Introduction
Hyperlipidemia is a lipid metabolism disorder that
causes abnormally elevated levels of cholesterol, triglycerides
(TG) and lipoproteins in the blood (1). Hyperlipidemia is widely accepted to
be a key risk factor for atherosclerosis, coronary heart disease
(CHD) and peripheral vascular disease (2,3).
Decreasing the lipid levels has been shown to reduce the risk of
coronary artery disease and aid in preventing additional coronary
events (4). In addition,
hyperlipidemia may result in solid organ injury, including damage
to the liver and kidneys (5)
Statins are considered to be the first-line therapy in the
reduction of lipid levels, and large clinical outcome trials have
consistently demonstrated their efficacy in low-density
lipoprotein-cholesterol (LDL-C) reduction and the prevention of
cardiovascular events (6).
However, the application of statins is restricted by the adverse
side-effects on liver function and creatine kinase activity,
particularly in older patients, those with multiple comorbid
diseases and those treated with high-dose statins or a combination
lipid-lowering therapy. Thus, research into an effective, safe,
alternative therapy for CHD patients with the added complication of
hyperlipidemia is of great clinical significance.
Xuezhikang (XZK), an extract from the red yeast rice
Monascus purpureus, has been used as a traditional Chinese
medicine for patients with cardiovascular diseases for over 2,000
years (7). XZK contains 13 types
of natural statins, as well as unsaturated fatty acids, flavonoids,
ergosterol, amino acids, alkaloids, trace elements of plant sterols
and a number of other biologically active substances (8); thus, XZK is regarded as a natural
polypill. A number of studies have demonstrated that fermented red
yeast rice not only moderately lowers cholesterol levels, but has
the additional advantage of causing fewer adverse effects compared
with other statin drugs (9–11).
In addition, a previous study demonstrated that XZK significantly
reduces the recurrence of coronary events and the occurrence of new
cardiovascular events and mortalities, in addition to improving
lipoprotein regulation and being a safe and well-tolerated drug
(12). Combining XZK with other
therapies in the treatment of fatty liver has been reported to
relieve clinical syndromes and improve liver function (5). However, the effects of XZK treatment
on the liver in patients with hyperlipidemia have not yet been
fully identified.
Therefore, the aim of the present study was to
investigate the effects of XZK treatment on the plasma levels of
total cholesterol (TC), TG and LDL-C. In addition, the effects of
XZK treatment on kidney function and the related molecular
mechanisms were evaluated in a rat model of hyperlipidemia.
Materials and methods
Materials
XZK capsules (0.6 g each) were obtained from Beijing
Peking University WBL Biotech Co., Ltd. (Beijing, China). Each
capsule contained a combination of lovastatin, also known as
monoclonin K (2.5–3.2 mg/capsule), and small quantities of
lovastatin hydroxyl acid, unsaturated fatty acids (primarily
linoleic acid, oleic acid, palmitic acid and stearic acid),
essential amino acids, ergosterol and a number of other components.
The bioavailability of lovastatin contained within XZK was 169%
compared with that of purified lovastatin (13).
Animal treatment
A total of 30 male Sprague-Dawley rats, weighing
180–200 g, were purchased from the Laboratory Animal Institute of
Jilin University (Changchun, China). Animals were housed in an
environmentally controlled breeding room, under the institutional
guidelines of the Experimental Animals of Jilin University for the
humane treatment of laboratory animals.
Animals were randomly divided into three groups,
with ten rats in each group. Rats in group I (control group) were
fed a normal diet, while rats in the other two groups were fed a
high cholesterol diet with vitamin D3 (Sigma-Aldrich, St Louis, MO,
USA) added to normal chow. After six weeks, blood was collected
from the tails of the rats and the lipid and lipoprotein levels
were determined. To ensure that all the rats in the high-fat
diet-fed groups were hyperlipidemic, the animals were subsequently
treated with the following pharmacological agents. Group III rats
(XZK group) were fed a high cholesterol diet plus vitamin D3 and
XZK (300 mg/kg, p.o.) daily for 42 days, while rats in Group II
(hyperlipidemic group) were fed a high cholesterol diet plus
vitamin D3 and administered saline daily for 42 days. The body
weight and food intake of each rat were recorded during the
experimental period. Animal surgery was performed under sodium
pentobarbital anesthesia. The experimental protocols were approved
by the Institutional Animal Ethics Committee of Jilin
University.
Blood samples and kidney tissues
The rats were monitored daily for general health and
weighed individually at the beginning and end of the experiment.
The daily feed intake and weight gain were recorded during the
experimental period.
At the end of the experiment, all the rats were
anesthetized with sodium pentobarbital (1.25 g/kg; Sigma-Aldrich)
following overnight fasting, and decapitated. Blood samples were
collected by cardiac puncture and were maintained at room
temperature for coagulation. The serum was obtained by
centrifugation at 3,000 × g at 4°C for 10 min, after which the
samples were stored at −70°C for later use. Kidneys were excised,
rinsed in ice-cold saline, blotted dry on filter paper and weighed.
Aliquots of the kidney were snap-frozen in liquid nitrogen for 24 h
and stored at −70°C prior to use. A portion of each kidney was
fixed in 10% formalin for histological analysis.
Measurement of lipid and lipoprotein
levels
Serum levels of TC, TG, high-density
lipoprotein-cholesterol (HDL-C) and LDL-C were measured
enzymatically using commercially available kits (Randox
Laboratories, San Francisco, CA, USA), according to the
manufacturer’s instructions.
Determination of renal antioxidant
activity and malondialdehyde (MDA) content
Catalase (CAT) activity was measured as the
reduction in H2O2 concentration by recording
the absorbance at a wavelength of 240 nm (14). Glutathione peroxidase (GSH-px)
activity was assayed as previously described (15), while the levels of superoxide
dismutase (SOD) and MDA were determined using diagnostic kits
(Jiangchen Science & Technology Co., Ltd., Nanjing, China) with
a UV-visible spectrophotometer (Shimadzu Corporation, Kyoto,
Japan), according to the manufacturer’s instructions.
Assay for renal function parameters
Blood samples were obtained from the rats for the
measurement of glucose, serum creatinine (Scr), blood urea nitrogen
(BUN), uric acid (UA), urine creatinine (Ucr) and albuminuria. The
serum levels were detected using diagnostic kits (Jiangchen Science
& Technology Co., Ltd.). The kidney index was calculated as
follows: 1,000 - kidney weight/body weight. Creatinine clearance
(Ccr) was calculated according to the following formula (16): Ccr (ml/kg/min) = urinary creatinine
(μM) × urinary volume (ml/kg/min)/Scr (μM).
Assays for renal histology
Renal tissue samples were fixed in 10% (v/v) neutral
buffered formalin and embedded in paraffin wax. Paraffinized
sections were cut into 5-μm sections, deparaffinized with xylene
and dehydrated with ethanol. The sections were stained with
hematoxylin and eosin (HE) for histopathological analysis with a
photomicroscope (Olympus, Tokyo, Japan). The operative procedures
complied with the standard protocols and the examination of the
slides was performed by a pathologist blinded to the experimental
profile.
Measurement of serum tumor necrosis
factor (TNF)-α and interleukin (IL)-6
A sandwich enzyme-linked immunosorbent assay (ELISA)
was used to determine the serum concentrations of TNF-α in all the
rats after six weeks of XZK treatment, using a rat TNF-α DuoSet
ELISA development kit (eBioscience, San Diego, CA, USA). Briefly,
96-well ELISA plates were coated with a monoclonal mouse anti-human
TNF-α antibody and incubated overnight at 4°C, following which the
plates were washed three times with phosphate-buffered saline
containing 0.05% Tween 20 (PBST). The plates were incubated with
blocking solution for 1 h at room temperature and the test samples
and recombinant TNF-α standards were added. Subsequently, the
plates were incubated at room temperature for a further 2 h,
following which they were washed five times with PBST.
Biotin-conjugated anti-human TNF-α antibodies were added, and the
plates were incubated at room temperature for 2 h and washed with
PBST. Avidin-horseradish peroxidase (HRP) was added and the
reaction was allowed to proceed at room temperature for 30 min. The
plates were washed five times with PBST, and
3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich, Sydney, Australia)
was added to develop the reaction, which was stopped by
H2SO4 (2N). The optical density was measured
at a wavelength of 450 nm with a VERSAmax automated microplate
reader (Molecular Devices, Inc., Sunnyvale, CA, USA). A standard
curve was constructed by plotting the optical density values
against the log of the TNF-α concentration.
The aforementioned ELISA protocol was similarly
completed using the rat IL-6 DuoSet ELISA development kit
(eBioscience) to determine the sera levels of IL-6.
Quantitative reverse transcription
polymerase chain reaction (RT-qPCR)
All glassware was treated with diethyl-pyrocarbonate
to inhibit RNases. Total RNA was extracted from the kidneys of 30
rats (n=10 per group) using TRIzol®, according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Reverse transcription was performed using a
TaqMan Reverse Transcription kit (Applied Biosystems, Foster
City, CA, USA) in a total reaction volume of 100 μl with 2 μg total
RNA. RT-qPCR was performed in a total reaction volume of 25 μl
using TaqMan fast mix (Applied Biosystems). Gene expression
was normalized against the expression level of β-actin. The
specific primers used were as follows: TNF-α forward,
5′-CGAGTCTGGGCAGGTCTACTTT-3′ and reverse,
5′-AAGCTGTAGGCCCCAGTGAGTT-3′; IL-6 forward,
5′-ATGCCTGACCTCAACTCCACT-3′ and reverse, 5′-GAGCAGCCCCAGGGAGAA-3′;
β-actin forward, 5′-GATCATTGCTCCTCCTGAGC-3′ and reverse,
5′-ACTCCTGCTTGCTGATCCAC-3′. The PCR thermal cycling conditions were
as follows: Initial denaturation at 95°C for 3 min, followed by 30
cycles of denaturation for 30 sec at 95°C, annealing for 40 sec at
58°C and extension for 30 sec at 72°C, with a final extension at
72°C for 10 min. The expression levels of the target genes were
normalized against that of β-actin in the cDNA samples. The
experiments were performed in triplicate.
Western blot analysis
All kidney specimens were weighed, lysed,
homogenized and centrifuged at 14,000 × g for 15 min at 4°C. The
kidney protein content of the supernatant was assayed using
Bradford reagent (Sigma-Aldrich, Steinheim, Germany). After
ensuring the linearity of the band density, samples (10 μg total
protein) were applied to 10–15% polyacrylamide gels, where the
proteins were separated using standard SDS-PAGE protocols and
transferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Subsequently, the membranes
were blocked with 5% bovine serum albumin (BSA) and incubated at
room temperature for 2 h. This was followed by a second 2-h
incubation in 5% BSA containing a rabbit anti-mouse TNF-α antibody
(1:1,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
a rabbit anti-mouse IL-6 antibody (1:500; Abcam, Cambridge, UK).
Following three washes with PBS, the membranes were incubated with
a goat anti-mouse secondary antibody conjugated to HRP (1:1,000;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) for 1 h at
room temperature. Visualized bands were detected using an enhanced
chemiluminescence reagent (Amersham Pharmacia Biotech, Little
Chalfont, UK), and densitometric analysis of the protein bands was
performed using Bio-Rad Quantity One software (Bio-Rad
Laboratories, Inc.). The TNF-α and IL-6 protein content was
expressed relative to β-actin in arbitrary units.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical comparisons of more than two groups were
performed using one-way analysis of variance, followed by Dunnett’s
post-hoc test. SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA)
and Prism 5.0d (GraphPad Software, Inc., San Diego, CA, USA)
software were used for statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
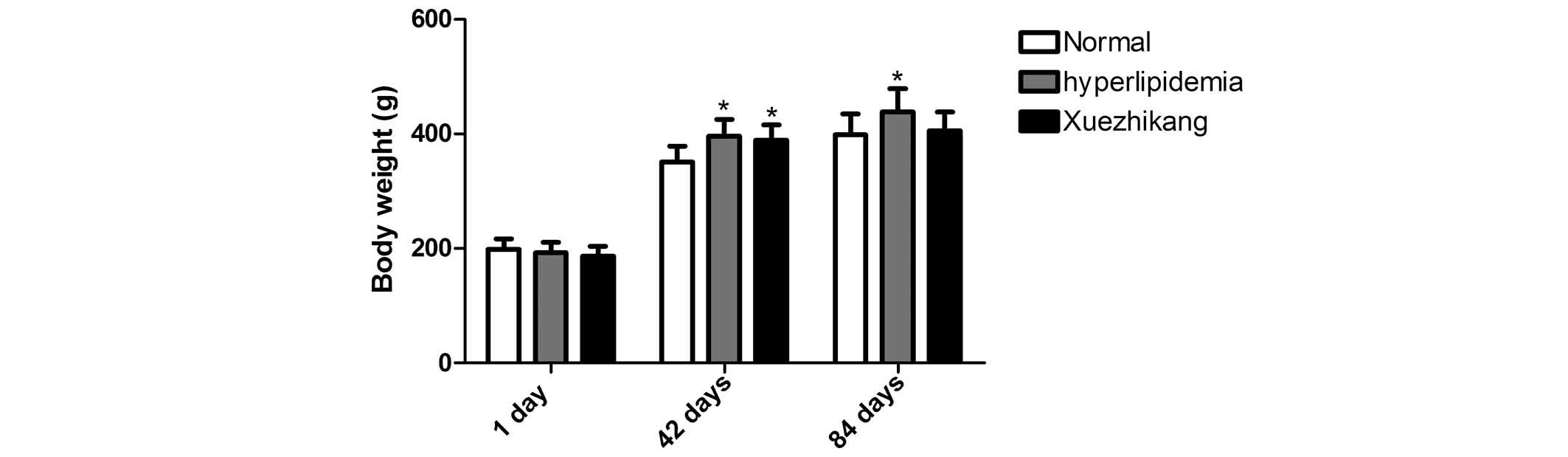
Food intake and body weight
In the course of the experiment, the daily food
intake of the XZK and hyperlipidemic rats was virtually identical,
and less than that of the rats fed a normal diet. After 42 days,
the hyperlipidemic group had a significantly increased body weight
compared with the rats fed a normal diet. In addition, after 84
days, the average body weight of the rats fed the high-fat diet was
significantly greater than that of the rats fed a normal diet
(P<0.01), indicating that the model was successful at inducing
hyperlipidemia. Administration of XZK reduced the weight gain
(P<0.01) compared with the rats fed the high-fat diet alone
(Fig. 1).
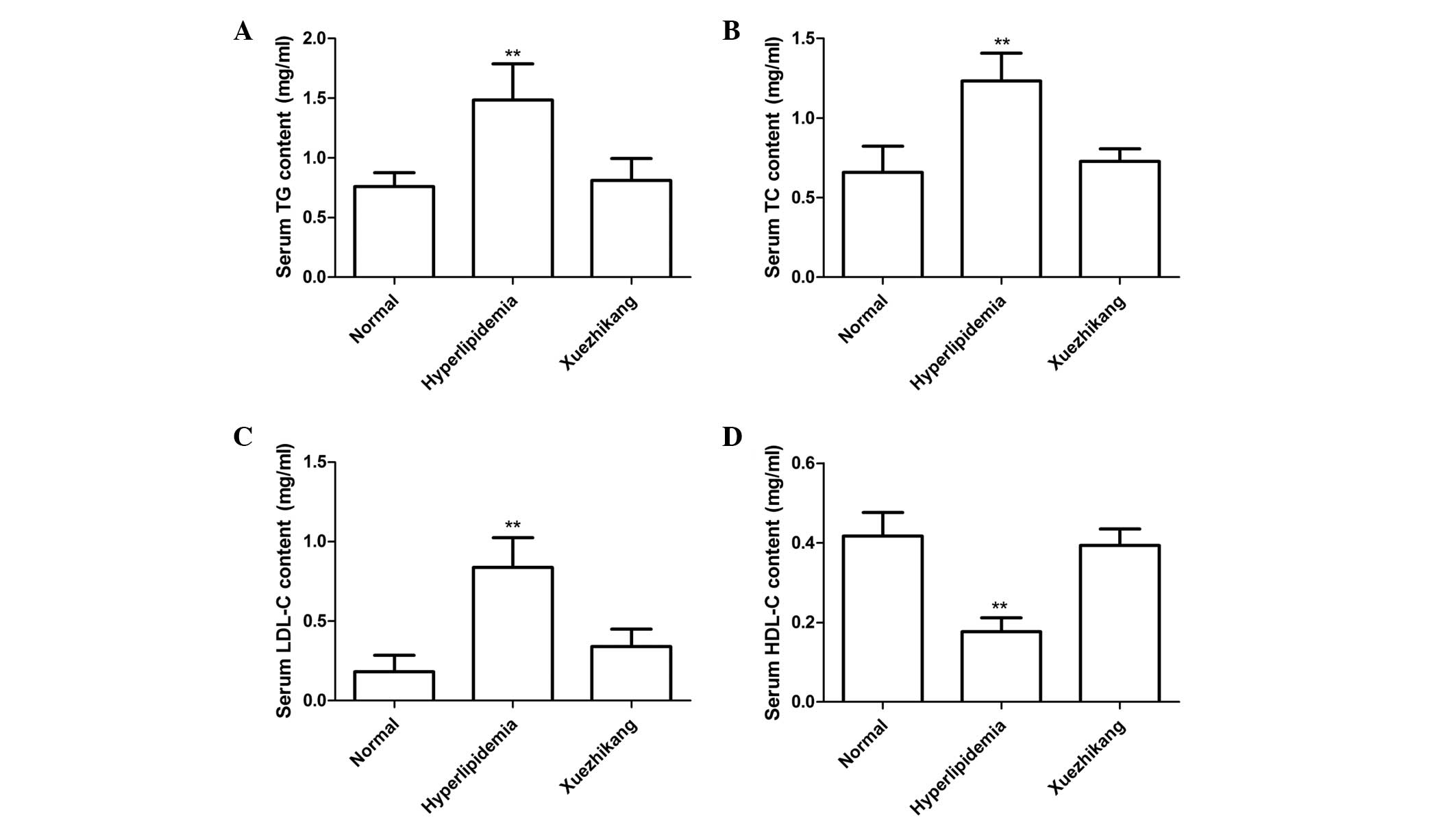
Serum lipid and lipoprotein
parameters
Compared with the rats fed the normal diet, rats fed
the high-fat diet had significantly increased serum levels of TC
(P<0.001) and TG (P<0.001). Administration of XZK (300 mg/kg)
significantly reduced the serum levels of TC and TG compared with
the rats fed the high-fat diet alone (P<0.001; Fig. 2A and B). In addition, following 12
weeks on the high-fat diet, the serum levels of LDL-C significantly
increased (P<0.001), while the serum levels of HDL-C decreased
(P<0.01), as compared with the control group. Administration of
XZK (300 mg/kg) markedly reduced the serum levels of LDL-C
(P<0.001) and increased the serum levels of HDL-C (P>0.05)
compared with the rats fed the high-fat diet alone (Fig. 2C and D).
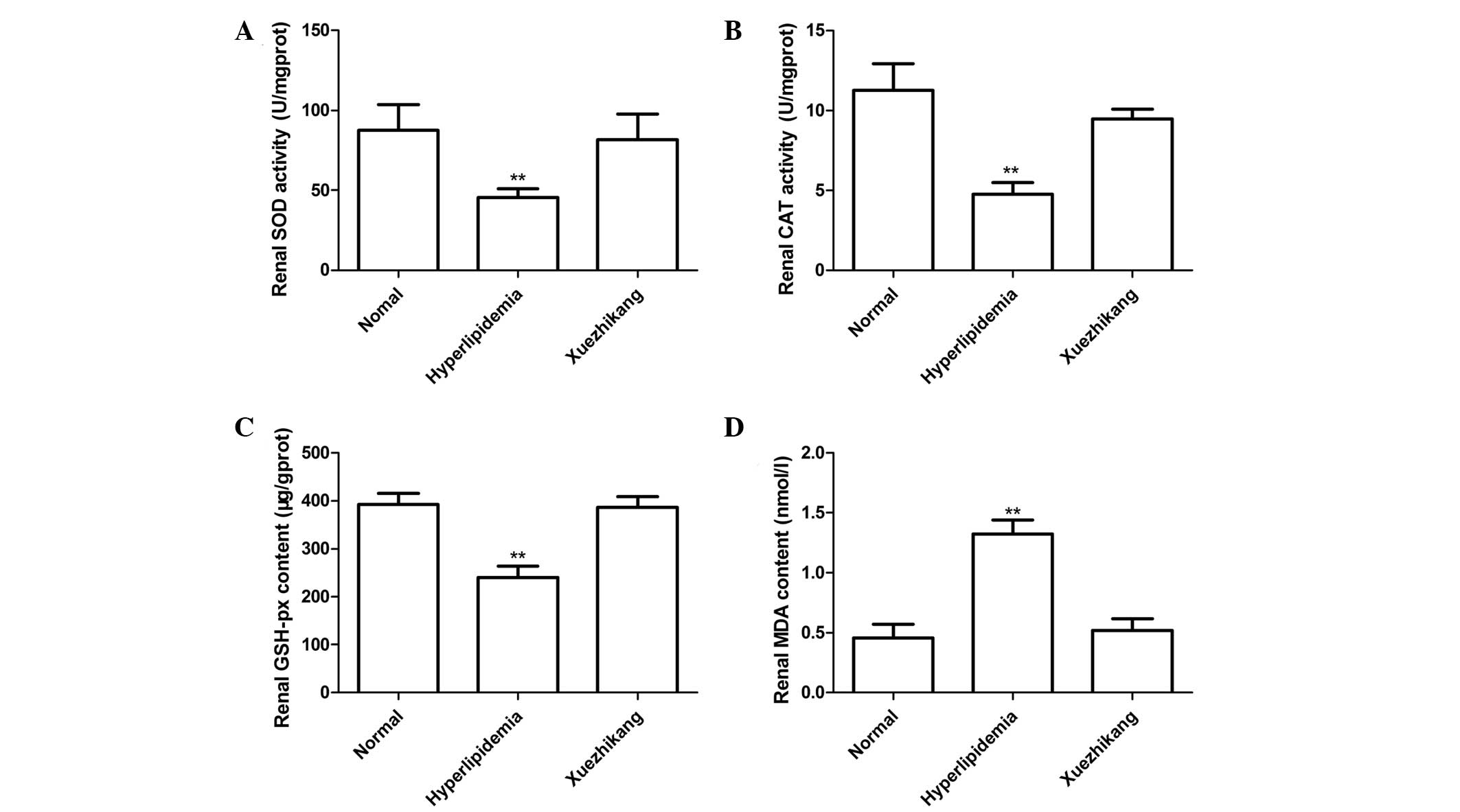
Effects of XZK on the renal antioxidant
status
Levels of renal SOD and CAT activity, and MDA and
GSH-px for all the groups are presented in Fig. 3. The high-fat diet was shown to
reduce the levels of SOD and CAT activity and the renal content of
GSH-px, but increase the level of MDA (P<0.01; Fig. 3) when compared with the rats fed
the normal diet. Daily administration of XZK (300 mg/kg)
significantly increased the activity of SOD and CAT, as well as the
levels of GSH-px, while reducing the levels of renal MDA
(P<0.01; Fig. 3) when compared
with the rats fed the high-fat diet alone.
Effects of XZK on renal function
As shown in Table
I, renal function was reflected by the levels of BUN, Scr, UA,
Ccr and albuminuria. In the rats fed the high-fat diet, the levels
of BUN, Scr, UA, Ccr and albuminuria were significantly higher
compared with those in the rats fed a normal diet (P<0.01).
However, administration of XZK significantly reversed these
changes, with the levels of BUN, Scr, UA, Ccr and albuminuria
reduced to near normal values (P<0.05).
 | Table IRenal functional parameters of the
normal, hyperlipidemia and Xuezhikang groups after six weeks. |
Table I
Renal functional parameters of the
normal, hyperlipidemia and Xuezhikang groups after six weeks.
| Renal parameters | Control | Hyperlipidemia | Xuezhikang |
|---|
| Albuminuria (μg/24
h) | 21.12±6.34 | 110.89±12.14b | 32.18±7.89 |
| BUN (mM) | 8.54±0.82 | 12.11±0.81b | 8.64±0.94 |
| Scr (μM) | 97.24±6.82 | 148.88±8.54b | 103.55±9.11 |
| Ucr (μM) | 487.34±26.44 | 1245±100.35b | 589.89±45.23a |
| UA (μM) | 58.87±6.94 | 83.23±10.45b | 56.77±8.45 |
| Ccr
(ml/kg/min) | 3.64±0.26 | 6.43±0.42b | 3.67±0.27 |
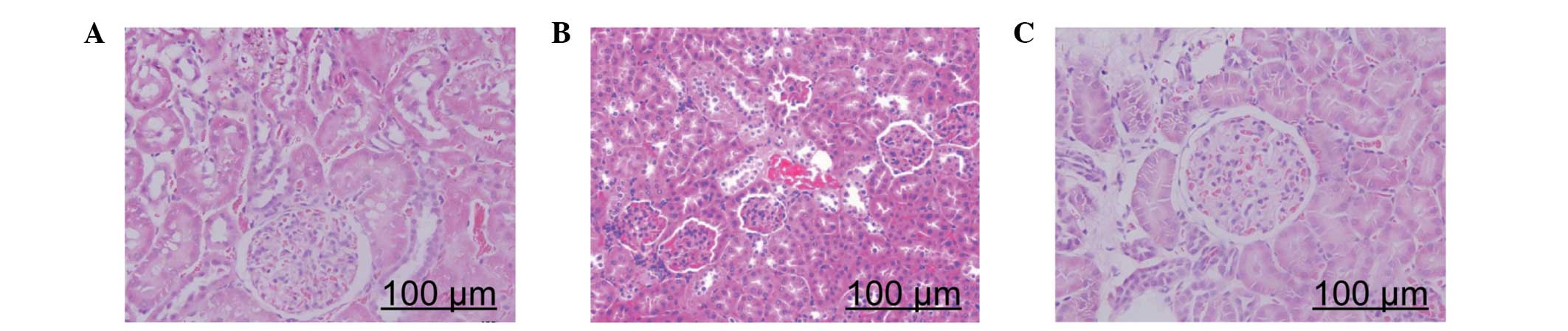
Histological evaluation
Photomicrographs of kidney samples stained with HE
are presented in Fig. 4. Kidney
samples from the rats fed the normal diet for 12 weeks appeared
normal (Fig. 4A). However,
pathological lesions were observed in the kidney samples of rats
fed the high-fat diet, which included epithelial cell necrosis of
the proximal tubules, leucocyte infiltration, vascular congestion
and tubular dilatation (Fig. 4B).
Notably, the kidney injury was markedly reduced following the
administration of XZK (Fig.
4C).
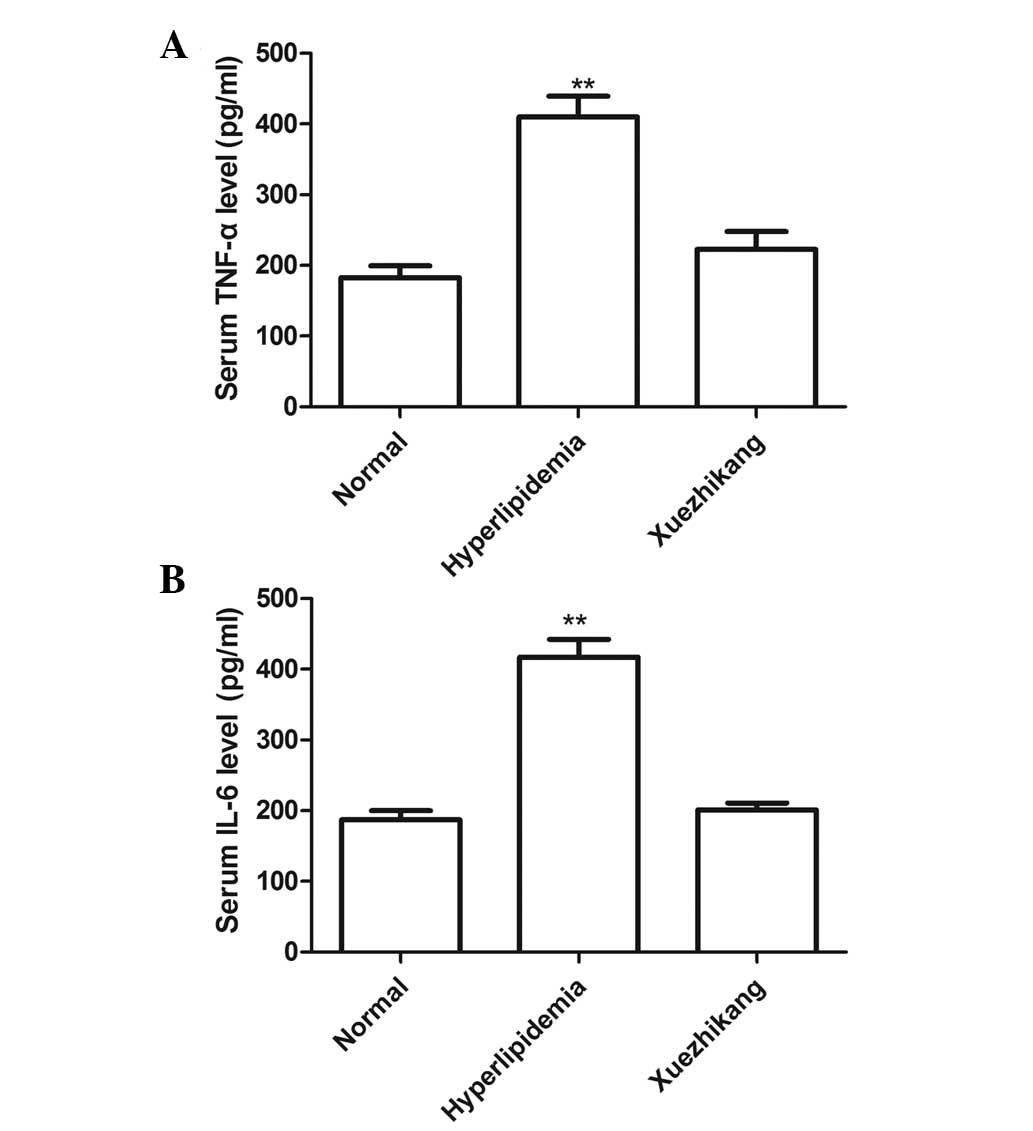
Serum levels of TNF-α and IL-6
To measure the serum expression levels of TNF-α and
IL-6 in all the groups, sandwich ELISAs were performed. As shown in
Fig. 5, the serum levels of TNF-α
and IL-6 in the high-fat diet group were higher than those of the
rats fed a normal diet (P<0.01; Fig. 5A and B). The serum levels of TNF-α
and IL-6 were reduced in the XZK treatment group when compared with
those of the high-fat diet group, and no statistically significant
difference was observed between the XZK treatment group and the
normal diet group.
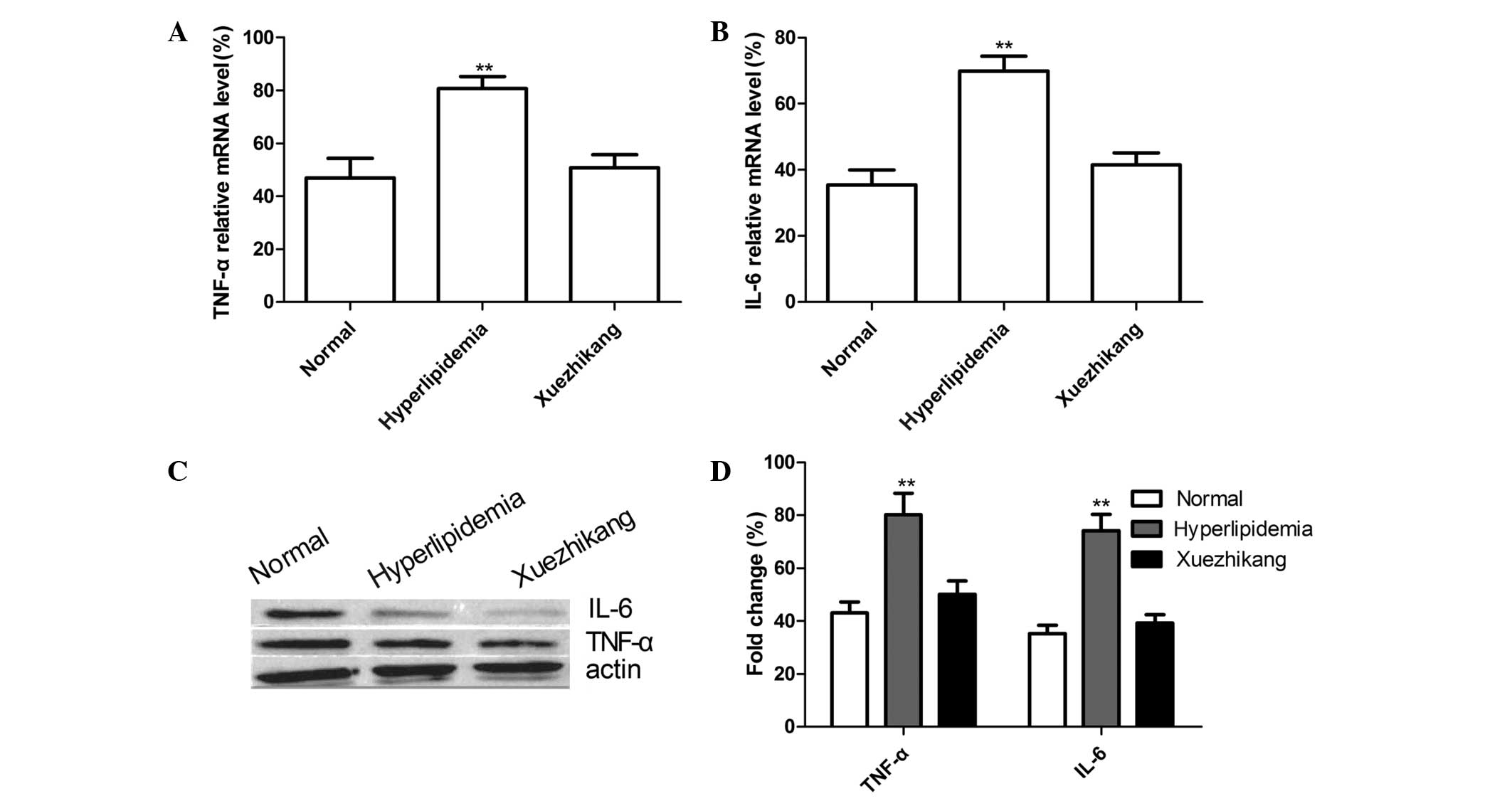
TNF-α and IL-6 expression levels in the
kidney tissue
To measure the mRNA and protein expression levels of
TNF-α and IL-6 in the kidney tissues of all the groups, RT-qPCR and
western blot analysis were performed, respectively. As shown in
Fig. 6A and B, the mRNA expression
levels of TNF-α and IL-6 in the high-fat diet group were
significantly higher compared with the normal diet group
(P<0.01). When compared with the high-fat diet group, the TNF-α
and IL-6 mRNA expression levels in the XZK treatment group were
significantly reduced (P<0.05). Additionally, the protein
expression levels of TNF-α and IL-6 were increased in the high-fat
diet group when compared with those in the normal diet group
(P<0.01), and the administration of XZK markedly reduced the
TNF-α and IL-6 protein expression levels when compared with the
high-fat diet group (P<0.05; Fig.
6C and D).
Discussion
Epidemiological and clinical evidence supports the
hypothesis that hyperlipidemia contributes to the formation and
progression of atherosclerosis, which is an important factor in the
pathogenesis of CHD (17).
Reducing lipid levels may slow the progression of the disease
(18). The Physician’s Health
Study investigated the probability of developing renal dysfunction
in 4,483 healthy male physicians, and found that the relative risk
for elevated creatinine levels was directly associated with
baseline blood lipid levels, and that dyslipidemia may cause
chronic renal disease (19). In
addition, the Helsinki Heart Study described an association between
cholesterol levels and progressive kidney disease in 2,702
middle-aged dyslipidemic males (20). A number of studies on patients with
hyperlipidemia have observed an association between the baseline
levels of serum cholesterol and the probability of nephropathy
progression (21–24).
The results of the present study revealed that the
levels of BUN, Scr, UA, Ccr and albuminuria in rats fed a high-fat
diet were significantly higher compared with those in rats fed a
normal diet. In addition, the high-fat diet was demonstrated to
cause kidney damage, which confirmed that hyperlipidemia may result
in a loss of kidney function and kidney injury. The results also
revealed that the hyperlipidemic rats developed higher serum levels
of TG, TC and LDL-C, as well as a reduced concentration of HDL-C,
which is consistent with the results of previous studies (25,26).
Statins are inhibitors of
3-hydroxyl-3-methylglutaryl Coenzyme A reductase, and have been
shown to be the most efficacious therapy for hyperlipidemia.
Statins not only reduce the plasma concentration of LDL-C, but also
reduce cardiovascular morbidity and mortality in patients with
dyslipidemia (27). However, for
the majority of patients, statins alone are insufficient to achieve
current guideline-recommended LDL-C goals. In addition, the safety
of statins at high doses is a concern, in older patients where they
may affect liver function (28). A
recent study showed that statins delay the progression of kidney
disease in a variety of animal experimental models (29). Thus, research into a safe yet
effective therapeutic agent for use in the place of statins for the
treatment of dyslipidemia and CHD is of great clinical
significance. XZK, an extract of red yeast rice, has been shown to
significantly reduce serum levels of TC, TG and LDL-C and increase
serum levels of HDL-C in hyperlipidemic rat models and patients;
the lipid modification effects of XZK appear to be similar to those
of pravastatin, simvastatin, lovastatin and atorvastatin (30). In addition, XZK has been
demonstrated to be a safe and effective treatment for the secondary
prevention of CHD in older individuals (5).
The results of the current study demonstrated that
the administration of XZK (300 mg/kg) significantly reduced the
levels of TC, TG and LDL-C, and increased the levels of HDL-C
(P<0.001) when compared with the rats fed a high-fat diet alone,
which is in agreement with the results of previous studies
(5,9–11).
In addition, the results demonstrated that administration of XZK
(300 mg/kg) restored the damaged kidney tissue and significantly
reduced the levels of SOD, BUN, Scr, UA Ccr and albuminuria, while
increasing the levels of MDA, CAT and GSH-px, as compared with the
rats fed a high-fat diet. These results are consistent with a
previous study (16), indicating
that XZK is a safe and effective therapeutic agent that is able to
reduce kidney injury caused by dyslipidemia.
Lipid deposition within arterial subintimal space is
considered to be an important step in the formation of
atherosclerotic plaques, preceding the increased expression of
adhesion molecules on the endothelial cell surface and the
subsequent leukocyte recruitment from the blood stream (31). Proinflammatory cytokines are
hypothesized to have an important role in
lipopolysaccharide-mediated endothelial damage, through the uptake
of oxidized LDL via increased expression levels of macrophage
scavenger receptors (32), and by
regulating plaque stability (33),
which may be important processes in the pathogenesis of
atherosclerosis (34). However,
the area in which the cytokines are produced is important. In the
plasma, TNF-α and IL-6 may induce endothelial cell damage (35), whereas cytokines produced in the
atherosclerotic plaque stimulate cell proliferation and the
migration of smooth muscle cells and macrophages (34), reducing the stability of the plaque
(33). Thus, the downregulation of
TNF-α and IL-6 is necessary in the treatment of cardiovascular
disease. In the present study, XZK was shown to reduce the serum
expression levels of TNF-α and IL-6 in the hyperlipidemia rat
model. In addition, the levels of TNF-α were higher in the
hyperlipidemia group than in the healthy control rats,
demonstrating that hyperlipidemia causes the upregulation of
proinflammatory cytokines and leads to liver tissue damage. Normal
expression levels of TNF-α and IL-6 in the blood of healthy
individuals aid the prevention of infection and enhance immune
function; however, overexpression may result in injury to tissues
(36). In the present study, the
renal expression levels of TNF-α and IL-6 were increased in the
hyperlipidemia group when compared with the normal control group,
and XZK treatment was shown to reduce TNF-α and IL-6 expression
compared with the hyperlipidemia group, indicating that TNF-α and
IL-6 may be involved in kidney damage, and XZK may function by
reducing the expression levels of TNF-α and IL-6.
In conclusion, the present study demonstrated that
the administration of XZK (300 mg/kg) reduces kidney injury caused
by hyperlipidemia, downregulates the levels of TG, TC and LDL-C, as
well as the expression levels of inflammatory transcription
factors, and upregulates HDL-C. These results are likely to
contribute towards improving the understanding of the molecular
pathogenesis and mechanisms underlying hyperlipidemia, and may aid
the development of XZK as an effective therapeutic agent for
hyperlipidemia.
Acknowledgements
The study was supported a grant from the Science and
Technology Research and Innovation Team of Jilin Province (no.
JL20130612).
References
|
1
|
Suk HY, Zhou C, Yang TT, et al: Ablation
of calcineurin Aβ reveals hyperlipidemia and signaling cross-talks
with phosphodiesterases. J Biol Chem. 288:3477–3488. 2013.
|
|
2
|
Pöss J, Custodis F, Werner C, Weingärtner
O, Böhm M and Laufs U: Cardiovascular disease and dyslipidemia:
beyond LDL. Curr Pharm Des. 17:861–870. 2011.PubMed/NCBI
|
|
3
|
Vaziri ND and Norris K: Lipid disorders
and their relevance to outcomes in chronic kidney disease. Blood
Purif. 31:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burst V and Benzing T: Dyslipidemia
treatment and cardiovascular disease in the renal patient. Curr
Pharm Des. 17:894–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu ZL, Xu ZM, Kou WR and Zhao SP: Advance
in basic and clinical research of Xuezhikang capsule. Chin J Integr
Med. 12:85–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prospective Studies Collaboration.
Lewington S, Whitlock G, Clarke R, et al: Blood cholesterol and
vascular mortality by age, sex, and blood pressure: a meta-analysis
of individual data from 61 prospective studies with 55,000 vascular
deaths. Lancet. 370:1829–1839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Q, Liu Z, Chen K, Xu H and Liu J: A
systematic review of Xuezhikang, an extract from red yeast rice,
for coronary heart disease complicated by dyslipidemia. Evid Based
Complement Alternat Med. 2012:6365472012.PubMed/NCBI
|
|
8
|
Ma J, Li Y, Ye Q, et al: Constituents of
red yeast rice, a traditional Chinese food and medicine. J Agric
Food Chem. 48:5220–5225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu XY, Li P, Yang YB and Liu ML:
Xuezhikang, extract of red yeast rice, improved abnormal
hemorheology, suppressed caveolin-1 and increased eNOS expression
in atherosclerotic rats. PLoS One. 8:e627312013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Journoud M and Jones PJ: Red yeast rice: a
new hypolipidemic drug. Life Sci. 74:2675–2683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Zhang J, Shi Y, Grimsgaard S,
Alraek T and Fønnebø V: Chinese red yeast rice (Monascus
purpureus) for primary hyperlipidemia: a meta-analysis of
randomized controlled trials. Chin Med. 1:42006. View Article : Google Scholar
|
|
12
|
Lu Z, Kou W, Du B, et al; Chinese Coronary
Secondary Prevention Study Group. Effect of Xuezhikang, an extract
from red yeast Chinese rice, on coronary events in a Chinese
population with previous myocardial infarction. Am J Cardiol.
101:1689–1693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong XZ, Li LD and Wu LM: Effects of
fenofibrate and xuezhikang on high-fat diet-induced non-alcoholic
fatty liver disease. Clin Exp Pharmacol Physiol. 34:27–35. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar
|
|
15
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
16
|
Pan D, Zhang D, Wu J, et al: A novel
proteoglycan from Ganoderma lucidum fruiting bodies protects
kidney function and ameliorates diabetic nephropathy via its
antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol.
63:111–118. 2014.
|
|
17
|
Tietge UJ: Hyperlipidemia and
cardiovascular disease: inflammation, dyslipidemia, and
atherosclerosis. Curr Opin Lipidol. 25:94–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin SS, Blaha MJ, Blankstein R, et al:
Dyslipidemia, coronary artery calcium, and incident atherosclerotic
cardiovascular disease: implications for statin therapy from the
multi-ethnic study of atherosclerosis. Circulation. 129:77–86.
2014. View Article : Google Scholar
|
|
19
|
Schaeffner ES, Kurth T, Curhan GC, et al:
Cholesterol and the risk of renal dysfunction in apparently healthy
men. J Am Soc Nephrol. 14:2084–2091. 2003.PubMed/NCBI
|
|
20
|
Mänttäri M, Tiula E, Alikoski T and
Manninen V: Effects of hypertension and dyslipidemia on the decline
in renal function. Hypertension. 26:670–675. 1995.PubMed/NCBI
|
|
21
|
Takemura T, Yoshioka K, Aya N, et al:
Apolipoproteins and lipoprotein receptors in glomeruli in human
kidney diseases. Kidney Int. 43:918–927. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abrass CK: Cellular lipid metabolism and
the role of lipids in progressive renal disease. Am J Nephrol.
24:46–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tonelli M, Moyé L, Sacks FM, et al;
Cholesterol and Recurrent Events Trial Investigators. Effect of
pravastatin on loss of renal function in people with moderate
chronic renal insufficiency and cardiovascular disease. J Am Soc
Nephrol. 14:1605–1613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peev V, Nayer A and Contreras G:
Dyslipidemia, malnutrition, inflammation, cardiovascular disease
and mortality in chronic kidney disease. Curr Opin Lipidol.
25:54–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y, Guo R, Wang X, et al: The
regulation of alfalfa saponin extract on key genes involved in
hepatic cholesterol metabolism in hyperlipidemic rats. PLoS One.
9:e882822014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arafa HM: Curcumin attenuates diet-induced
hypercholesterolemia in rats. Med Sci Monit. 11:BR228–BR234.
2005.PubMed/NCBI
|
|
27
|
Martin SS, Blaha MJ, Blankstein R, et al:
Dyslipidemia, coronary artery calcium, and incident atherosclerotic
cardiovascular disease: implications for statin therapy from the
multi-ethnic study of atherosclerosis. Circulation. 129:77–86.
2014. View Article : Google Scholar
|
|
28
|
Tolman KG: The liver and lovastatin. Am J
Cardiol. 89:1374–1380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Campese VM: Dyslipidemia and progression
of kidney disease: role of lipid-lowering drugs. Clin Exp Nephrol.
18:291–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang Q, Liu Z, Chen K, Xu H and Liu J: A
systematic review of xuezhikang, an extract from red yeast rice,
for coronary heart disease complicated by dyslipidemia. Evid Based
Complement Alternat Med. 2012:6365472012.PubMed/NCBI
|
|
31
|
Lin YC, Chiang CH, Chang LT, et al:
Simvastatin attenuates the additive effects of TNF-α and IL-18 on
the connexin 43 up-regulation and over-proliferation of cultured
aortic smooth muscle cells. Cytokine. 62:341–351. 2013.PubMed/NCBI
|
|
32
|
Li Z, Yang S, Lin H, et al: Probiotics and
antibodies to TNF inhibit inflammatory activity and improve
nonalcoholic fatty liver disease. Hepatology. 37:343–350. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Libby P, Sukhova G, Lee RT and Galis ZS:
Cytokines regulate vascular functions related to stability of the
atherosclerotic plaque. J Cardiovasc Pharmacol. 25(Suppl 2):
S9–S12. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ross R: The pathogenesis of
atherosclerosis: a perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Zee KJ, Kohno T, Fischer E, Rock CS,
Moldawer LL and Lowry SF: Tumor necrosis factor soluble receptors
circulate during experimental and clinical inflammation and can
protect against excessive tumor necrosis factor alpha in vitro and
in vivo. Proc Natl Acad Sci USA. 89:4845–4849. 1992.
|
|
36
|
Fan XF, Deng YQ, Ye L, et al: Effect of
Xuezhikang Capsule on serum tumor necrosis factor-alpha and
interleukin-6 in patients with nonalcoholic fatty liver disease and
hyperlipidemia. Chin J Integr Med. 16:119–123. 2010. View Article : Google Scholar : PubMed/NCBI
|