Introduction
Celiac disease (CD) is an autoimmune-mediated
enteropathy, characterized by gluten-triggered small bowel mucosal
lesions in genetically susceptible individuals. The current
diagnostic criteria for CD require intestinal mucosal villous
atrophy and the presence of serum anti-transglutaminase type 2
(anti-TG2) antibodies, both of which disappear when the patient
adopts a gluten-free diet (GFD) (1). Patients with CD have an increased
risk of developing other autoimmune disorders, in particular, type
1 diabetes (T1D) (2). T1D and
these pathologies are considered to be related by a common genetic
background. All of these diseases are associated with
organ-specific autoantibodies that can be detected prior to the
development of clinical symptoms. In particular, the first
association between CD and T1D was suggested in 1969, and the
genetic risk factors associated with the two diseases include human
leukocyte antigen (HLA) genes and non-HLA genes (3).
In this context, previous studies have suggested
that the TNF superfamily member TNF-related apoptosis-inducing
ligand (TRAIL), a mediator of the immune system with anti-cancer
activity (4,5), also plays an important role in the
control of autoimmune diseases (6), and in particular in T1D (7–9). As
compared with other TNF family members, TRAIL has a complex biology
since it interacts with four transmembrane receptors (TRAIL-R1,
-R2, -R3 and -R4) (4) and one
soluble receptor (osteoprotegerin) (10).
On these bases, the aim of the present study was to
analyze the serum levels of TRAIL in patients with celiac disease,
studying a pediatric retrospective cohort, including patients with
overt CD prior to and following 6–12 months on a gluten-free diet,
patients with potential CD, patients with CD associated with other
autoimmunities, and patients with eosinophilic esophagitis.
Materials and methods
Study population
Sera of pediatric individuals followed at the
Institute for Maternal and Child Health - IRCCS ‘Burlo Garofolo’ of
Trieste, Italy were obtained from: i) patients with CD at onset
(n=100) and from the same CD patients after six (n=49) and 12
(n=13) months of gluten-free diet; ii) patients with potential CD
(n=45); iii) patients with CD associated with other auto-immune
diseases (n=17); and iv) patients with eosinophilic esophagitis
(n=15). Parents/caregivers of all patients provided informed
consent to blood sample drawing and storage for research purposes,
in accordance with the Declaration of Helsinki of 1975. Data
reported in Table I include the
age and gender of the patients and the diagnosis. The study was
approved by the Independent Bioethics Committee of the Institute
for Maternal and Child Health-IRCCS ‘Burlo Garofolo’ (Trieste,
Italy).
 | Table IDescription of the sample of patients
on which circulating levels of TRAIL were measured. |
Table I
Description of the sample of patients
on which circulating levels of TRAIL were measured.
| Pathologies | No. (%) | Age, yearsa | Male gender (%) | TRAIL, pg/mla | P-valueb |
|---|
| CD | 100 (56.5) | 6.3 (3.7–9.9) | 35 (35) | 100.0
(79.3–124.2) | Reference |
| Potential CD | 45 (25.4) | 6.6 (3.9–10.7) | 20 (44) | 93.6
(79.8–113.3) | 0.315 |
| CD + other
autoimmunity | 17 (9.6) | 9.1 (4.1–16.5) | 4 (24) | 86.0 (75.1–92.4) | 0.047 |
| Eosinophilic
esophagitis | 15 (8.5) | 12.8 (10.1–15.6) | 12 (80) | 88.1
(75.6–138.0) | 0.684 |
| Total | 177 (100.0) | 7.1 (3.9–11.0) | 71 (40) | 94.7
(78.9–119.9) | |
TRAIL ELISA assay
Serum levels of TRAIL were measured in duplicate
with a Human TRAIL/TNFSF10 Quantikine ELISA kit (R&D Systems,
Minneapolis, MN, USA) following the manufacturer’s instructions, as
previously described (11,12). Selected samples were run in each
ELISA plate as internal controls, confirming the reproducibility of
determinations over time.
Statistical analysis
Box plots were used to represent the distribution of
values in different groups of patients. After verifying that TRAIL
serum values did not distribute normally (skewness and kurtosis
joint normality test), the non-parametric Mann-Whitney rank-sum
test was applied to compare the TRAIL values among different
populations. The Wilcoxon signed-rank test for paired samples was
used to compare the levels of TRAIL at onset with those following 6
and 12 months on a gluten-free diet. Correlation coefficients were
calculated using Spearman’s rank correlation coefficient rho. A
P-value <0.05 was considered to indicate a statistically
significant difference. All analyses were conducted using Stata/IC
11.2 software for Windows (Stata Corp LP, College Station, TX,
USA).
Results
Determination of the levels of
circulating TRAIL in patients with CD at onset
As mentioned in Materials and methods, the cohort of
individuals analyzed in the present study included: 100 patients
with CD at onset, 45 patients with potential CD, 17 patients with
CD associated with another autoimmunity, and 15 patients with
eosinophilic esophagitis (Table
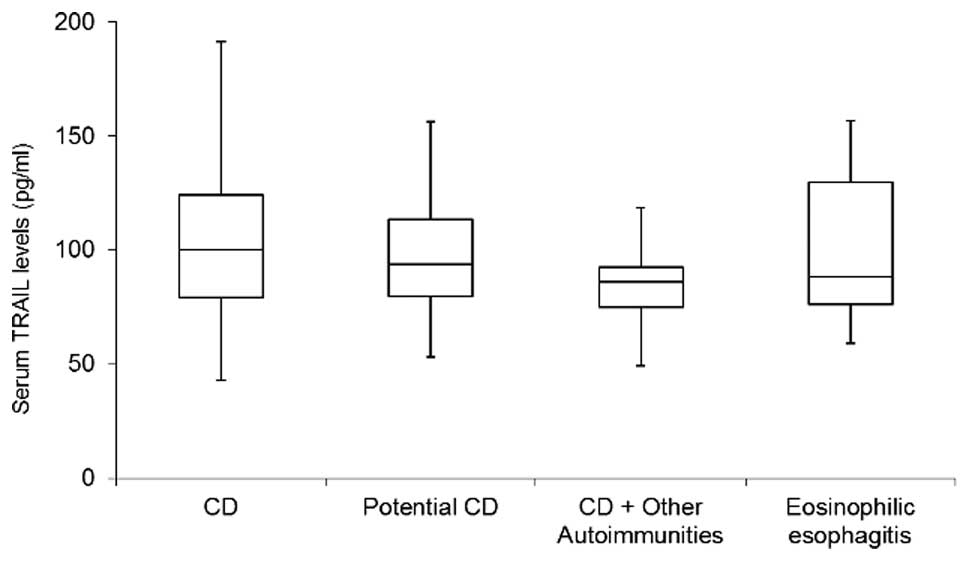
I). No significant differences among the groups were observed
in relation to the male to female ratio and no significant
correlation was observed between serum TRAIL levels and the age of
the patients. The circulating levels of TRAIL assessed in patients
with CD were not significantly different when compared with those
in patients with potential CD, nor when compared with those in
patients with eosinophilic esophagitis (Table I, Fig.
1). Notably, the analysis of TRAIL levels between patients with
CD with and without other concomitant autoimmune disorders (T1D,
Hashimoto’s thyroiditis, Addison’s disease, vitiligo, autoimmune
atrophic gastritis and psoriasis) revealed significantly lower
levels in patients with other autoimmune disorders (P=0.047). This
suggests that the concomitant presence of other autoimmune diseases
impacts on the circulating levels of TRAIL in CD.
Determination of the levels of
circulating TRAIL in patients with CD at follow-up
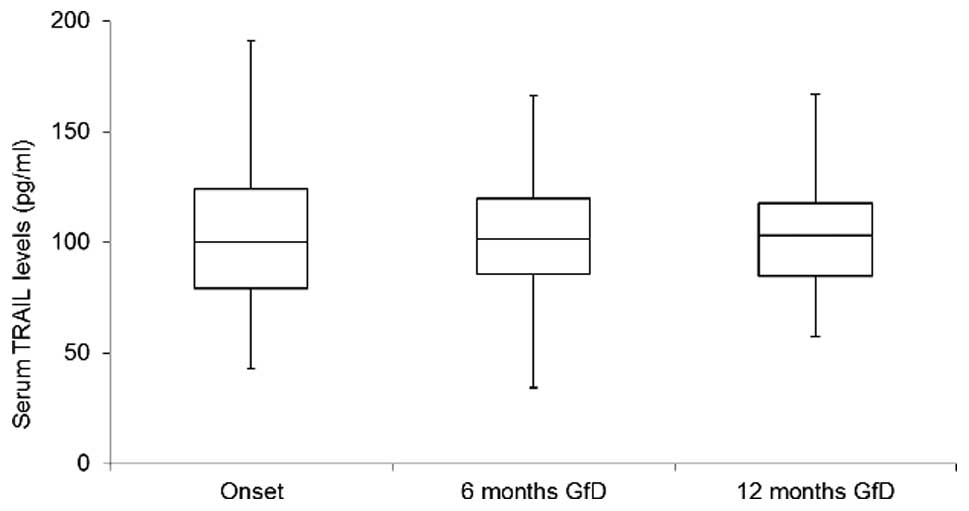
In the next group of experiments, in the cohort of
CD patients, the serum levels of TRAIL were evaluated after 6 and
12 months on a gluten-free diet. TRAIL values at disease onset were
not significantly different from the levels measured after six
months on a gluten-free diet (n=49; P=0.773; Fig. 2). These data were confirmed also
for a small subset of patients (n=13) for which data were also
available after 1 year of a gluten-free diet (difference between
onset and 12 months: P=0.279; difference between 6 and 12 months:
P=0.553).
Discussion
By analyzing a cohort of pediatric patients affected
by CD, the current study has demonstrated that the circulating
levels of TRAIL are not significantly different in CD patients with
respect to those in patients with potential CD or eosinophilic
esophagitis, while the circulating levels of TRAIL are
significantly lower in patients with CD and concomitant autoimmune
disorders when compared with those in CD patients with no such
disorders. In addition, the levels of circulating TRAIL remained
stable over a 6–12 month follow-up performed on a fraction of the
same CD patients that continued to receive a gluten-free diet.
From these results, it may be inferred that the
behavior of circulating TRAIL profoundly differs between T1D and
CD. In fact, it has previous been demonstrated that circulating
levels of TRAIL significantly decrease in T1D, with the lowest
levels of TRAIL being documented in T1D patients with diabetic
ketoacidosis (DKA) and depending on the severity of the disease
(10). Moreover, TRAIL serum
levels at T1D onset showed an inverse correlation with the insulin
requirement at different time points up to 21 months of follow-up
(10) and these data were in
agreement with an independent study performed on T2D, which showed
that circulating levels of TRAIL increased in patients with insulin
therapy (13).
The present study suggests that the association
between the circulating levels of TRAIL and the severity of the
auto-immune reaction should be further explored, taking into
account that CD patients with other concomitant auto-immune
diseases showed significantly lower levels as compared with those
in CD patients without concomitant auto-immune diseases. The lack
of a correlation between the natural history and/or the response to
therapy in CD, and the circulating levels of TRAIL in CD is
suggested by the lack of differences observed between CD patients
prior to and following a gluten-free diet. By contrast, the fact
that TRAIL levels are significantly lower in T1D patients with DKA
at onset and with a higher insulin requirement, points to a
possible association between TRAIL and the metabolic stress
underlying T1D (10). Thus, unlike
with other cytokines, which appear to be modulated in CD (14–16),
circulating levels of TRAIL do not appear to change, despite the
ability of TRAIL to modulate the immune response in different
experimental settings. It remains to be demonstrated whether TRAIL
might be modulated at the gut level.
Acknowledgements
CC, TN and PS designed the study; AN performed the
experiments; LM performed the statistical analyses; CC and TN wrote
the first draft of the manuscript under the supervision of PS; and
all authors contributed to the final editing of the manuscript.
References
|
1
|
Green PH and Cellier C: Celiac disease. N
Engl J Med. 357:1731–1743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scaramuzza AE, Mantegazza C, Bosetti A and
Zuccotti GV: Type 1 diabetes and celiac disease: The effects of
gluten free diet on metabolic control. World J Diabetes. 4:130–134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hooft C, Roels H and Devos E: Diabetes and
coeliac disease. Lancet. 2:11921969.PubMed/NCBI
|
|
4
|
Secchiero P and Zauli G: TNF-related
apoptosis-inducing ligand and the regulation of hematopoiesis. Curr
Op Hematol. 15:42–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Secchiero P, Gonelli A, Celeghini C, et
al: Activation of the nitric oxide synthase pathway represents a
key component of tumor necrosis factor-related apoptosis-inducing
ligand-mediated cytotoxicity on hematologic malignancies. Blood.
98:2220–2228. 2001. View Article : Google Scholar
|
|
6
|
Vinay DS and Kwon BS: The tumour necrosis
factor/TNF receptor superfamily: therapeutic targets in autoimmune
diseases. Clin Exp Immunol. 164:145–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Bartolo BA, Chan J, Bennett MR,
Cartland S, Bao S, Tuch BE and Kavurma MM: TNF-related
apoptosis-inducing ligand (TRAIL) protects against diabetes and
atherosclerosis in ApoE−/− mice. Diabetologia.
54:3157–3167. 2011.PubMed/NCBI
|
|
8
|
Zauli G, Toffoli B, di Iasio MG, Celeghini
C, Fabris B and Secchiero P: Treatment with recombinant tumor
necrosis factor-related apoptosis-inducing ligand alleviates the
severity of streptozotocin-induced diabetes. Diabetes.
59:1261–1265. 2010. View Article : Google Scholar
|
|
9
|
Tornese G, Iafusco D, Monasta L, et al:
The levels of circulating TRAIL at the onset of type 1 diabetes are
markedly decreased in patients with ketoacidosis and with the
highest insulin requirement. Acta Diabetol. 51:239–246. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zauli G, Melloni E, Capitani S and
Secchiero P: Role of full-length osteoprotegerin in tumor cell
biology. Cell Molecular Life Sci. 66:841–851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volpato S, Ferrucci L, Secchiero P, et al:
Association of tumor necrosis factor-related apoptosis-inducing
ligand with total and cardiovascular mortality in older adults.
Atherosclerosis. 215:452–458. 2011. View Article : Google Scholar
|
|
12
|
Secchiero P, Corallini F, Ceconi C,
Parrinello G, Volpato S, Ferrari R and Zauli G: Potential
prognostic significance of decreased serum levels of TRAIL after
acute myocardial infarction. PloS One. 4:e44422009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Secchiero P, Corallini F, Beltrami AP, et
al: An imbalanced OPG/TRAIL ratio is associated to severe acute
myocardial infarction. Atherosclerosis. 210:274–277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiang G, Zhang J, Ling Y and Zhao L:
Circulating level of TRAIL concentration is positively associated
with endothelial function and increased by diabetic therapy in the
newly diagnosed type 2 diabetic patients. Clin Endocrinol (Oxf).
80:228–234. 2013. View Article : Google Scholar
|
|
15
|
Lahdenperä AI, Hölttä V, Ruohtula T, et
al: Up-regulation of small intestinal interleukin-17 immunity in
untreated coeliac disease but not in potential coeliac disease or
in type 1 diabetes. Clin Exp Immunol. 167:226–234. 2012.PubMed/NCBI
|
|
16
|
Pagliari D, Cianci R, Frosali S, Landolfi
R, Cammarota G, Newton EE and Pandolfi F: The role of IL-15 in
gastrointestinal diseases: A bridge between innate and adaptive
immune response. Cytokine Growth Factor Rev. 24:455–466. 2013.
View Article : Google Scholar : PubMed/NCBI
|