groups. The patients returned
for follow-up visits on days 15, 30 and 60. In the period prior to
the third follow-up visit, four patients terminated digoxin therapy
and in four patients digoxin was stopped by the general
practitioner due to presumed digoxin-related side effects (one
patient had an irregular heart beat and three patients had minor
gastrointestinal side effects).
 | Table IDemographic and clinical features of
the patient cohort. |
Table I
Demographic and clinical features of
the patient cohort.
| Characteristic | Control, n=20 | ILDD, n=24 | CLDD, n=23 |
|---|
| Male, n (%) | 15 (75) | 18 (75) | 17 (74) |
| Age in years, mean ±
SD | 65±8 | 66±6 | 66±7 |
| BMI in
kg/m2, mean ± SD | 22.0±3.1 | 21.2±2.8 | 21.5±2.6 |
| Systolic BP in mmHg,
mean ± SD | 155±18 | 152±16 | 153±15 |
| Diastolic BP in mmHg,
mean ± SD | 74±16 | 75±18 | 76±17 |
| Cardiothoracic ratio
>0.55, n (%) | 20 (100) | 24 (100) | 23 (100) |
| NYHA class, n
(%) |
| II | 9 (45) | 11 (46) | 11 (48) |
| III | 10 (50) | 12 (50) | 11 (48) |
| IV | 1 (5) | 1 (4.2) | 1 (4.3) |
| ACE-inhibitors user,
n (%) | 5 (25) | 6 (25) | 5 (22) |
| ARB antagonists user,
n (%) | 5 (25) | 6 (25) | 7 (30) |
| α-blockers user, n
(%) | 5 (25) | 7 (29) | 6 (26) |
| β-blockers user, n
(%) | 10 (50) | 12 (50) | 11 (48) |
| Statins user, n
(%) | 1 (5) | 2 (8) | 2 (8.7) |
| CCB user, n (%) | 20 (100) | 24 (100) | 23 (100) |
| Nitrate user, n
(%) | 10 (50) | 12 (50) | 11 (48) |
| EPO user, n (%) | 20 (100) | 24 (100) | 23 (100) |
| Calcitriol user, n
(%) | 20 (100) | 24 (100) | 23 (100) |
| History of
glomerulonephritis, n (%) | 14 (70) | 17 (71) | 16 (70) |
| History of
hypertension, n (%) | 4 (20) | 5 (21) | 5 (22) |
| History of diabetes,
n (%) | 2 (10) | 2 (8.3) | 2 (8.7) |
| Time on dialysis,
weeks | 5 (2) | 5 (2) | 5 (2) |
| Years on
dialysis | 5.8 | 5.9 | 6.0 |
| Hemoglobin in g/dl,
mean ± SD | 90.5±21.1 | 89.8±20.6 | 90.1±20.3 |
| Calcium in mmol/l,
mean ± SD | 2.09±0.27 | 2.06±0.25 | 2.04±0.23 |
| Phosphorus in mmol/l,
mean ± SD | 2.06±0.71 | 2.03±0.69 | 2.08±0.70 |
Effect of intermittent and continuous low
doses of digoxin on the heart function of patients with heart
failure on hemodialysis
To investigate the effect of intermittent and
continuous low doses of digoxin on the heart function of patients
with heart failure on hemodialysis, the expression level of BNP was
analyzed by ELISA and the changes in LVEDD, CO, LVEF and HR were
assessed by two-dimensional echocardiography. According to
repeated-measures analysis of variance, LVEDD, BNP levels and HR
were significantly decreased between days 0 and 60 in the ILDD and
CLDD groups compared with the values in the disease control group
(all P<0.05). In contrast to the significant decrease in LVEDD,
BNP levels and HR, the values of LVEF and CO were increased between
days 0 and 60 in the ILDD and CLDD groups compared with the values
in the disease control group (all P<0.05); however, over the
entire time-period, the values of BNP, LVEDD, CO, LVEF and HR were
not significantly different between the ILDD and CLDD groups
(P>0.05) (Fig. 1).
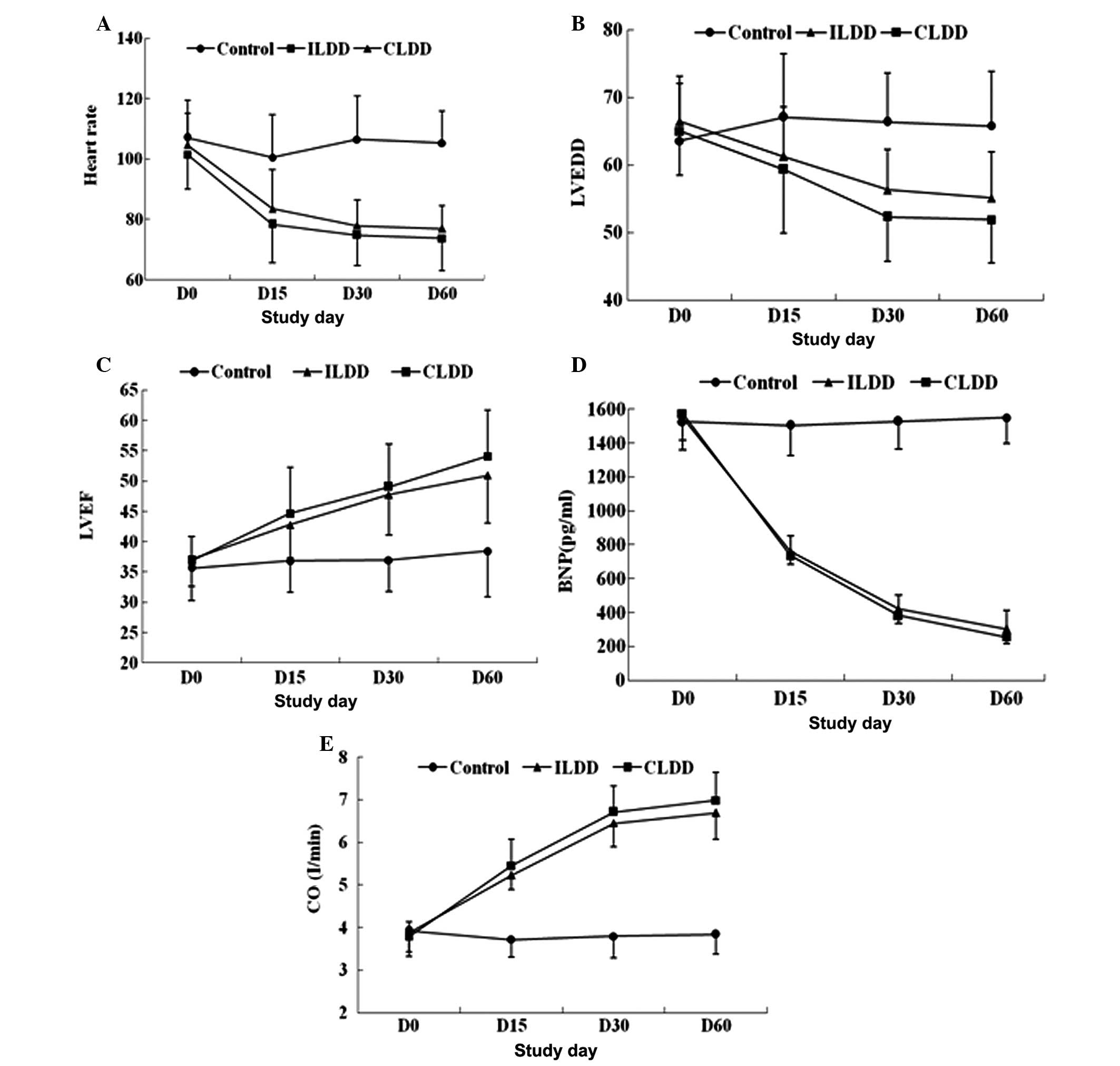 | Figure 1Effect of intermittent and continuous
low doses of digoxin on the heart function of patients with heart
failure undergoing maintenance hemodialysis. (A) Heart rate:
ILDD/CLDD vs. control, P<0.001, ILDD vs. CLDD, P=0.072.
(B) LVEDD: ILDD/CLDD vs. control, P<0.001, ILDD vs. CLDD,
P=0.051. (C) LVEF, ILDD/CLDD vs. control P<0.001: ILDD vs. CLDD,
P=0.149. (D) BNP: ILDD/CLDD vs. control, P<0.001, ILDD vs. CLDD,
P=0.592. (E) CO: ILDD/CLDD vs. control, P<0.001: ILDD vs. CLDD,
P=0.094. Data were analyzed by repeated-measures analysis of
variance. ILDD, intermittent low doses of digoxin; CLDD, continuous
low doses of digoxin; LVEDD, left ventricular end diastolic
diameter; LVEF, left ventricular ejection fraction; BNP, brain
natriuretic peptide; CO, cardiac output. |
Symptoms of digoxin toxicity and SDCs
following intermittent and continuous low-dose digoxin
administration
To evaluate the safety of digoxin in patients with
heart failure on maintenance hemodialysis, the most common symptoms
of digoxin toxicity were monitored in the ILDD and CLDD groups. In
the ILDD group, the 24 patients had no apparent signs or symptoms
of toxicity; however, four patients developed definite digoxin
toxicity in the CLDD group and digoxin administration was therefore
discontinued in those four patients (Table II). The mean SDC was 0.55 ng/ml in
the ILDD group, while in the CLDD group it was 0.71 ng/ml (Table III). Taken with previous results
that increasing SDCs were associated with increased mortality
(7), the findings suggested that
intermittent low-dose digoxin is safer than continuous low-dose
digoxin.
 | Table IISymptoms of digoxin toxicity in the
ILDD and CLDD groups. |
Table II
Symptoms of digoxin toxicity in the
ILDD and CLDD groups.
| Symptoms | ILDD, n=24 | CLDD, n=23 |
|---|
| Loss of appetite, n
(%) | 0/24 (0) | 1/23 (4.3) |
| Nausea, n (%) | 0/24 (0) | 1/23 (4.3) |
| Vomiting, n (%) | 0/24 (0) | 0/23 (0) |
| Diarrhea, n (%) | 0/24 (0) | 1/23 (4.3) |
| Dizziness and
headaches, n (%) | 0/24 (0) | 0/23 (0) |
| Blurred or
yellowish-green vision, n (%) | 0/24 (0) | 0/23 (0) |
| Confusion, n (%) | 0/24 (0) | 0/23 (0) |
| Fast or slow heart
rate, n (%) | 0/24 (0) | 0/23 (0) |
| Irregular heart beat,
n (%) | 0/24 (0) | 1/23 (4.3) |
| Feelings of a racing,
pounding or forcefully beating heart, n (%) | 0/24 (0) | 0/23 (0) |
| Fatigue and weakness,
n (%) | 0/24 (0) | 0/23 (0) |
 | Table IIISerum digoxin concentrations of the
ILDD and CLDD groups. |
Table III
Serum digoxin concentrations of the
ILDD and CLDD groups.
| Detection time | ILDD (ng/ml) | CLDD (ng/ml) |
|---|
| Digoxin use 15
days | 0.55±0.18 | 0.73±0.17 |
| Digoxin use 30
days | 0.52±0.13 | 0.71±0.15 |
| Digoxin use 60
days | 0.57±0.11 | 0.69±0.18 |
Discussion
Digoxin, as an inexpensive drug, has been used for
>200 years for the treatment of cardiovascular disease, and it
still has an important place in the management of patients with
CHF. A clinical study demonstrated reductions in one-year mortality
when digoxin was used at a low SDC of 0.5–0.9 ng/ml, corresponding
to ≤125 μg/day (9). Other
retrospective analyses of the Digitalis Investigation Group trial
also showed that digoxin used at similarly low SDCs had beneficial
effects on morbidity and mortality (10,11);
thus, low-dose digoxin has been clearly shown to have beneficial
clinical effects.
Digoxin is eliminated from the body by the kidneys
(12). The drug can remain in the
body for 36–48 h in individuals with normal kidney function but may
take between three and five days to clear in patients with renal
insufficiency. This means that the drug may accumulate in patients
with renal function impairment, with patients on dialysis being
more likely to develop high SDCs and digoxin toxicity than other
patients (12). Digoxin is mostly
stored in the skeletal tissues rather than the blood and is not
effectively removed by dialysis or exchange transfusion, which
results in the underuse of digoxin in patients with heart failure
on dialysis; therefore, evaluating the safety of prescribing
digoxin is important for patients who are undergoing long-term
renal replacement therapy.
Ahmed et al (4) reported that a low dose of 125 μg
digoxin every other day may be the preferred option in frail,
elderly heart-failure patients with impaired kidney function. Chan
et al (7), however, studied
4,549 incident hemodialysis patients who were digoxin users, and
the results showed that the median prescribed dosage was 62.5
μg/day (125 μg every other day) and the median serum level was 1.0
ng/ml within this particular cohort of digoxin users. In addition,
digoxin use was associated with a 28% increased risk of mortality,
while an increasing serum digoxin level was also associated with
mortality. This increased mortality risk with serum digoxin level
was most apparent in patients with lower predialysis serum
K+ levels. These results suggest that the use of digoxin
by patients who are on hemodialysis is associated with increased
mortality, particularly among those with low predialysis
K+ concentrations.
In the present study, the effect of ILDD on the
heart function of dialysis patients with CHF was observed. The
results showed that the LVEDD, BNP level and HR were significantly
decreased while the LVEF and CO were increased between days 0 and
60 in the digoxin groups compared with those in the control group.
No significant difference was found for the expression of BNP,
LVEDD, CO, LVEF and HR between days 0 and 60 in the CLDD and ILDD
groups. ELISA was subsequently used to measure SDCs, and showed
that the median serum level was 0.55 ng/ml in the ILDD group and
0.71 ng/ml in the CLDD group. No evident digoxin intoxication was
found in the ILDD group. These findings suggested that intermittent
lower doses of digoxin are effective and safe in patients with CHF
on hemodialysis.
According to the possible mechanistic explanations
for digoxin action, digoxin not only directly inhibits the
Na+/K+-ATPase pump in the membrane of the
cardiac myocyte but also inhibits the
rennin-angiotensin-aldosterone system by inhibiting the
Na+/K+-ATPase in the renal tubules (4). The effects of digoxin have long been
known to be dependent on digoxin dose and SDC (4); however, the favorable effects of
digoxin at low SDCs on natural end-points, such as all-cause
hospitalizations and mortality, are likely to be mediated via the
neurohormonal-modulating properties of digoxin (4). It has been suggested that the
neurohormonal properties of digoxin are best exerted at low SDCs
(4).
In the present study, consistent with previous
studies (4,7), it was demonstrated that a lower dose
of digoxin could improve the heart function of patients on
dialysis. Furthermore, the results indicated that ILDD and CLDD
were similarly effective in patients on dialysis, with the SDC in
the ILDD group being 0.55 ng/ml. Digoxin is a drug with a narrow
therapeutic-to-toxic margin, whereby patient-level
pharmacokinetics, metabolism and clearance factors aggregate to
introduce variability in drug responsiveness (7). Higher SDCs have been shown to
increase mortality, yet lower SDCs reduce morbidity and mortality
(13). Chan et al (7) reported that each 1 ng/ml increase in
SDC significantly increased the risk of mortality by 19%. The
results in the present study showed that the SDC in the ILDD group
was considerably lower than that in the CLDD group. The most common
symptoms of digoxin toxicity were monitored in the ILDD and CLDD
groups. In the ILDD group, the 24 patients had no apparent signs or
symptoms of toxicity; however, four patients developed definite
digoxin toxicity in the CLDD group. When these findings are
considered together, an intermittent low dose of digoxin is
believed to be safe and has clearly been shown to have beneficial
clinical effects on patients with CHF undergoing hemodialysis.
In conclusion, despite the small sample in this
study, the results of the present analysis demonstrate that the use
of digoxin in intermittent lower doses plays an important role in
improving quality of life and easing the burden on dialysis
patients with heart failure. These data may provide a new stimulus
to further evaluate the safety and effectiveness of lower-dose
digoxin in dialysis patients with symptomatic heart failure through
a large, multicenter randomized clinical trial in the future.
References
|
1
|
Pervaiz MH, Dickinson MG and Yamani M: Is
digoxin a drug of the past? Cleve Clin J Med. 73:821–824.
826829–832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lambert C and Rouleau JL: How to
digitalize and to maintain optimal digoxin levels in congestive
heart failure. Cardiovasc Drugs Ther. 2:717–726. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed A and Waagstein F: Low-dose digoxin
and reduction in mortality and morbidity in heart failure. Int J
Cardiol. 136:91–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed A: Digoxin and reduction in
mortality and hospitalization in geriatric heart failure:
importance of low doses and low serum concentrations. J Gerontol A
Biol Sci Med Sci. 62:323–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Digitalis Investigation Group. The effect
of digoxin on mortality and morbidity in patients with heart
failure. N Engl J Med. 336:525–533. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
K/DOQI clinical practice guidelines on
cardiovascular disease in dialysis patients: Overview of the
epidemiology of cardiovascular disease. Am J Kidney Dis. 45(Suppl
3): S8–S9. 2005.
|
|
7
|
Chan KE, Lazarus JM and Hakim RM: Digoxin
associates with mortality in ESRD. J Am Soc Nephrol. 21:1550–1559.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The Criteria Committee of the New York
Heart Association. Nomenclature and Criteria for Diagnosis of
Diseases of the Heart and Great Vessels. 9th ed. Little, Brown
& Co; Boston, USA: pp. 253–256. 1994
|
|
9
|
Digitalis Investigation Group. Ahmed A,
Waagstein F, Pitt B, et al: Effectiveness of digoxin in reducing
one-year mortality in chronic heart failure in the Digitalis
Investigation Group trial. Am J Cardio. 103:82–87. 2009.PubMed/NCBI
|
|
10
|
Ahmed A, Rich MW, Love TE, et al: Digoxin
and reduction in mortality and hospitalization in heart failure: a
comprehensive post hoc analysis of the DIG trial. Eur Heart J.
27:178–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed A, Pitt B, Rahimtoola SH, et al:
Effects of digoxin at low serum concentrations on mortality and
hospitalization in heart failure: a propensity-matched study of the
DIG trial. Int J Cardiol. 123:138–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iisalo E: Clinical pharmacokinetics of
digoxin. Clin Pharmacokinet. 2:1–16. 1977. View Article : Google Scholar
|
|
13
|
Cayley WE Jr: Digoxin in chronic heart
failure. Fam Pract. 21:469–475. 2004. View Article : Google Scholar
|















