Introduction
Spinal cord injury (SCI) is the main cause of
paraplegia, but the effective therapies for it are lacking.
Experimental studies have shown that SCI consists of primary and
secondary damage. The majority of the primary damage of SCI is due
to external mechanical stress (1–5). The
degree of secondary damage is influenced by a number of
injury-promoting factors including edema, ischemia, increased
levels of neurotransmitters, increased intracellular
Ca2+ levels and the presence of free radicals or nitric
oxide (NO). In particular, excitatory amino acids (EAAs) are
thought to play an important role in the secondary autodestruction
of neural tissue following SCI. EAA levels increase following
spinal cord trauma in proportion to the severity of injury and this
increase exacerbates paralysis in rats, whereas the administration
of antagonists of N-methyl-D-aspartate (NMDA) receptors, which are
postsynaptic receptors for EAAs, significantly alleviates this
paralysis (6–9).
Studies have shown that embryo spinal cord
transplantation is able to repair SCI, although a large amount of
neuronal apoptosis remains in the spinal cord, and indicate that
the NMDA receptor antagonist MK-801 may reduce the cell death by
decreasing the concentration of EAAs and preventing extracellular
calcium ion influx (10–13). In recent years, the concept of
apoptosis in the central nervous system has been introduced to
explain the process of ischemia and trauma. Also, it has been found
that some neurons and glial cells are apoptotic following SCI
(10,14,15).
In addition, the overstimulation of glutamate receptors is toxic to
neurons and glial cells and is involved in processes culminating in
programmed cell death (3,4). Dizocilpine maleate (MK801) is a
potent non-competitive NMDA receptor antagonist that blocks the
excitotoxic sequelae of ischemia in tissue cultures and animal
models of cerebral ischemia, reduces infarct size and improves
neurological outcome (5). The
efficacies of several NMDA antagonist drugs have been studied in
various models of SCI, conducive SCI or in ischemic lesions of the
rat spinal cord (11,12,16–19);
however, to the best of our knowledge, studies using MK801 to
prevent apoptosis in rats that have undergone fetal spinal cord
(FSC) transplantation following spinal hemisection have not been
reported. Terminal deoxynucleotidyl transferase-mediated
dUTP-biotin nick end labeling (TUNEL) reaction and
immunohistochemical analysis of B-cell lymphoma-2 (Bcl-2) were used
to investigate the effect of the NMDA receptor antagonist MK-801 on
apoptosis in rats that have undergone FSC transplantation to treat
spinal hemisection.
Materials and methods
Experimental animals
Adult Wistar rats (male and female, 180–250 g) were
obtained from Vital River Laboratories (Beijing, China) and used in
this study. Seventy-two Wistar rats were randomly divided into
three groups: Spinal cord hemisection injury with a combination of
FSC transplantation and MK-801 treatment (group A); spinal cord
hemisection injury with FSC transplantation site (group B); and
spinal cord injury with insertion of a Gelfoam pledget (group C).
The rats were sacrificed 1, 3, 7 and 14 days subsequent to the
surgery. Six animals from each group were sacrificed at each
time-point. The animals were transcardially perfused with
heparinized saline (0.9%) followed by a solution consisting of 4%
paraformaldehyde in 10 mM phosphate-buffered saline (PBS) solution,
pH 7.4. The spinal cord was dissected out, and blocks were prepared
for cryostat sectioning. Blocks rostral and caudal to the lesion
area and blocks containing the lesion/transplant 1 cm respectively
were cut in serial transverse 20-μm sections. The apoptosis of
spinal slices from the spinal cord was examined by TUNEL reaction
and the expression of Bcl-2 was evaluated by immunohistochemistry.
The positive cells were quantitatively analyzed using an image
analysis system (Tiger Image Analysis system; ICT Research
Institute of Chongqing University, Chongqing, China). The animals
used in this study were maintained in accordance with the Guide for
the Care and Use of Laboratory Animals published by the U.S.
National Institutes of Health (20) and the Policy of Animal Care and Use
Committee of the Beijing Ditan Hospital of Capital Medical
University (Beijing, China).
Preparation of spinal cord
transplants
Timed-pregnant rats were used to provide embryonic
spinal cord transplants for insertion into the injured spinal cord
of allogeneic rats. Pregnant female rats were anesthetized at 14
days of gestation (E14). The fetuses were removed individually as
donor tissue was required and maintained in sterile culture medium.
The FSCs were dissected and 1–3 mm3 segments of the cord
were prepared for transplantation.
Transplantation methods
Adult rats were anesthetized with 1% pentobarbital
sodium (35 mg/kg body weight, intraperitoneal). The backs of the
rats were shaved, and the rats were placed on a stereotaxic frame.
A laminectomy was performed at the T12-T13 vertebral level. An
incision was made with a fine scalpel blade through the meningeal
membranes. Lumbar enlargement spinal cord tissue was removed by
vacuum aspiration through the meningeal cut, creating a nearly
complete hemisection cavity (2×1×1 mm3) sparing only
left lateral parts. In group C, the rats were hemisected only and
Gelfoam of the same size as the lesion was grafted into the lesion
cavity. In group B, a solid piece of FSC tissue, ~2×1×1
mm3 in length, was drawn up into a sterile glass pipette
and gently placed into the cavity subsequent to hemostasis being
achieved. The overlying dura was closed with 9-0 Prolene suture and
Durafilm was placed over the lesion site. The paraspinal
musculature and subcutaneous tissues were closed subsequently with
an absorbable suture. In group A, the rats were treated in the same
manner as those in group B and were also injected with MK-801 at a
dose of 3 mg/kg by tail vein 30 min and 6 h following the surgical
procedures.
Nissl staining
Staining of paraffin sections of 10 μm after 2 times
of xylene and graded alcohol dewaxing to water, then add working
fluid dye for 10 mins at 37°C. Then add distilled water for about
30 secs after washing and dehydration of 70% alcohol added in 2
mins, then add 0.01% ethanol and eosin solution under the
microscope color separation, color separation after the slice with
alcohol dehydration, xylene transparent, finally using DPX sealing
sections. The staining results for Nissl bodies and nucleolus were
purple blue, pink background. The solution consists of 1% azure II
aqueous solution, 1% cresyl violet solution, 0.2 M acetate, 0.2 M
sodium acetate, absolute ethyl alcohol, double distilled water.
Make a total volume of 100 ml in pH 4.0.
Fluorescence TUNEL
Frozen 20-μm sections were analyzed according to the
instructions provided by the manufacturer of the Fluorescein In
situ Cell Death Detection kit (Boehringer Mannheim Inc.,
Mannheim, Germany). The positively labeled cells were identified
using light microscopy (Olympus, Tokyo, Japan). An apoptosis index
(AI) was used to estimate the fraction of apoptotic cells according
to the following formula: AI = number of cells with apoptotic
nuclei per low-power field (lpf)/total number of cell nuclei per
lpf.
Immunohistochemical analysis of
Bcl-2
Antibodies to Bcl-2 (PR-0257; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used according to the
methods provided with the streptavidin-peroxidase kit (Zhongshan
Goldenbridge, Beijing, China). Positively labeled cells were
identified using light microscopy. The positive cells were
identified in nine slices (three slices from the lesion area,
rostral and caudal segments, respectively), randomly. The positive
cells (cells/mm2) were quantitatively analyzed using a
computer image analysis system (Tiger 920G Image Analysis system,
ICT Research Institute of Chongqing University, Chongqing,
China).
Statistical analysis
Quantitative image analysis was performed on
sections immunostained for Bcl-2 and in the determination of the AI
using the image analysis system. The means and standard errors of
each intervention group were calculated, and a one-way analysis of
variance was performed. Differences between two groups were
examined for significance using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
Nissl staining
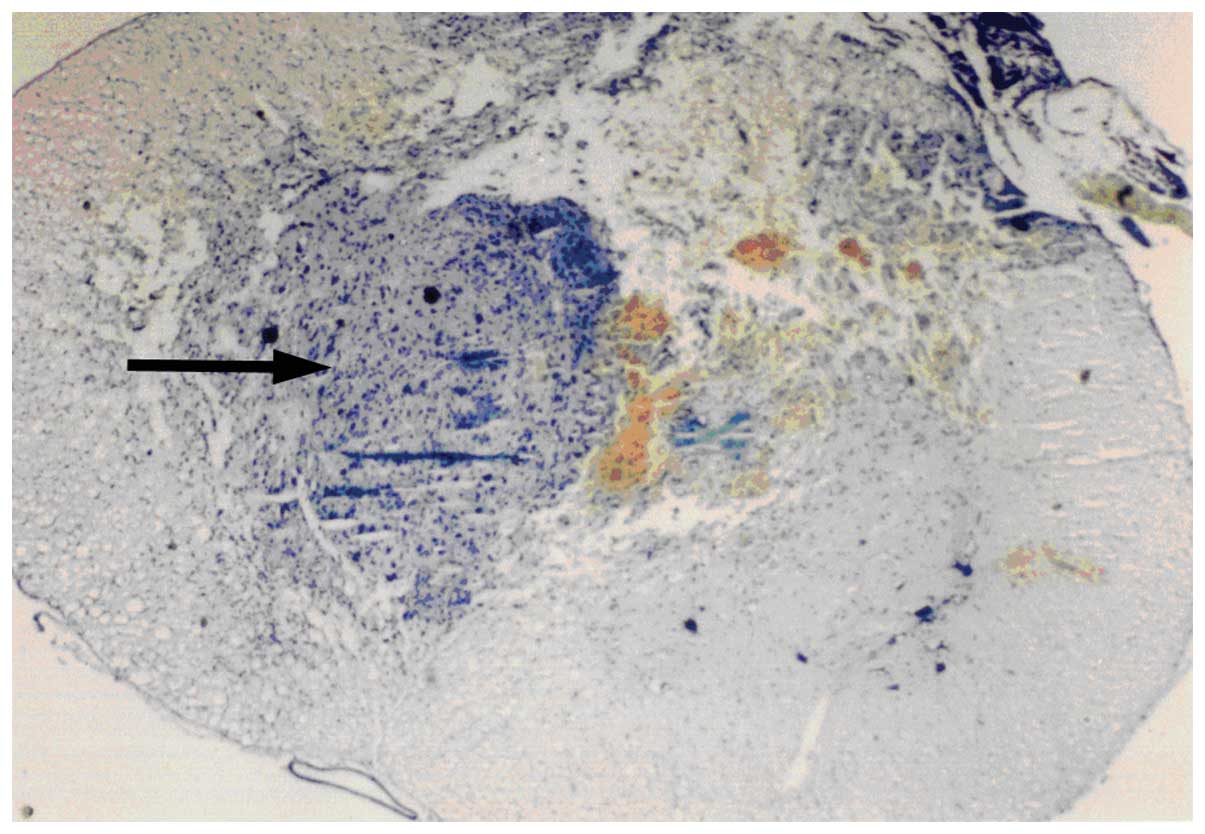
A microphotograph at 14 days after spinal cord
injury of a fetal spinal cord tissue transplant is shown in
Fig. 1. The arrow shows the
transplant from the fetal spinal cord tissue.
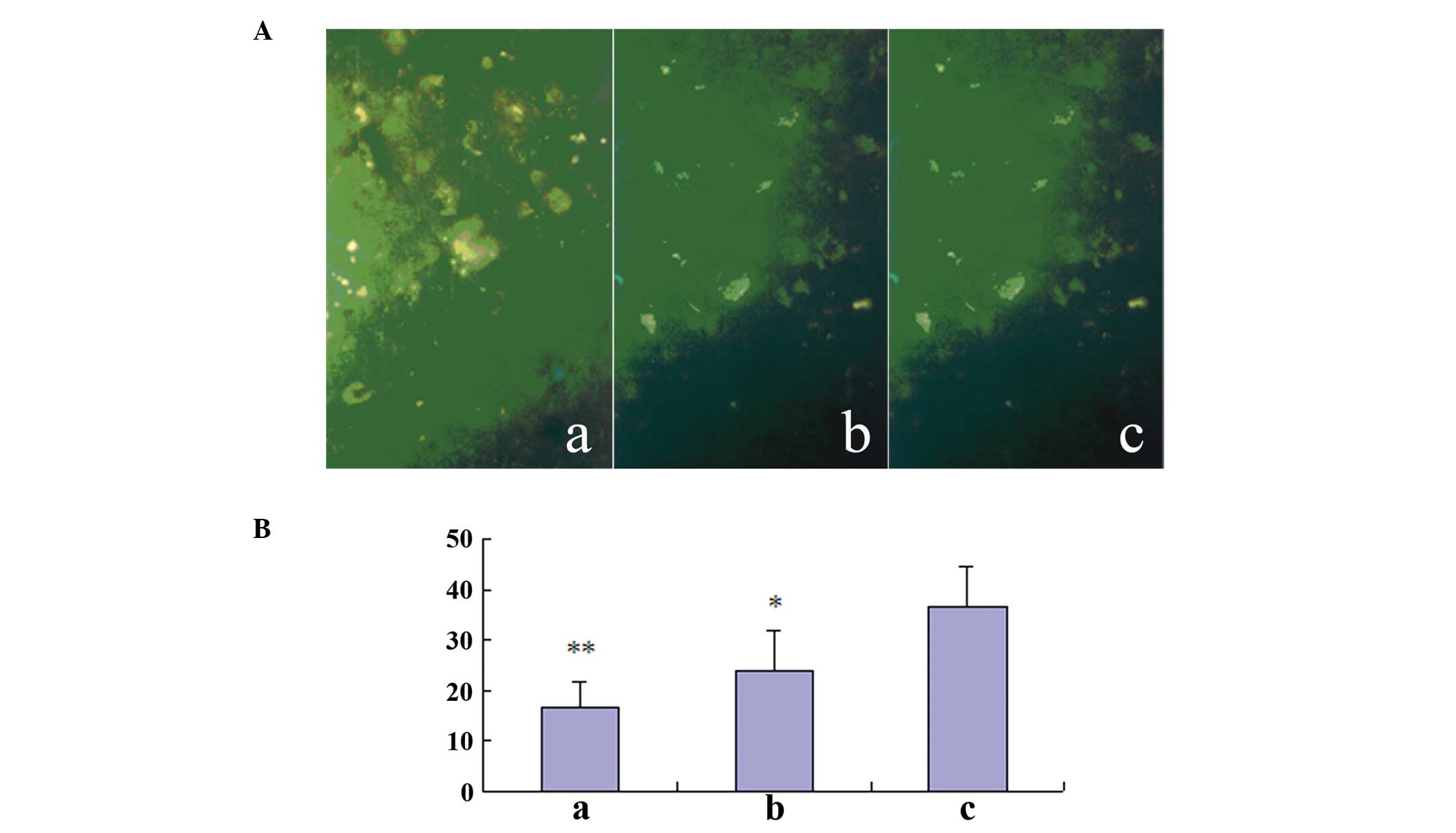
TUNEL-positive cell counts
TUNEL-positive cell nuclei were observed to be
present throughout the gray matter of the spinal cord.
Approximately 25% of the spinal cord cells at day 1 were labeled by
the TUNEL reaction. At 3 days after SCI, the number of apoptotic
cells increased significantly and was at a maximum. These cells
were present in the three segments (lesion area, rostral and
caudal). From day 7 onward, the number of apoptotic cells gradually
decreased. A number of TUNEL-labeled cells were still observed at
day 14. Significant differences were identified between the
transplantation plus MK-801 treatment (group A), transplantation
(group B) and control (group C) groups (Table I; Fig.
2).
 | Table IApoptosis index following spinal cord
injury in rats. |
Table I
Apoptosis index following spinal cord
injury in rats.
| | Days following
surgical procedure |
|---|
| |
|
|---|
| Groups | n | 1 | 3 | 7 | 14 |
|---|
| A | 6 | 12.20±4.52 | 16.46±5.16a | 9.58±3.46b | 7.60±2.50 |
| B | 6 | 15.76±6.84 | 23.59±7.85b | 12.54±4.59 | 10.37±4.52 |
| C | 6 | 28.45±7.81 | 36.27±8.28 | 20.96±5.12 | 9.65±3.56 |
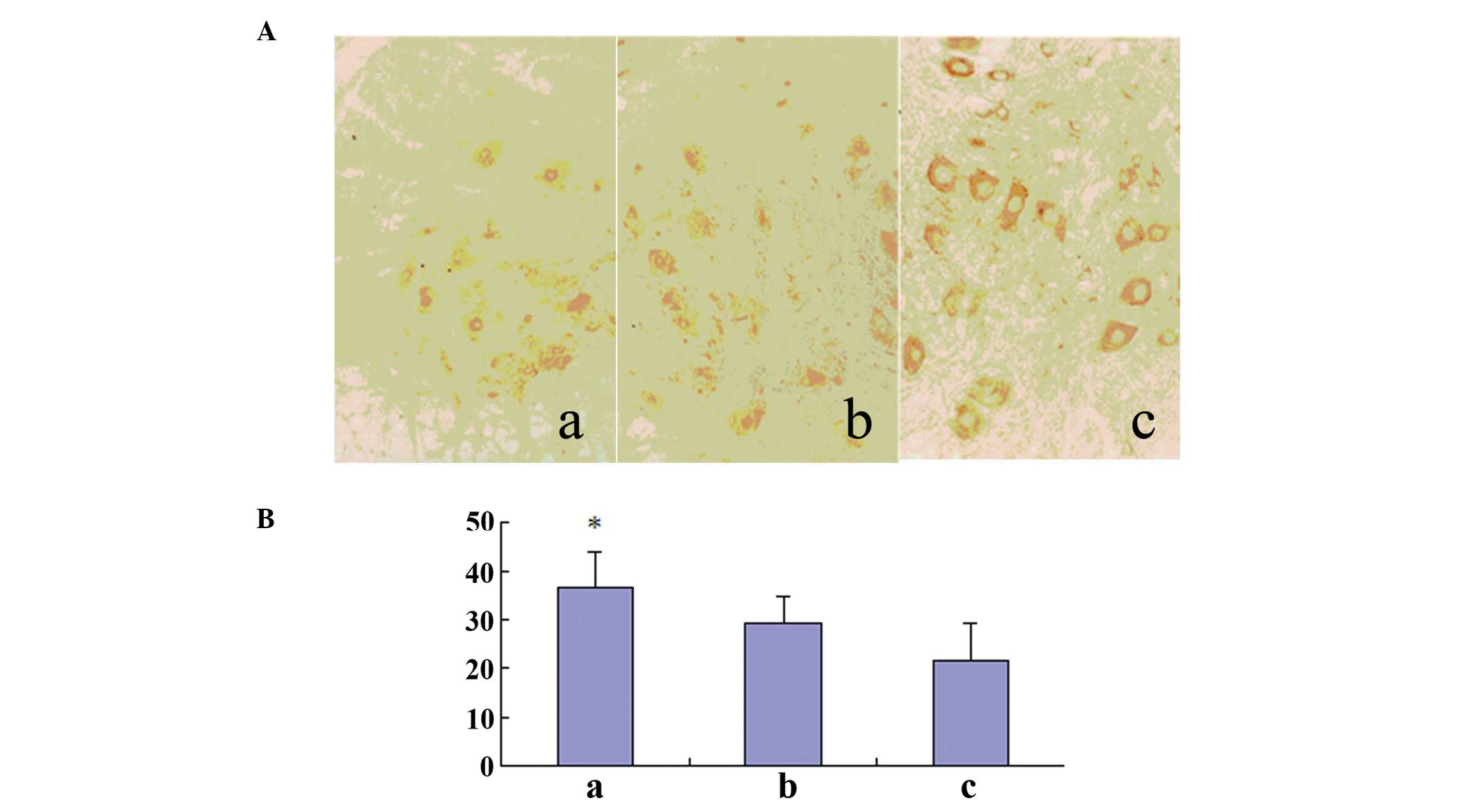
Bcl-2-positive cell counts
One day after SCI, Bcl-2 immunostained cells
(including neurons and glial cells) were detected in the gray
matter of the three segments (lesion, upper lesion and lower
lesion). The number of positively immunostained cells in groups A
and B reached a maximum on day 7. The immunostaining was present in
neurons and glia cells. The number of positive cells was maintained
at a high level until day 14 and then decreased. The number of
Bcl-2-positive cells in Group A was significantly higher than that
in the other two groups (Table
II; Fig. 3).
 | Table IIBcl-2-positive cells following spinal
cord injury in rats (mm2). |
Table II
Bcl-2-positive cells following spinal
cord injury in rats (mm2).
| | Days following
surgical procedure |
|---|
| |
|
|---|
| Groups | n | 1 | 3 | 7 | 14 |
|---|
| A | 6 | 21.57±6.84 | 34.60±7.53a | 40.50±8.68b | 18.36±6.85a |
| B | 6 | 19.85±4.83 | 29.35±5.38 | 33.46±6.21a | 15.35±5.34b |
| C | 6 | 18.56±2.15 | 21.48±7.83 | 9.13±3.47 | 8.87±2.74 |
Discussion
Timed-pregnant rats were used to provide embryonic
(at 14 days) spinal cord for transplantation into the adult spinal
cord lesion site of allogeneic rats. Such transplants can prevent
the retrograde cell death of immature axotomized neurons and
support the growth of axons into and through the site of injury
(21–26). The base of support in animals with
transplants has been found to be similar to control values
(27). Animals with a hemisection
rotate their hindlimbs further laterally than control animals do
during locomotion. Spinal cord transplants at the site of adult
spinal cord injury (13) result in
enhanced sparing or recovery of motor function (28–31).
This type of transplant is suggested to induce recovery of function
as a consequence of the anatomical plasticity it elicits. The
experimental process in which fetuses were removed individually and
maintained in sterile culture medium to provide donor tissue, with
dissection of the FSCs into 1–3-mm3 segments for
transplantation, was simple and easy to operate, with good
repeatability.
The mechanism by which MK-801 prevents apoptosis was
investigated in the present study. Programmed cell death (PCD) is a
naturally occurring physiological process that plays a crucial role
in the development and maintenance of the brain and spinal cord by
eliminating unwanted or unnecessary cells. A number of studies have
shown that apoptosis is not only present in the development of the
nervous system, but also in trauma and degenerative diseases of the
nervous system (32,33,34).
Since the 1980s, numerous experimental studies of the treatment of
spinal cord injury by FSC have been performed (35,36).
It is hypothesized that the transplanted FSC tissue should bridge
the spinal lesion and provide chemical and/or mechanical guidance
for host neurons to grow across the lesion, bridge the spinal
lesion and provide additional cellular elements to repair the
damaged circuitry, and provide factors that should rescue neurons
that would otherwise die and/or modulate neural circuits to improve
function.
Bcl-2 is a unique cytoplasmic protein that is
ubiquitous and localized to intracellular sites of oxygen free
radical generation, including the mitochondria, endoplasmic
reticulum and nuclear membranes. The specific mechanism by which
Bcl-2 prevents apoptosis has been investigated. It has been shown
in vitro and in vivo that overexpression of the Bcl-2
oncogene function suppresses lipid peroxidation completely, and
thus limits the generation of reactive oxygen species. Thus, it
appears that Bcl-2 regulates an antioxidant pathway that limits
free radical generation and thereby prevents apoptosis (37–39).
Biochemical and pathological changes in the spinal
cord may worsen following injury (40). Necrosis plays a substantial role
following injury. In recent years, however, several studies have
demonstrated that postinjury spinal cord cell death is due in part
to apoptosis, as determined morphologically and biochemically by
light and electron microscopic examination, agarose gel
electrophoresis and ining (41).
Overactivation of EAAs has been shown to induce neuronal
degeneration and may contribute to neuronal loss in several disease
states. The results of several studies have suggested that exposure
to EAAs induces neuronal apoptosis in the brain and in neuronal
culture (42,43). NMDA receptor antagonists inhibit
neuronal apoptosis in the brain following transient ischemia
(44). However, very few studies
have been conducted to examine whether EEAs induce neuronal
apoptosis in the spinal cord following FSC transplantation. Studies
have shown that neurons in the dorsal horn of the spinal cord
undergo apoptosis following peripheral nerve insult and that
administration of MK-801 reduces the degree of apoptotic cell loss
(45). It is suggested that in
spinal neurons, apoptosis is induced in a transsynaptic manner by
an early signal from injured afferent fibers via activation of
spinal NMDA receptors (46). The
extrinsic Fas pathway is sufficient to induce complete apoptosis in
certain cell types as well as oligodendrocytes, astrocytes and
microglia through caspase-8 activation. A further study has
demonstrated that treatment with MK-801 significantly reduces Fas
ligand positive staining (47).
TUNEL-like staining in the perilesional spinal cord tissue was also
examined. Almost no apoptotic cells were detected in the spinal
cord from sham-operated mice. At 24 h after trauma, tissues from
mice with SCI demonstrated a marked appearance of dark brown
apoptotic cells and intercellular apoptotic fragments. By contrast,
tissues obtained from mice treated with MK-801 demonstrated no
apoptotic cells or fragments. This confirmed the well documented
neuroprotective effects of MK-801 and lends support to the
potential importance of NMDA antagonists as therapeutic agents in
the treatment of acute SCI (11,12).
In the present study, the administration of an NMDA
receptor antagonist reduced the number of apoptotic cells in white
and gray matter in rats that had undergone FSC transplantation
following spinal hemisection. This finding suggests that EAAs,
which are released from damaged cells, promote delayed cell death
due to apoptosis in the spinal cord following injury (34–36).
The mechanism by which EAAs induce apoptosis remains unclear. The
glutamate-Ca2+-NO hypothesis is believed to be the
mechanism of EAA-mediated neuronal cell death. According to this
hypothesis, the influx of extracellular Ca2+ ions is
enhanced by EAAs released from damaged cells, resulting in the
activation of NO synthase (16–18).
Production of NO is then increased in the injured neural tissue.
The NO diffuses into the surrounding neurons and glial cells,
promotes energy failure, and induces DNA damage in cells. When the
injured cells cannot be repaired, delayed cell death may eventually
occur due to apoptosis. By contrast, it has been suggested that the
excessive Ca2+ ion influx activates nuclear endonuclease
directly and results in apoptosis (19).
In conclusion, the present study has shown that the
NMDA receptor antagonist MK-801 downregulates TUNEL-positive cells
and upregulates Bcl-2 positive cells, prevents apoptosis in rats
that have undergone FSC transplantation following spinal
hemisection and may reduce the toxicity of EAAs. In addition,
MK-801 promotes the survival of transplanted FSCs and reduces
apoptosis following SCI, consistent with previous studies (39,48).
Acknowledgements
This study is supported by the National Natural
Science Foundation of China (no. 30300075), the China Postdoctoral
Science Foundation (nos. 2003034384 and 20080440995) and the
Sichuan Science Fund for Outstanding Youths (no. 05ZQ026-020).
References
|
1
|
Lu Y and Wang MY: Neural stem cell grafts
for complete spinal cord injury. Neurosurgery. 71:N13–N15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun HH, Gao F, Liu B, Yu HT, Kong N and
Liu GM: Inhibition of Nogo expression to promote repair after
spinal cord injury. Chin Med J (Engl). 125:4044–4048.
2012.PubMed/NCBI
|
|
3
|
Sung WH, Chiu TY, Tsai WW, Cheng H and
Chen JJ: The effect of virtual reality-enhanced driving protocol in
patients following spinal cord injury. J Chin Med Assoc.
75:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma A: Pharmacological management of
acute spinal cord injury. J Assoc Physicians India. 60(Suppl):
13–18. 2012.
|
|
5
|
Londhey VA: Acute spinal cord injury-the
unchanged challenges! J Assoc Physicians India. 60(Suppl): 5–6.
2012.PubMed/NCBI
|
|
6
|
Boulenguez P and Vinay L: Strategies to
restore motor functions after spinal cord injury. Curr Opin
Neurobiol. 19:587–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacDermott AB, Mayer ML, Westbrook GL, et
al: NMDA-receptor activation increases cytoplasmic calcium
concentration in cultured spinal cord neurons. Nature. 321:519–522.
1986. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiva S, Moellering D, Ramachandran A, et
al: Redox signalling: from nitric oxide to oxidized lipids. Biochem
Soc Symp. 107–120. 2004.PubMed/NCBI
|
|
9
|
Park E, Velumian AA and Fehlings MG: The
role of excitotoxicity in secondary mechanisms of spinal cord
injury: a review with an emphasis on the implications for white
matter degeneration. J Neurotrauma. 21:754–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hama A and Sagen J: Combinations of
intrathecal gamma-amino-butyrate receptor agonists and
N-methyl-d-aspartate receptor antagonists in rats with neuropathic
spinal cord injury pain. Eur J Pharmacol. 683:101–108. 2012.
View Article : Google Scholar
|
|
11
|
Kim Y, Cho HY, Ahn YJ, Kim J and Yoon YW:
Effect of NMDA NR2B antagonist on neuropathic pain in two spinal
cord injury models. Pain. 153:1022–1029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunanyan AS, Petrosyan HA, Alessi V and
Arvanian VL: Repetitive spinal electromagnetic stimulation opens a
window of synaptic plasticity in damaged spinal cord: role of NMDA
receptors. J Neurophysiol. 107:3027–3039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunkel-Bagden E and Bregman BS: Spinal
cord transplants enhance the recovery of locomotor function after
spinal cord injury at birth. Exp Brain Res. 81:25–34. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Z, Yu W, Liu HB, et al:
Neuroprotective effects of curculigoside against NMDA-induced
neuronal excitoxicity in vitro. Food Chem Toxicol. 50:4010–4015.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berry JN, Sharrett-Field LJ, Butler TR and
Prendergast MA: Temporal dependence of cysteine protease activation
following excitotoxic hippocampal injury. Neuroscience.
222:147–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang LN, Yang JP, Ji FH, et al:
Brain-derived neurotrophic factor modulates N-methyl-D-aspartate
receptor activation in a rat model of cancer-induced bone pain. J
Neurosci Res. 90:1249–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi SS, Hahm KD, Min HG and Leem JG:
Comparison of the spinal neuropathic pain induced by intraspinal
injection of N-methyl-d-aspartate and quisquate in rats. J Korean
Neurosurg Soc. 50:420–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo F, Maeda Y, Ko EM, et al: Disruption
of NMDA receptors in oligodendroglial lineage cells does not alter
their susceptibility to experimental autoimmune encephalomyelitis
or their normal development. J Neurosci. 32:639–645. 2012.
View Article : Google Scholar
|
|
19
|
García-Alías G, Petrosyan HA, Schnell L,
et al: Chondroitinase ABC combined with neurotrophin NT-3 secretion
and NR2D expression promotes axonal plasticity and functional
recovery in rats with lateral hemisection of the spinal cord. J
Neurosci. 31:17788–17799. 2011.PubMed/NCBI
|
|
20
|
Guide for the Care and Use of Laboratory
Animals 1996. National Institutes of Health publication;
Washignton, D.C: pp. 85–23. 1996
|
|
21
|
Schnell L, Hunanyan AS, Bowers WJ, et al:
Combined delivery of Nogo-A antibody, neurotrophin-3 and the
NMDA-NR2d subunit establishes a functional ‘detour’ in the
hemisected spinal cord. Eur J Neurosci. 34:1256–1267.
2011.PubMed/NCBI
|
|
22
|
Becker D and McDonald JW III: Approaches
to repairing the damaged spinal cord: overview. Handb Clin Neurol.
109:445–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
All AH, Bazley FA, Gupta S, et al: Human
embryonic stem cell-derived oligodendrocyte progenitors aid in
functional recovery of sensory pathways following contusive spinal
cord injury. PLoS One. 7:e476452012. View Article : Google Scholar
|
|
24
|
Kosaka Y, Kin H, Tatetsu M, Uema I and
Takayama C: Distinct development of GABA system in the ventral and
dorsal horns in the embryonic mouse spinal cord. Brain Res.
1486:39–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin S, Wang Y, Zhang C and Xu J:
Modification of the neurotrophin-3 gene promotes cholinergic
neuronal differentiation and survival of neural stem cells derived
from rat embryonic spinal cord in vitro and in vivo.
J Int Med Res. 40:1449–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faden AI, Lemke M, Simon RP and Noble LJ:
N-methyl-D-aspartate antagonist MK801 improves outcome following
traumatic spinal cord injury in rats: behavioral, anatomic, and
neurochemical studies. J Neurotrauma. 5:33–45. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima K, Yamashita K, Uesugi S and Ito
H: Temporal and spatial profile of apoptotic cell death in
transient intracerebral mass lesion of the rat. J Neurotrauma.
16:143–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen TA, Yang F, Cole GM and Chan SO:
Inhibition of caspase-3-like activity reduces glutamate induced
cell death in adult rat retina. Brain Res. 904:177–188. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matute C, Domercq M and Sánchez-Gómez MV:
Glutamate-mediated glial injury: mechanisms and clinical
importance. Glia. 53:212–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bakiri Y, Hamilton NB, Káradóttir R and
Attwell D: Testing NMDA receptor block as a therapeutic strategy
for reducing ischaemic damage to CNS white matter. Glia.
56:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lou J, Lenke LG, Xu F and O’Brien M: In
vivo Bcl-2 oncogene neuronal expression in the rat spinal cord.
Spine (Phila Pa 1976). 23:517–523. 1998. View Article : Google Scholar
|
|
32
|
Nakahara S, Yone K, Sakou T, et al:
Induction of apoptosis signal regulating kinase 1 (ASK1) after
spinal cord injury in rats: possible involvement of ASK1-JNK and
p38 pathways in neuronal apoptosis. J Neuropathol Exp Neurol.
58:442–450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abe Y, Yamamoto T, Sugiyama Y, et al:
Apoptotic cells associated with Wallerian degeneration after
experimental spinal cord injury: a possible mechanism of
oligodendroglial death. J Neurotrauma. 16:945–952. 1999. View Article : Google Scholar
|
|
34
|
Profyris C, Cheema SS, Zang D, et al:
Degenerative and regenerative mechanisms governing spinal cord
injury. Neurobiol Dis. 15:415–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Itoh Y, Mizoi K and Tessler A: Embryonic
central nervous system transplants mediate adult dorsal root
regeneration into host spinal cord. Neurosurgery. 45:849–856. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medalha CC, Jin Y, Yamagami T, Haas C and
Fischer I: Transplanting neural progenitors into a complete
transection model of spinal cord injury. J Neurosci Res.
92:607–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kane DJ, Sarafian TA, Anton R, et al:
Bcl-2 inhibition of neural death: decreased generation of reactive
oxygen species. Science. 262:1274–1277. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheung NS, Carroll FY, Larm JA, et al:
Kainate-induced apoptosis correlates with c-Jun activation in
cultured cerebellar granule cells. J Neurosci Res. 52:69–82. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Casha S, Yu WR and Fehlings MG:
Oligodendroglial apoptosis occurs along degenerating axons and is
associated with FAS and p75 expression following spinal cord injury
in the rat. Neuroscience. 103:203–218. 2001. View Article : Google Scholar
|
|
40
|
Tator CH and Fehlings MG: Review of the
secondary injury theory of acute spinal cord trauma with emphasis
on vascular mechanisms. J Neurosurg. 75:15–26. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sellers DL, Maris DO and Horner PJ:
Postinjury niches induce temporal shifts in progenitor fates to
direct lesion repair after spinal cord injury. J Neurosci.
29:6722–6733. 2009. View Article : Google Scholar
|
|
42
|
Farooque M, Hillered L, Holtz A and Olsson
Y: Effects of moderate hypothermia on extracellular lactic acid and
amino acids after severe compression injury of rat spinal cord. J
Neurotrauma. 14:63–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu R, Tao Y, Wu C, et al: Domoic acid
induced spinal cord lesions in adult mice: evidence for the
possible molecular pathways of excitatory amino acids in spinal
cord lesions. Neurotoxicology. 29:700–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marutani E1, Kosugi S, Tokuda K, et al: A
novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor
antagonist prevents ischemic neuronal death. J Biol Chem.
287:32124–32135. 2012. View Article : Google Scholar
|
|
45
|
Kovac AD, Kwidzinski E, Heimrich B,
Bittigau P, Deller T, Nitsch R and Bechmann I: Entorhinal cortex
lesion in the mouse induces transsynaptic death of perforant path
target neurons. Brain Pathol. 14:249–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han RZ, Hu JJ, Weng YC, et al: NMDA
receptor antagonist MK-801 reduces neuronal damage and preserves
learning and memory in a rat model of traumatic brain injury.
Neurosci Bull. 25:367–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Azkue JJ, Zimmermann M, Hsieh TF, et al:
Peripheral nerve insult induces NMDA receptor-mediated, delayed
degeneration in spinal neurons. Eur J Neurosci. 10:2204–2206. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Esposito E, Paterniti I, Mazzon E, et al:
MK801 attenuates secondary injury in a mouse experimental
compression model of spinal cord trauma. BMC Neurosci. 12:31–52.
2011. View Article : Google Scholar : PubMed/NCBI
|