Introduction
Mycobacterium tuberculosis (Mtb) is a major
international public health problem, with one-third of the world’s
population latently infected by Mtb. In these cases, active
tuberculosis (TB) disease will develop following failure of their
immune system (1,2). The age-standardized prevalence rates
of total diabetes and prediabetes were 9.7% (males, 10.6%; females,
8.8%) and 15.5% (males, 16.1%; females, 14.9%), respectively, in
China (3). Immune function may
decrease in diabetes mellitus (DM) subjects who are susceptible to
pulmonary tuberculosis (PTB). Therefore, increased research should
focus on cases of DM with PTB (DMPTB).
Serum 25-hydroxyvitamin D3
[25(OH)D3] is a valid measure of the vitamin D status
(4).
1,25(OH)2D3 is an active form of vitamin
D3 that functions as an immunomodulator in immune
homeostasis (5,6), and has been shown to restrain the
growth of Mtb (7). Deficient serum
vitamin D levels have been associated with the incidence of TB
(6,8,9) and
DM (10,11), while sufficient serum
25(OH)D3 levels have been shown to protect against TB or
DM (5,12). The interaction of
1,25(OH)2D3 with its receptor on T
lymphocytes may play an important role in the regulation of the
immune status (13). A number of
studies have indicated that 1,25(OH)2D3 may
be a successful anti-TB therapeutic agent (7,14).
Antimicrobial peptide cathelicidin (LL-37), an
endogenous antimicrobial peptide synthesized by neutrophils,
monocytes, T cells and other immune cells in a vitamin D-dependent
manner, has been demonstrated to function against Mtb infection
using in vitro and in vivo models (15–17).
In addition, vitamin D can restrict the acquired immune response
against TB by regulating the production of cytokines.
1,25(OH)2D3 has been shown to differentially
modulate the production of cytokines in response to Mtb antigens by
predominantly suppressing interferon (IFN)-γ production in a
dose-dependent manner (18).
Furthermore, a number of studies (19,20)
have indicated that interleukin (IL)-17A plays a critical role in
the prevention of Mtb infection via the induction of mature
granuloma formation.
However, the status of 25(OH)D3 and LL-37
is not clear in patients with PTB complicated with DM. To the best
of our knowledge, T helper (Th)-associated cytokines, including
IFN-γ, IL-4 and IL-17, have not been analyzed in patients with
DMPTB. Therefore, the aim of the present study was to investigate
the status of 25(OH)D3, LL-37 and Th-associated
cytokines, in order to evaluate the association with PTB or
DMPTB.
Materials and methods
Study population and specimen
collection
In total, 90 participants were enrolled in the
study. The study protocol was approved by the Clinical Research
Ethics Committee of Shandong Provincial Chest Hospital (Jinan,
China), and informed consent was obtained from every participant
who provided blood samples voluntarily for the study. DM was
diagnosed by the following parameters: Fasting plasma glucose (FPG)
level of ≥126 mg/dl (7.0 mmol/l), symptoms of hyperglycemia, casual
plasma glucose level of ≥200 mg/dl (11.1 mmol/l) and 2 h plasma
glucose level of ≥200 mg/dl (11.1 mmol/l), measured during an oral
glucose tolerance test. PTB was diagnosed by a positive sputum
acid-fast bacillus smear or a typical PTB image from a chest
computed tomography scan, and an effective outcome following
anti-TB therapy. Individuals were excluded from the study if they
exhibited a clinical manifestation of infection or had been
administered corticosteroids, diuretics or supplementary vitamin
D3 in the three months previously. The patients were
divided into three groups, which included 30 normal controls (NC
group), 30 PTB patients (PTB group) and 30 DM and PTB patients
(DMPTB group). The NC group comprised volunteers, while the PTB and
DMPTB groups consisted of inpatients from the Shandong Provincial
Chest Hospital. Patient characteristics, including age, gender and
FPG, were collected. The blood sample from patients fasted for 10 h
was collected using EDTA K2 tubes and separated in gel
coagulating tubes (Shandong Chengwu Yongkang Medical Products
Company, Chengwu, China). All fresh specimens were transported on
ice, and the plasma and serum samples were separated within 20 min
by centrifugation at 1,500 × g at 4°C for 15 min. The EDTA plasma
samples required for LL-37 analysis and the serum samples used for
vitamin D3 analysis were stored in fresh polypropylene
tubes at −70°C. The serum samples required for cytokines analysis
were stored at −20°C.
25(OH)D3 measurement
Serum samples were stored at −70°C. Reagents,
including methanol, methyl cyanides, n-hexane and anhydrous ethanol
(Merck KGaA, Darmstadt, Germany), standard substance (SRM972,
Sigma-Aldrich, St. Louis, MO, USA), internal standard product
(Advanced Medical Isotopes Corporation, Kennewick, WA, USA) and
quality control (RECIPE, Munich, Germany). Serum
25(OH)D3 levels were measured by liquid
chromatography-tandem mass spectrometry on an API 4000 mass
spectrometer (Applied Biosystems Life Technologies, Foster City,
CA, USA) in the Jinyu Medical Test Center (Guangzhou, China).
Human LL-37 measurement
A solid-phase enzyme-linked immunosorbent assay
(HK321, HyCult Biotechnology, Uden, Netherlands) was used to
measure the levels of LL-37. According to the manufacturer’s
instructions, the samples and standards were incubated in
microtiter wells coated with mouse monoclonal antibodies
recognizing against human LL-37 that were provided with the kit. A
biotinylated tracer antibody that was conjugated to
streptovidin-peroxidase was used to bind to the human LL-37. The
conjugated streptovidin-peroxidase reacted with the substrate and
tetramethylbenzidine, and the enzyme reaction was stopped following
the addition of oxalic acid. Absorbance was measured at 450 nm with
a spectrophotometer. A standard curve was obtained by plotting the
absorbance values against the corresponding concentrations of the
human LL-37 standards. The concentration of human LL-37 in the
samples, which were run concurrently with the standards, was
determined from the standard curve.
Cytokine measurement
A human Th1/Th2/Th9/Th17/Th22 13 Plex FlowCytomix
kit (BMS817FF, eBioscience, Inc., San Diego, CA, USA) was used to
determine the levels of the various cytokines by flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). Analysis
was performed with FlowCytomixTM Pro 3.0 software
(eBioscience, Inc.). The principle of this test was the fluorescent
bead immunoassay. Fluorescent beads were conjugated to each target
analyte followed by addition of a biotin-conjugated secondary
detection antibody and a dye that functions as a reporter. In this
experiment streptavidin-conjugated phycoerythrin dye was used and
the different intensities of the fluorescent beads bound to the
antibody were measured. The concentration of the various analytes
was calculated using a standard curve.
Statistical analysis
Results are expressed as the mean ± standard
deviation and statistical analyses were performed using SPSS 14.0
for Windows (SPSS, Inc. Chicago, IL, USA). Comparisons between mean
values were analyzed by one-way analysis of variance using the
least significant difference test and Tamhane’s T2 test, while
categorical variables were analyzed using the χ2 test.
Correlation analyses were assessed using Pearson’s test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Participants and clinical parameters
A total of 90 participants were enrolled in the
study, which comprised three groups (NC, PTB and DMPTB). In the NC
group, the mean age was 38.83±12.88 years, and the male/female
ratio was 14/16. In the PTB group, the mean age was 36.83±16.02
years, and there were 18 males and 12 females. In the DMPTB group,
the mean age was 43.90±12.85 years, and the male/female ratio was
14/16. No statistically significant differences were observed among
the groups with regard to the mean age or male/female ratio
(P>0.05). However, the FPG level in the DMPTB group (11.05±3.62
mmol/l) was significantly higher compared with the NC (4.73±0.55
mmol/l, P<0.001) and PTB groups (4.80±0.54 mmol/l, P<0.001).
A statistically significant difference was not observed in the FPG
between the NC and PTB groups (P=0.952). The data are summarized in
Table I.
 | Table IClinical characteristics of the
participants. |
Table I
Clinical characteristics of the
participants.
| Group | Cases, n | Age, years (mean ±
SD) | Gender, n
(male/female) | FPG, mmol/l |
|---|
| NC | 30 | 38.83±12.88 | 14/16 | 4.73±0.55 |
| PTB | 30 | 36.83±16.02 | 18/12 | 4.80±0.54 |
| DMPTB | 30 | 43.90±12.85 | 14/16 | 11.05±3.62a |
Concentration of serum
25(OH)D3 and plasma LL-37
Serum 25(OH)D3 concentrations in the NC
group (17.49±7.50 ng/ml) were markedly higher compared with the
levels in the PTB (12.04±6.08 ng/ml; P<0.01) and DMPTB groups
(11.36±4.85 ng/ml; P<0.01). No statistically significant
difference was observed in the 25(OH)D3 concentration
between the PTB and DMPTB groups. In addition, the plasma level of
LL-37 in the NC group (32.20±10.14 ng/ml) was significantly lower
compared with the PTB (44.53±16.88 ng/ml) and DMPTB groups
(57.52±34.17 ng/ml; P<0.01). The concentration of plasma LL-37
was not significantly different between the DMPTB and PTB groups
(P=0.950; Table II; Fig. 1A and B).
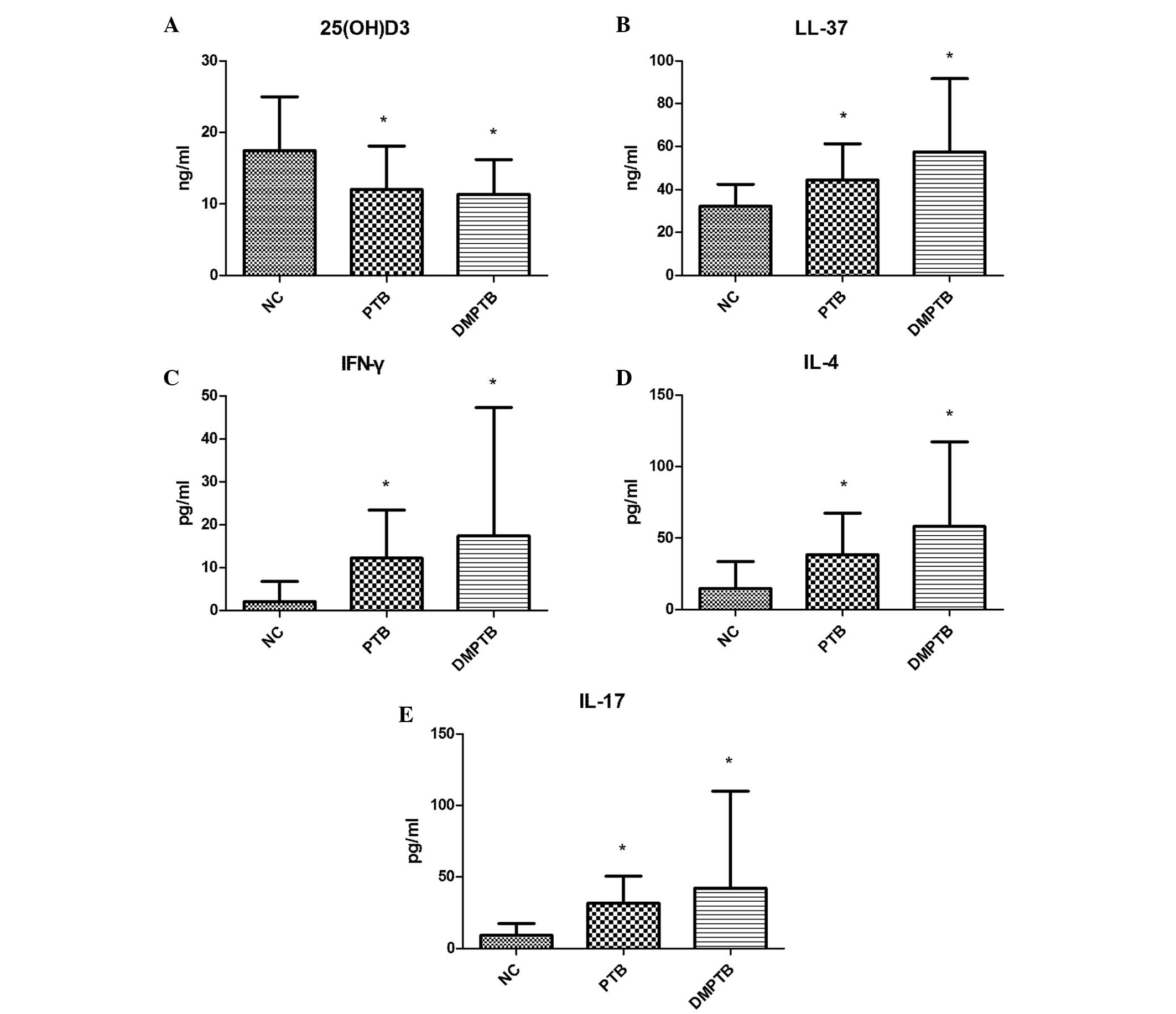 | Figure 1Concentration of (A) serum
25(OH)D3, (B) plasma LL-37, serum (C) IFN-γ, (D) IL-4
and (E) IL-17 in the NC, PTB and DMPTB groups. Serum levels of
25(OH)D3 significantly decreased, while plasma levels of
LL-37 and serum levels of IFN-γ, IL-4 and IL-17 markedly increased
in the PTB and DMPTB groups when compared with the NC group.
*P<0.05, vs. NC. NC, normal control; PTB, pulmonary
tuberculosis; DMPTB, diabetes mellitus and pulmonary tuberculosis;
25(OH)D3, 25-hydroxyvitamin D3; IL,
interleukin; IFN, interferon; LL-37, antimicrobial peptide
cathelicidin. |
 | Table IILevels of 25(OH)D3, LL-37
and cytokines in the blood. |
Table II
Levels of 25(OH)D3, LL-37
and cytokines in the blood.
| Index | NC (n=30) | PTB (n=30) | DMPTB (n=30) |
|---|
| 25(OH)D3,
ng/ml | 17.49±7.50 | 12.04±6.08a | 11.36±4.85a |
| LL-37, ng/ml | 32.20±10.14 | 44.53±16.88a | 57.52±34.17a |
| IFN-γ, pg/ml | 2.09±4.66 | 12.23±11.18b | 17.43±29.90b |
| IL-4, pg/ml | 14.72±18.86 | 38.37±28.98b | 58.18±58.96b |
| IL-17, pg/ml | 9.33±8.15 | 31.56±19.07b | 42.24±67.70b |
Levels of Th-associated cytokines
Serum levels of IFN-γ, IL-4 and IL-17 in the PTB
group (12.23±11.18, 38.37±28.98 and 31.56±19.07 pg/ml,
respectively) and DMPTB group (17.43±29.90, 58.18±58.96 and
42.24±67.70 pg/ml, respectively) were found to be significantly
higher compared with the levels in the NC group (2.09±4.66,
14.72±18.86 and 9.33±8.15 pg/ml, respectively; P<0.05). In
addition, no statistically significant difference was observed
between the PTB and DMPTB groups (P>0.05; Table II; Fig. 1C–E).
Correlations among vitamin D, LL-37 and
Th-associated cytokines in the PTB and DMPTB patients
Correlations among the levels of vitamin D, LL-37
and Th-associated cytokines are summarized in Table III. The IFN-γ level was found to
be negatively correlated with the LL-37 concentration (r=−0.379,
P<0.001), but strongly positively correlated with IL-4 (r=0.616,
P<0.001) and IL-17 (r=0.790, P<0.001). In addition, a
significant positive correlation was observed between the levels of
IL-4 and IL-17 (r=0.580, P<0.001). However, no significant
correlation was identified between the vitamin D concentration and
the levels of LL-37 (r=0.226, P=0.082), IFN-γ (r=−0.075, P=0.568),
IL-4 (r=0.030, P=0.818) and IL-17 (r=0.064, P=0.627). Furthermore,
no statistically significant correlation was identified between the
level of LL-37 and IL-4 (r=−0.182, P=0.164) or IL-17 (r=−0.052,
P=0.694).
 | Table IIICorrelations among vitamin D, LL-37
and Th-associated cytokines in patients with PTB and DMPTB. |
Table III
Correlations among vitamin D, LL-37
and Th-associated cytokines in patients with PTB and DMPTB.
| Index | LL-37 | IL-4 | IL-17 | IFN-γ |
|---|
| IFN-γ | r=−0.379 | r=0.616 | r=0.790 | - |
| P<0.001 | P<0.001 | P<0.001 | - |
| IL-4 | r=−0.182 | - | r=0.580 | r=0.616 |
| P=0.164 | - | P<0.001 | P<0.001 |
| Vitamin D | r=0.226 | r=0.030 | r=0.064 | r=−0.075 |
| P=0.082 | P=0.818 | P=0.627 | P=0.568 |
| IL-17 | r=−0.052 | r=0.580 | - | r=0.790 |
| P=0.694 | P<0.001 | - | P<0.001 |
Discussion
The host immune system plays an important role in
the development of PTB. Novel immune strategies are crucial to the
diagnosis and therapy of PTB, and the correlation between vitamin D
levels and the immune status has resulted in an increasing number
of studies investigating vitamin D.
Vitamin D is a type of hormone that functions in the
maintenance of immune homeostasis (6,8). A
limited number of studies have been published investigating the
levels of vitamin D and LL-37 in patients with DM and PTB. Thus,
the present study analyzed the levels of vitamin D and LL-37 in NC,
PTB and DMPTB patients. The concentration of vitamin D in the NC
group was significantly higher compared with the PTB and DMPTB
groups. A previous study (21)
reported that the mean 25(OH)D3 levels for the entire
population were in the ‘insufficient’ range (21.3±9.78 ng/ml), but
were observed to be lower in PTB patients (21). Vitamin D deficiencies were observed
in the PTB and DMPTB patients in the present study. Previous
studies (4,15) have demonstrated that individuals
who are vitamin D deficient or have insufficient levels of vitamin
D are more susceptible to Mtb infections. Hypovitaminosis D may
predispose individuals to multidrug-resistant TB (MDR-TB) and
increase the time in which MDR-TB sputum smear negativity is
achieved (22).
In the present study, the plasma level of LL-37 was
44.53±16.88 ng/ml in the PTB group. Yamshchikov et al found
that the mean serum LL-37 concentration for PTB patients was 49.5
ng/ml, and that the serum vitamin D level exhibited no correlation
with the plasma LL-37 level (15).
Stored serum specimens used in the study by Yamshchikov differed
from the EDTA plasma samples of fresh blood transported on ice used
in the present study. Temperature can influence the results of the
blood LL-37. However, the results of the LL-37 blood levels were
similar in the two studies. In the current study, the serum
25(OH)D3 and plasma LL-37 levels were not shown to
correlate.
Plasma levels of LL-37 in the PTB (44.53±16.88
ng/ml) and DMPTB groups (57.52±34.17 ng/ml) were significantly
higher compared with the NC group in the present study (P<0.01).
Yamshchikov et al hypothesized that higher LL-37
concentrations correlated with acid fast bacilli sputum smear
positivity. Thus, the circulating LL-37 level may be a potential
biomarker in patients with active TB disease (15). The results of the present study
also support the hypothesis that elevated plasma levels of LL-37 in
PTB patients may be a potential biomarker for PTB. Furthermore, an
additional study indicated that administration of oral
4-phenylbutyrate with vitamin D3 induces LL-37 peptide
expression in functional immune cells and enhances intracellular
Mtb death in macrophages (23).
In the present study, IFN-γ, IL-4 and IL-17 levels
in the PTB and DMPTB patients were found to markedly increase when
compared with the NC group. IFN-γ exhibited a positive correlation
with IL-17, and was shown to negatively correlate with LL-37.
Previous studies have demonstrated that vitamin D may downregulate
the recruitment and activation of T cells at infection sites,
including those of PTB (24,25).
Furthermore, a previous study reported that patients with DMPTB
were characterized by elevated frequencies of Th1 and Th17 cells,
which may contribute to the increased immune pathology observed in
Mtb infections (26). The present
study considered IFN-γ and IL-17 as a compensatory response that
enhanced the anti-inflammatory reaction and as an excessive immune
reaction that accelerated the damage in the PTB and DMPTB patients.
An associated study indicated that the specific secretion of
soluble immunological factors, in addition to IFN-γ, may be used to
evaluate Mtb infection and TB (27). Thus, IFN-γ is a good marker for Mtb
infection (28).
Vitamin D supplements for chronic inflammation have
been a prospective research subject in recent years. A number of
studies have demonstrated that vitamin D supplements are pertinent
in the treatment of TB (5,21). In addition, vitamin D has been
demonstrated to significantly hasten sputum culture conversion in
participants with the TT genotype of the TaqI vitamin D receptor
polymorphism (29). Therefore,
future studies investigating vitamin D therapy for DM with PTB are
required. The use of LL-37 and IL-17 as new biomarkers need more
specimens in order to verify their feasibility for future
studies.
In conclusion, lower serum levels of vitamin D were
observed in patients with PTB, particularly those also suffering
from DM. In addition, the plasma levels of LL-37, serum IFN-γ and
IL-17 increased in the compensatory response observed in PTB
patients. Therefore, LL-37 and IL-17 may serve as potential
biomarkers for the diagnosis of TB, and vitamin D may be used as a
potential adjuvant treatment for TB.
Acknowledgements
The authors thank the patients and control subjects
who participated in the study, and Ms. Xinxin Li (Department of
Hanguang Central Laboratory, Shandong Provincial Chest Hospital,
Jinan, Shandong) for the technical assistance. The study was
supported in part by a grant from the National Natural Science
Foundation of China (no. 81272181).
References
|
1
|
O’Garra A, Redford PS, McNab FW, Bloom CI,
Wilkinson RJ and Berry MP: The immune response in tuberculosis.
Annu Rev Immunol. 31:475–527. 2013. View Article : Google Scholar
|
|
2
|
Zuñiga J, Torres-García D, Santos-Mendoza
T, Rodriguez-Reyna TS, Granados J and Yunis EJ: Cellular and
humoral mechanisms involved in the control of tuberculosis. Clin
Dev Immunol. 2012:1939232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X,
Zhao Z, Li Q, Zhou Z, Shan G and He J: China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friis H, Range N, Pedersen ML, Mølgaard C,
Changalucha J, Krarup H, Magnussen P, Søborg C and Andersen AB:
Hypovitaminosis D is common among pulmonary tuberculosis patients
in Tanzania but is not explained by the acute phase response. J
Nutr. 138:2474–2480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arnedo-Pena A, Juan-Cerdán JV,
Romeu-Garcia A, Garcia-Ferrer D, Holguín-Gómez R, Iborra-Millet J,
Herrero-Carot C, Piñana MJ, Bellido-Blasco J, Ferrero-Vega JA,
Adsuara LS, Silvestre ES, Ferrer NM and Bartual VR: Latent
tuberculosis infection, tuberculin skin test and vitamin D status
in contacts of tuberculosis patients: a cross-sectional and
case-control study. BMC Infect Dis. 11:3492011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun RF, Adams JS and Hewison M:
Immunomodulation by vitamin D: implications for TB. Expert Rev Clin
Pharmacol. 4:583–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cadranel J, Garabedian M, Milleron B,
Guillozo H, Akoun G and Hance AJ: 1,25(OH)2D2
production by T lymphocytes and alveolar macrophages recovered by
lavage from normocalcemic patients with tuberculosis. J Clin
Invest. 85:1588–1593. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baeke F, Takiishi T, Korf H, Gysemans C
and Mathieu C: Vitamin D: modulator of the immune system. Curr Opin
Pharmacol. 10:482–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desai NS, Tukvadze N, Frediani JK, Kipiani
M, Sanikidze E, Nichols MM, Hebbar G, Kempker RR, Mirtskhulava V,
Kalandadze I, Seydafkan S, Sutaria N, Chen TC, Blumberg HM, Ziegler
TR and Tangpricha V: Effects of sunlight and diet on vitamin D
status of pulmonary tuberculosis patients in Tbilisi, Georgia.
Nutrition. 28:362–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi HS, Kim KA, Lim CY, Rhee SY, Hwang
YC, Kim KM, Kim KJ, Rhee Y and Lim SK: Low serum vitamin D is
associated with high risk of diabetes in Korean adults. J Nutr.
141:1524–1528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chagas CE, Borges MC, Martini LA and
Rogero MM: Focus on vitamin D, inflammation and type 2 diabetes.
Nutrients. 4:52–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalgård C, Petersen MS, Weihe P and
Grandjean P: Vitamin D status in relation to glucose metabolism and
type 2 diabetes in septuagenarians. Diabetes Care. 34:1284–1288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biyoudi-Vouenze R, Cadranel J, Valeyre D,
Milleron B, Hance AJ and Soler P: Expression of
1,25(OH)2D3 receptors on alveolar lymphocytes
from patients with pulmonary granulomatous diseases. Am Rev Respir
Dis. 143:1376–1380. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kota SK, Jammula S, Kota SK, Tripathy PR,
Panda S and Modi KD: Effect of vitamin D supplementation in type 2
diabetes patients with pulmonary tuberculosis. Diabetes Metab
Syndr. 5:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamshchikov AV, Kurbatova EV, et al:
Vitamin D status and antimicrobial peptide cathelicidin (LL-37)
concentrations in patients with active pulmonary tuberculosis. Am J
Clin Nutr. 92:603–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rivas-Santiago B, Rivas, Santiago CE,
Castañeda-Delgado JE, León-Contreras JC, Hancock RE and
Hernandez-Pando R: Activity of LL-37, CRAMP and antimicrobial
peptide-derived compounds E2, E6 and CP26 against Mycobacterium
tuberculosis. Int J Antimicrob Agents. 41:143–148. 2013. View Article : Google Scholar
|
|
17
|
Martineau AR, Newton SM, Wilkinson KA,
Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ
and Wilkinson RJ: Neutrophil-mediated innate immune resistance to
mycobacteria. J Clin Invest. 117:1988–1994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vidyarani M, Selvaraj P, Jawahar MS and
Narayanan PR: 1,25 Dihydroxyvitamin D3 modulated
cytokine response in pulmonary tuberculosis. Cytokine. 40:128–134.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okamoto Yoshida Y, Umemura M, et al:
Essential role of IL-17A in the formation of a mycobacterial
infection-induced granuloma in the lung. J Immunol. 184:4414–4422.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umemura M, Yahagi A, Hamada S, et al:
IL-17-mediated regulation of innate and acquired immune response
against pulmonary Mycobacterium bovis bacille Calmette-Guerin
infection. J Immunol. 178:3786–3796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel
M and Mahmood F: Vitamin D accelerates clinical recovery from
tuberculosis: results of the SUCCINCT Study [Supplementary
Cholecalciferol in recovery from tuberculosis]. A randomized,
placebo-controlled, clinical trial of vitamin D supplementation in
patients with pulmonary tuberculosis’. BMC Infect Dis. 13:222013.
View Article : Google Scholar
|
|
22
|
Rathored J, Sharma SK, Singh B, et al:
Risk and outcome of multidrug-resistant tuberculosis: vitamin D
receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis.
16:1522–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mily A, Rekha RS, Kamal SM, Akhtar E,
Sarker P, Rahim Z, Gudmundsson GH, Agerberth B and Raqib R: Oral
intake of phenylbutyrate with or without vitamin D3
upregulates the cathelicidin LL-37 in human macrophages: a dose
finding study for treatment of tuberculosis. BMC Pulm Med.
13:232013. View Article : Google Scholar
|
|
24
|
Selvaraj P, Harishankar M, Singh B,
Banurekha VV and Jawahar MS: Effect of vitamin D3 on
chemokine expression in pulmonary tuberculosis. Cytokine.
60:212–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prabhu Anand S, Selvaraj P and Narayanan
PR: Effect of 1,25 dihydroxyvitamin D3 on intracellular
IFN-gamma and TNF-alpha positive T cell subsets in pulmonary
tuberculosis. Cytokine. 45:105–110. 2009. View Article : Google Scholar
|
|
26
|
Kumar NP, Sridhar R, Banurekha VV, Jawahar
MS, Nutman TB and Babu S: Expansion of pathogen-specific T-helper 1
and T-helper 17 cells in pulmonary tuberculosis with coincident
type 2 diabetes mellitus. J Infect Dis. 208:739–748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Zhang Y, Hu S, Jin D, Chen X, Jin Q
and Liu H: Different patterns of cytokines and chemokines combined
with IFN-γ production reflect Mycobacterium tuberculosis infection
and disease. PLoS One. 7:e449442012. View Article : Google Scholar
|
|
28
|
Whitworth HS, Scott M, Connell DW, Dongés
B and Lalvani A: IGRAs - the gateway to T cell based TB diagnosis.
Methods. 61:52–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martineau AR, Timms PM, Bothamley GH,
Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC,
Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD,
Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK,
Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V,
Drobniewski FA, Eldridge SM and Griffiths CJ: High-dose vitamin
D3 during intensive-phase antimicrobial treatment of
pulmonary tuberculosis: a double-blind randomised controlled trial.
Lancet. 377:242–250. 2011. View Article : Google Scholar : PubMed/NCBI
|















