Introduction
Hypoxia is a very important factor affecting the
health and life activities of individuals at high altitude, which
has serious impacts on physiology and induces pathological changes
in the body (1). There is a close
link between these changes and drug pharmacokinetics, for example,
blood pH has an impact on the absorption and distribution of drugs
(2); hypoxemia and acid-base
imbalance have significant effects on the absorption, distribution,
metabolism and excretion of drugs. Therefore, arterial blood gas
analysis is of great significance for pharmacokinetics. In
addition, protein binding rates influence the distribution of drugs
and plasma drug concentrations; cardiac function affects blood
rheology and distribution; and liver and renal function influence
drug metabolism and excretion (3),
which seriously affect the therapeutic effects and side-effects of
drugs (2). As a result, studies
concerning the physiological and pathological changes in rats from
low altitude that are acutely exposed to high altitude lay an
important foundation for further research on the impacts of high
altitude and low oxygen levels on drug pharmacokinetics. They may
provide guidance for the adjustment of drug usage and dosage in
individuals who are acutely exposed to high altitude or live at
high altitude for a long time, with the aim of achieving optimal
treatment outcome and reduced drug side-effects.
In the present study, the physiological and
pathological effects in Wistar rats of acute exposure to a high
altitude of 4,010 m followed by a return to low altitude were
investigated in order to identify the associations between high
altitude and drug pharmacokinetics.
Materials and methods
Equipment and reagents
An automatic blood gas system (ABL80; Radiometer
Medical, Brønshoj, Denmark), automatic biochemistry analyzer (LX20;
Beckman Coulter, Inc., Brea, CA, USA), high speed centrifuge
(TGL-16B; Shenzhen Anke High-Tech Co., Ltd., Shenzhen, China),
hand-held GPS (Planet Neptun 500E, China Magellan Corporation,
Beijing) and electron microscope (BX-51; Olympus, Tokyo, Japan)
were used.
Animals
A total of 21 healthy and clean male Wistar rats
(Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China;
Certificate number: 2007000524909) were used in the study. The rats
weighed 200±20 g and all originated from a low altitude area. They
were randomly divided into group A, which was maintained at low
altitude (Shanghai, 31°30′NW, 121°52′EL; 55 m above sea level;
24°C; pressure, 95.6 kPa; relative humidity, 73%); group B, which
was acutely exposed to high altitude (Maqu, Gansu, 33°97′NW,
102°04′EL; 4,010 m above sea level; −2°C; pressure, 62.1 kPa;
relative humidity, 48%) and group C, which was taken to high
altitude and then back to low altitude (n=7 per group). This study
was approved by the Ethical Committee of Lanzhou General Hospital
of Lanzhou Command (Lanzhou, China).
Animal treatment methods
The rats in group A were normally fed at low
altitude (Shanghai; 55 m) and had an average weight of 203 g. The
rats in group B were acutely exposed to high altitude (4,010 m),
where they were fed normally for 72 h, and had an average weight of
198 g. The rats in group C were fed normally during acute exposure
to high altitude (4,010 m) for 72 h, and then returned to low
altitude where they were normally fed for a further 24 h; these
rats had an average weight of 207 g. The rats were transported by
aviation in a semi-enclosed polypropylene cage. In each group,
examination of the rats began after fasting for 12 h.
Blood gas analysis
In this study, 1 ml blood was taken from the
abdominal aorta following anesthetization by the injection of 1 ml
10% chloral hydrate into the peritoneal cavity. Blood gas analysis
was carried out immediately using the automatic blood gas system.
The indicators measured were pH, buffer base (BB), base excess
(BE), content of total carbon dioxide (ctCO2), oxygen
saturation of arterial blood (sO2), carbon dioxide
tension of arterial blood (pCO2), oxygen tension of arterial blood
(pO2), hemoglobin (Hb) level, sodium ion concentration
(cNa+), potassium ion concentration (cK+) and chloride
ion concentration (cCl−).
Biochemical indicator analysis
In this study, 3 ml blood was collected from the
superior vena orbitalis posterior into centrifuge tubes that
contained heparin sodium. The samples were centrifuged at 664 × g
for 10 min at room temperature and then analyzed with the automatic
biochemistry analyzer (4,5). The biochemical indicators were
lactate dehydrogenase (LHD), alanine aminotransferase (ALT),
aspartate aminotransferase (AST), alkaline phosphatase (ALP), total
protein (TP), total bilirubin (TBIL), glucose (GLU), urea and uric
acid (UA) (4,6,7).
Pathological changes
Cerebrum, lungs and kidneys were collected and put
into phosphate-buffered solution containing 10% formaldehyde
solution for longer than 24 h. The samples were fixed thoroughly.
Both kidneys were cut in half horizontally from the midline from
the lateral border to the renal hilum (deep into the kidney
calices) and were then stained with hematoxylin and eosin.
Pathological changes were observed under the electron
microscope.
Data analysis
All data are presented as the mean ± standard
deviation. Analysis of statistical significance was performed, and
P<0.05 was considered to indicate a statistically significant
result. An analysis of variance (SNK-q test) was used to perform
multiple comparison between the three groups. The analysis was
carried out using SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA).
Results
Comparison of blood gas analysis
The results of the blood gas analysis are presented
in Table I. Compared with the
values in group A, the pH, BB, BE, ctCO2,
sO2, pO2 and cNa+ values of group
B were significantly decreased by 2.43, 630, 311, 11.48, 91.38,
76.22 and 2.82% respectively, while the pCO2 and
cCl− values significantly increased by 47.40 and 6.76%,
respectively. In group C, the pH, BB, BE, sO2,
pO2, Hb and cNa+ values were significantly
decreased by 3.24, 542.00, 296.00, 92.89, 89.46, 32.32 and 4.20%,
respectively, while the pCO2 and cCl− values
were significantly increased by 75.49 and 4.25%, respectively. The
Hb level of group C was decreased by 25.82% compared with that in
group B.
 | Table IComparison of blood gas analysis (mean
± standard deviation; n=7). |
Table I
Comparison of blood gas analysis (mean
± standard deviation; n=7).
| Blood gas
indicators | Group A | Group B | Group C |
|---|
| pH | 7.40±0.03b,c | 7.22±0.17a | 7.16±0.07a |
| BB (mmol/l) | −0.84±0.91b,c | −6.15±3.89a | −5.40±2.48a |
| BE (mmol/l) | −1.11±0.90b,c | −4.57±3.49a | −4.4±1.96a |
| ctCO2
(mmol/l) | 24.74±0.80b | 21.90±1.30a,c | 23.5±0.68b |
| sO2
(%) | 92.5±0.97b,c | 7.97±4.68a | 6.57±5.52a |
| pCO2
(mmHg) | 38.32±3.56b,c | 56.5±20.24a | 67.25±10.34a |
| pO2
(mmHg) | 83.04±2.88b,c | 19.75±15.94a | 8.75±6.13a |
| Hb (g/dl) | 12.22±0.37c | 11.15±1.99c | 8.27±1.02a,b |
| cNa+
(mmol/l) | 144.57±0.78b,c | 140.50±4.50a | 138.5±2.51a |
| cK+
(mmol/l) | 5.07±0.34 | 4.97±0.41 | 5.27±0.73 |
| cCl−
(mmol/l) | 99.28±0.75b,c | 106.00±3.91a | 103.50±2.38a |
Statistical analysis demonstrated that the pH, BB,
BE, sO2 and pO2 were significantly reduced
while ctCO2 was significantly increased following the
acute exposure of the rats to high altitude.
Comparison of main biochemical
indicators
Biochemical indicators are main indicators for
evaluating the function of major organs. The biochemical indicator
results are presented in Table
II. Analysis of the results shows that the LHD and TP levels of
group B were significantly decreased by 58.44 and 26.82%,
respectively, compared with those in group A, while the TBIL and
ALP levels were severely increased by 338.00 and 24.94%,
respectively. The LHD and TP levels of group C were significantly
decreased by 5.98 and 17.41%, respectively, compared with those in
group A, while the TBIL and urea levels increased by 478 and
36.20%, respectively. The ALP level of group C decreased by 19.19%
compared with that in group B, whereas the TP, TBIL and urea levels
significantly increased by 12.85, 31.93 and 40.32%,
respectively.
 | Table IIComparison of biochemical indicators
(mean ± standard deviation; n=7). |
Table II
Comparison of biochemical indicators
(mean ± standard deviation; n=7).
| Biochemical
indicators | Group A | Group B | Group C |
|---|
| LHD (U/l) |
873.5±186.13b,c |
363.00±116.25a |
297.20±99.64a |
| AST (U/l) | 138.14±13.43 | 163.00±8.18 | 160.80±30.76 |
| ALT (U/l) | 54.71±5.9c | 65.66±14.36 | 72.00±5.24a |
| TP (g/l) | 64.85±2.67b,c | 47.46±6.59a,c | 53.56±9.22a,b |
| TBIL (μmol/l) | 0.65±0.26b,c | 2.85±0.45a,c | 3.76±0.37a,b |
| ALP (U/l) |
240.28±22.23b |
300.20±34.81a,c |
242.60±23.75b |
| GLU (mmol/l) | 5.11±1.05 | 6.20±2.99 | 6.80±0.96 |
| Urea (mmol/l) | 5.11±1.05c | 4.96±1.03c | 6.96±1.46a,b |
| UA (μmol/l) | 77.14±7.56 | 84.82±30.36 | 74.12±17.12 |
Comparison of pathological changes
Results of hematoxylin and eosin
staining in lung alveoli
The results show significant pathological changes in
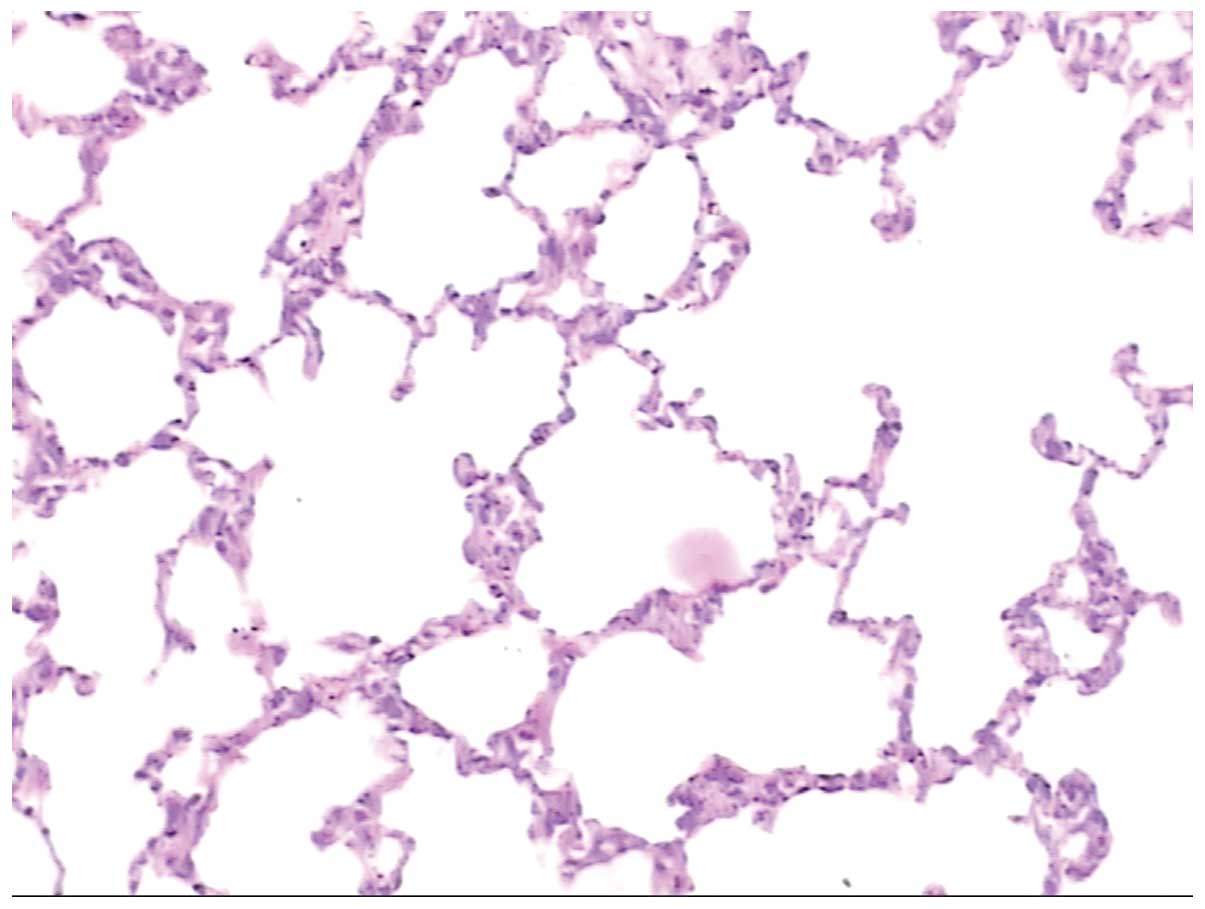
the alveoli among the groups. Fig.
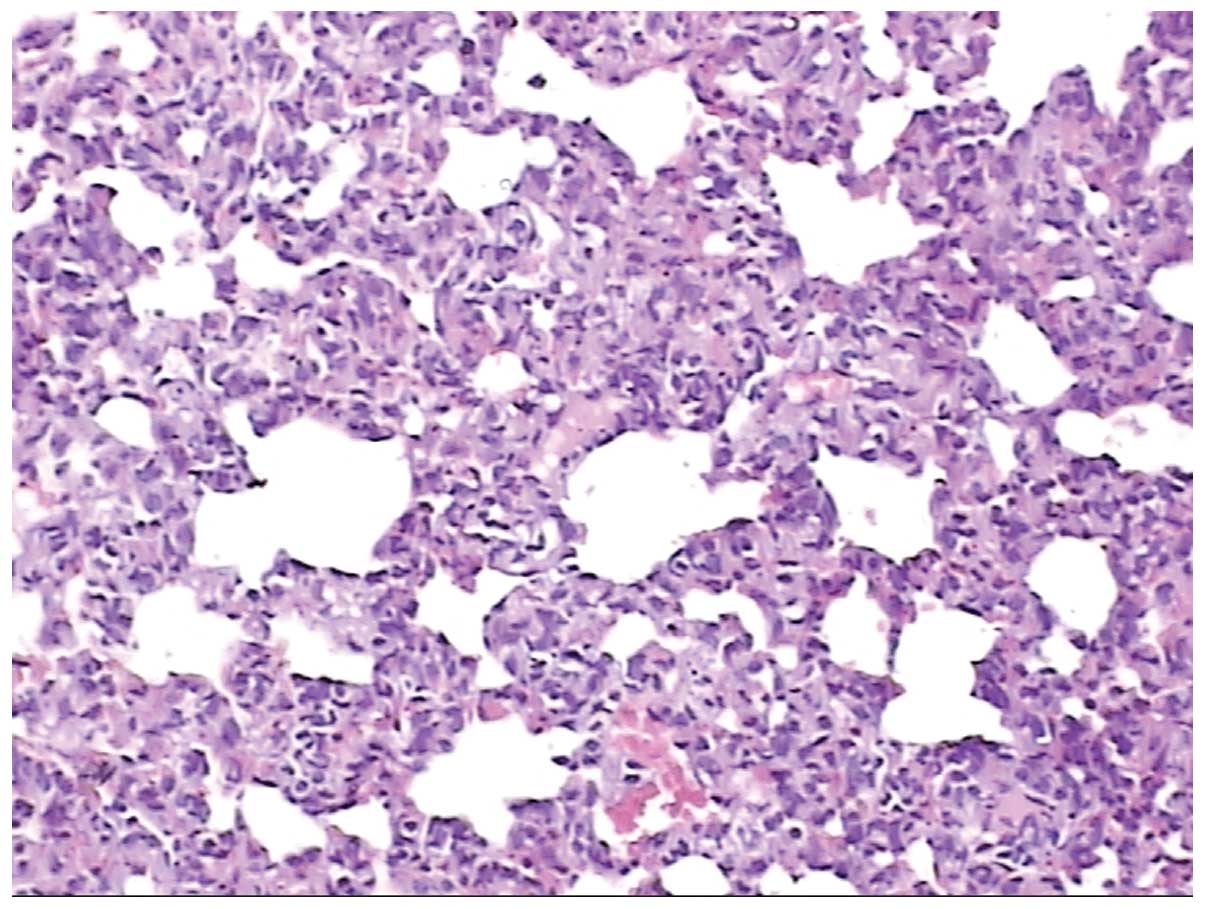
1 shows normal alveolar tissue, whereas Fig. 2 shows pathological alveolar tissue
observed following acute exposure to high altitude. Following acute
exposure to high altitude, the alveolar wall became hyperemic,
edematous and incrassate; the alveolar epithelium became
hyperplastic and neutrophilic granulocyte infiltrates were present.
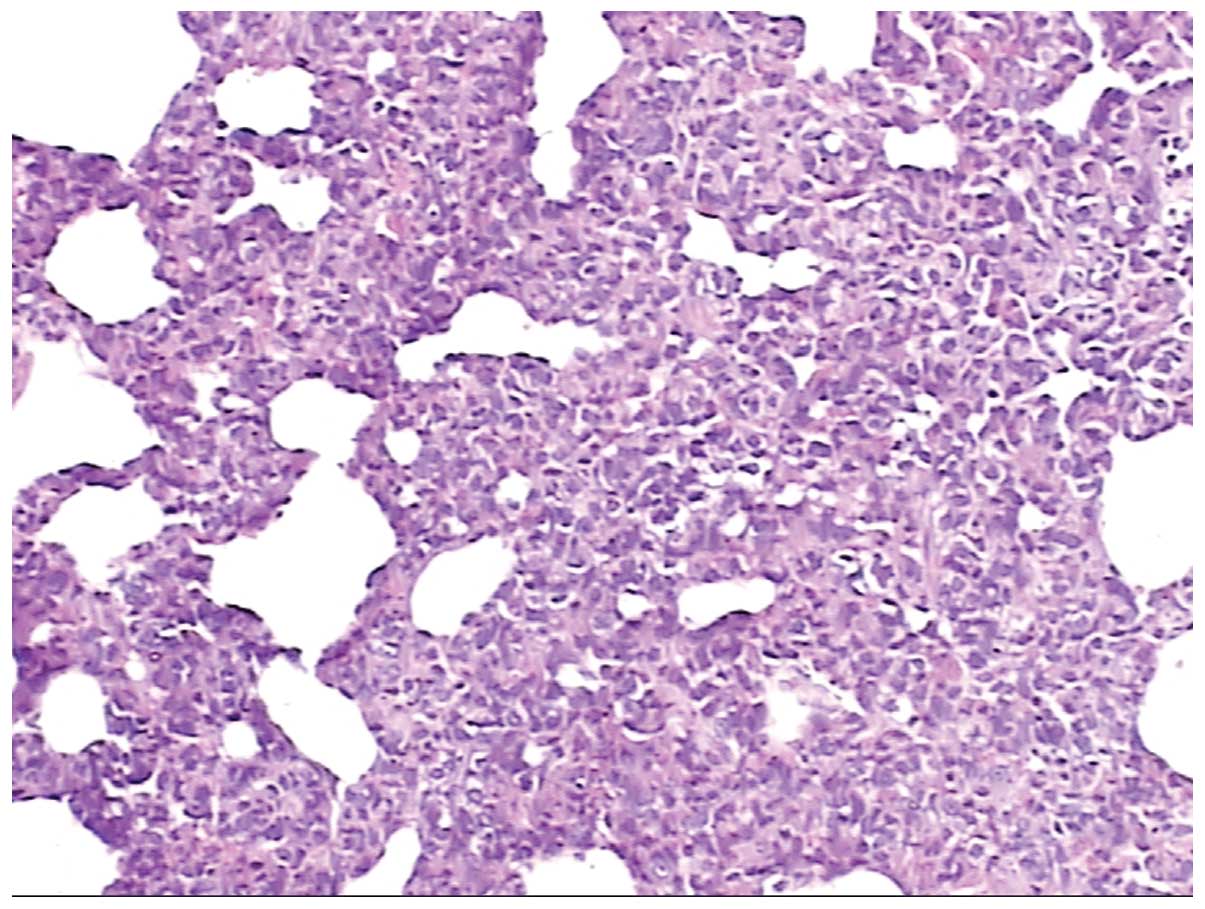
Fig. 3 shows pathological alveolar
tissue from a rat that had returned to low altitude from high
altitude. The alveolar wall was hyperemic, edematous and
incrassate; the alveolar epithelium was hyperplastic, and the
alveolar septum was widened.
Results of hematoxylin and eosin
staining of brain tissue
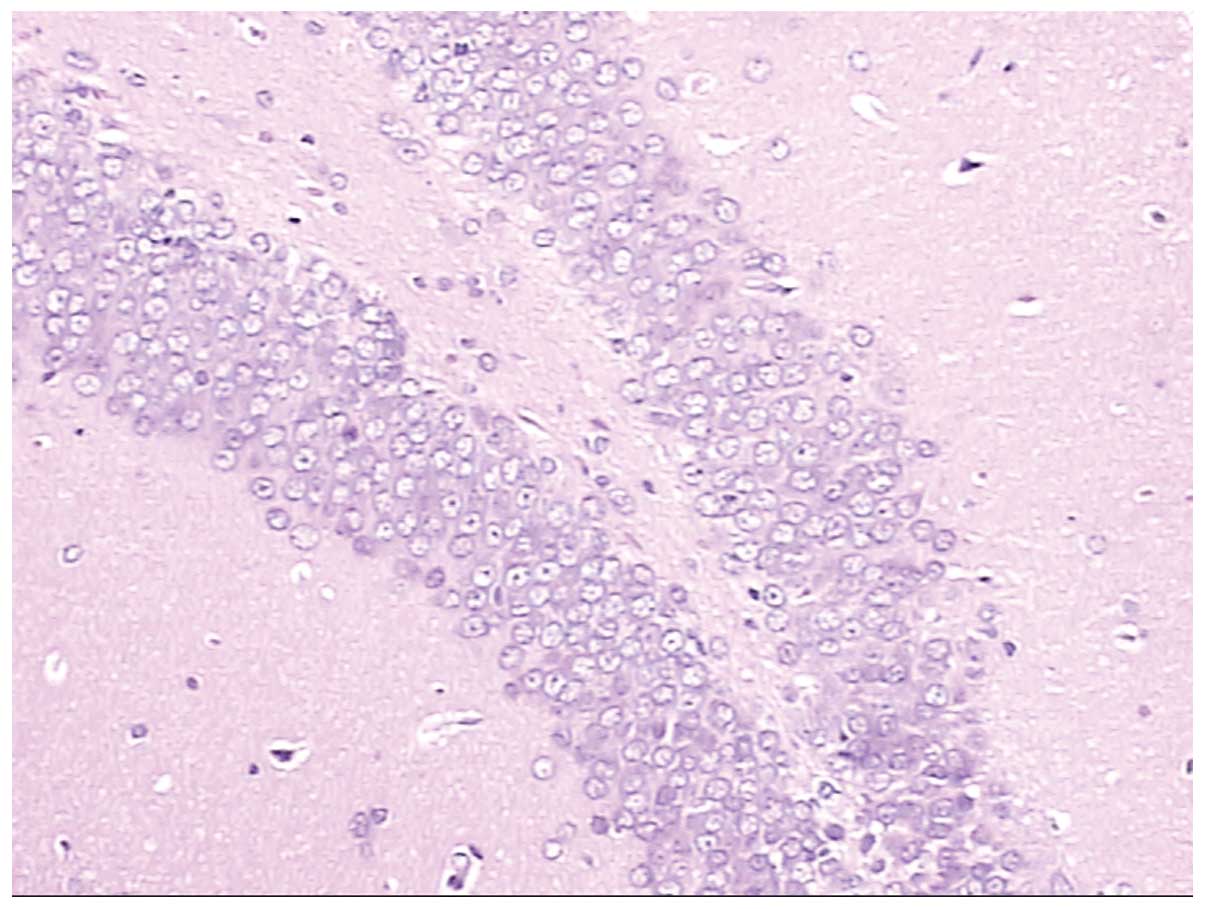
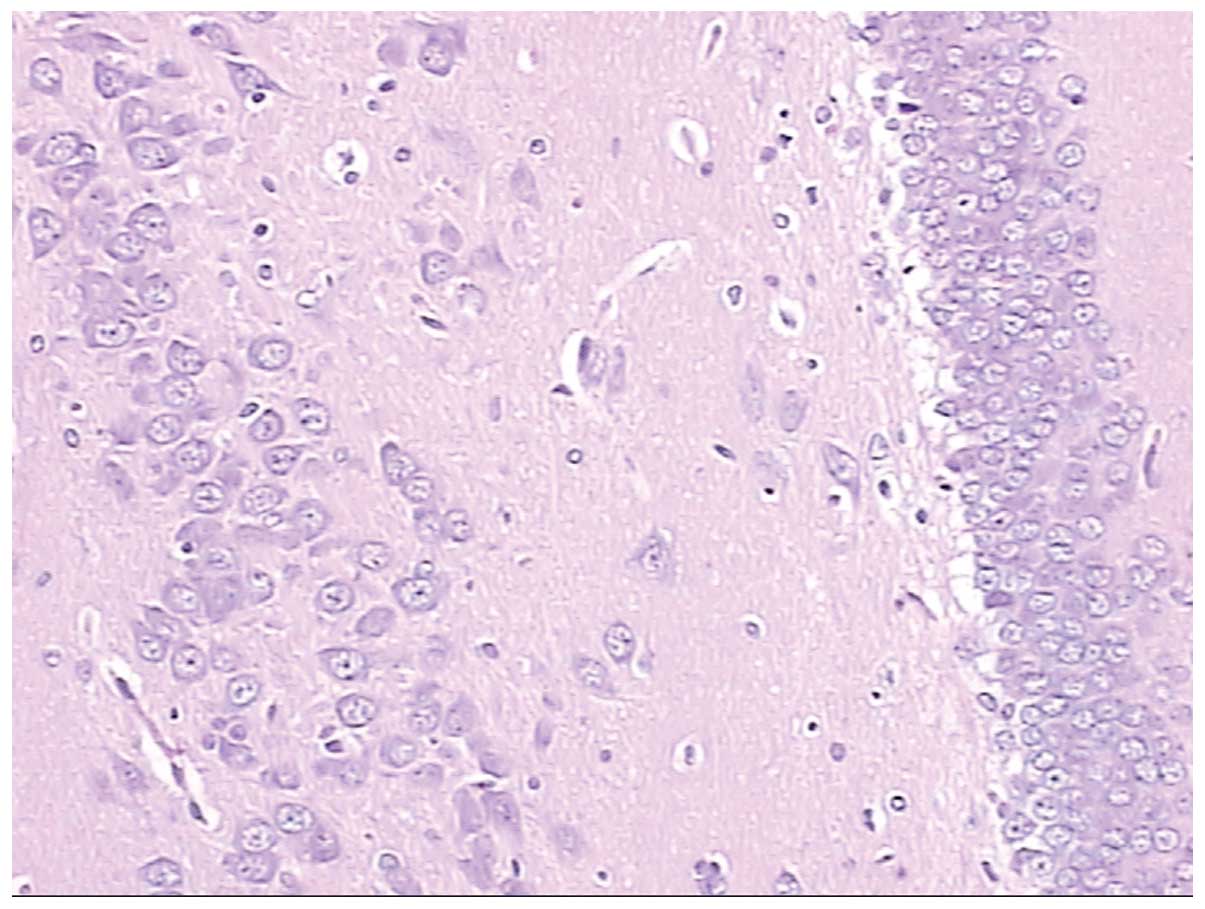
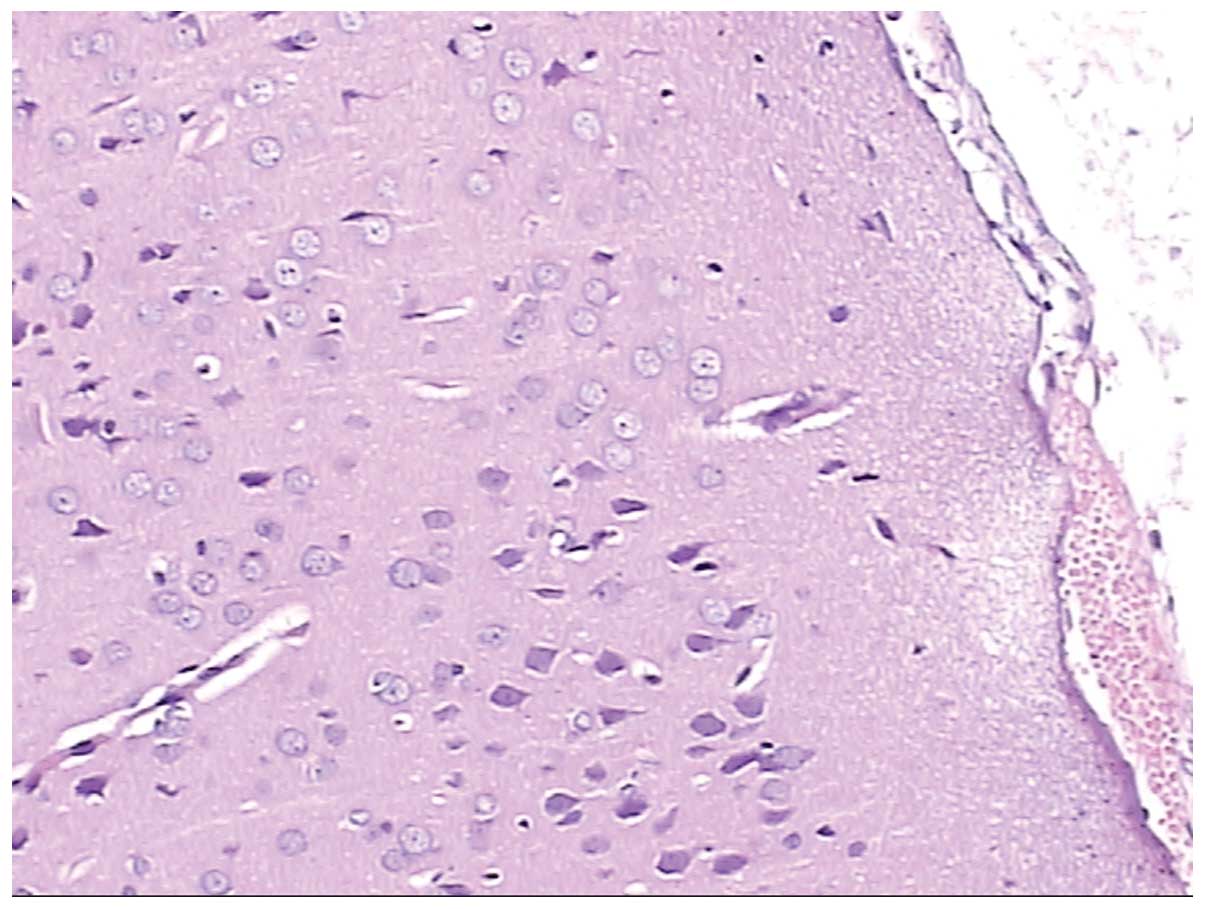
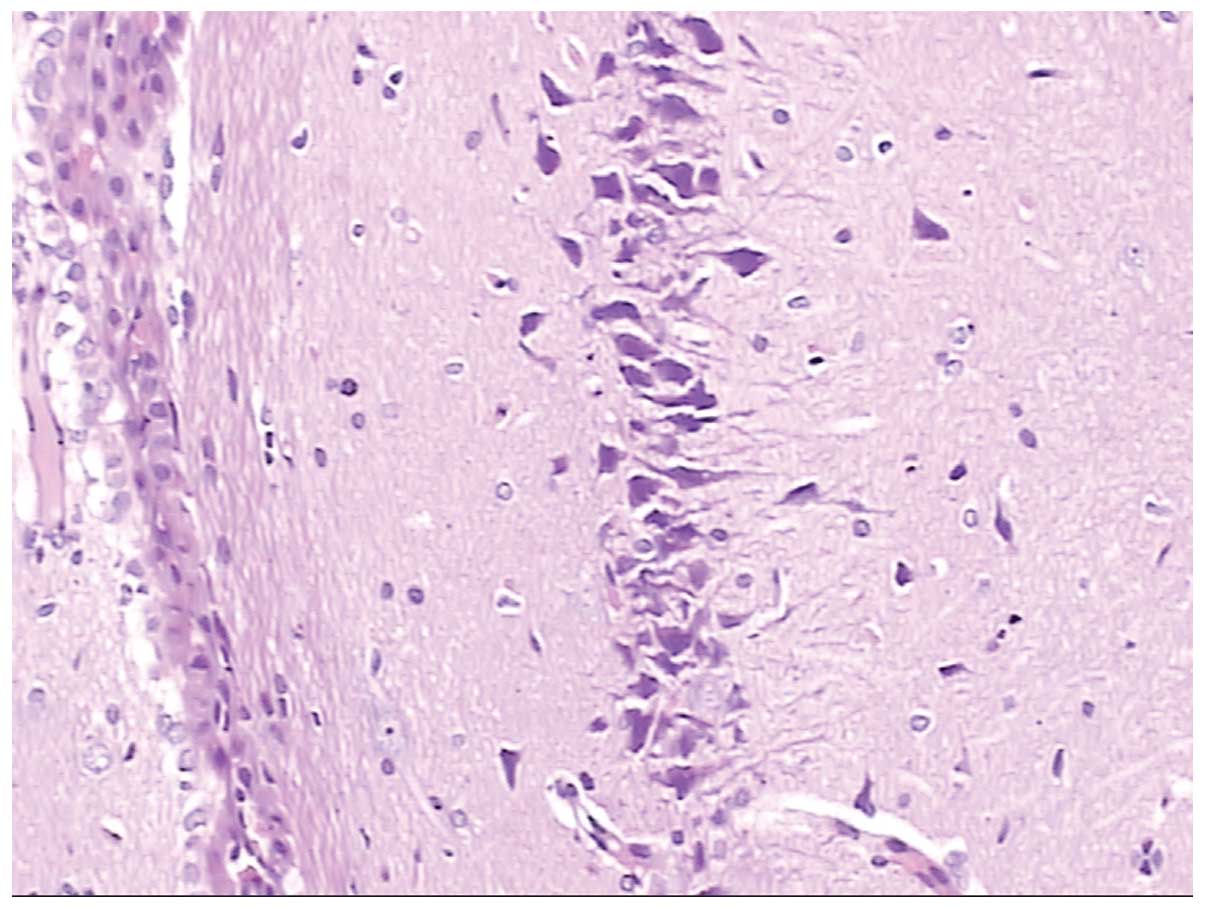
Figs. 4 and
5 show normal brain neurons and
hippocampal tissue. Figs. 6 and
7 show pathological brain neurons
and hippocampal tissue, respectively, following acute exposure to
high altitude. It may be observed that the brain neurons were
edematous and enlarged perivascular space was present, and the
hippocampal neurons were metamorphic and karyopyknotic.
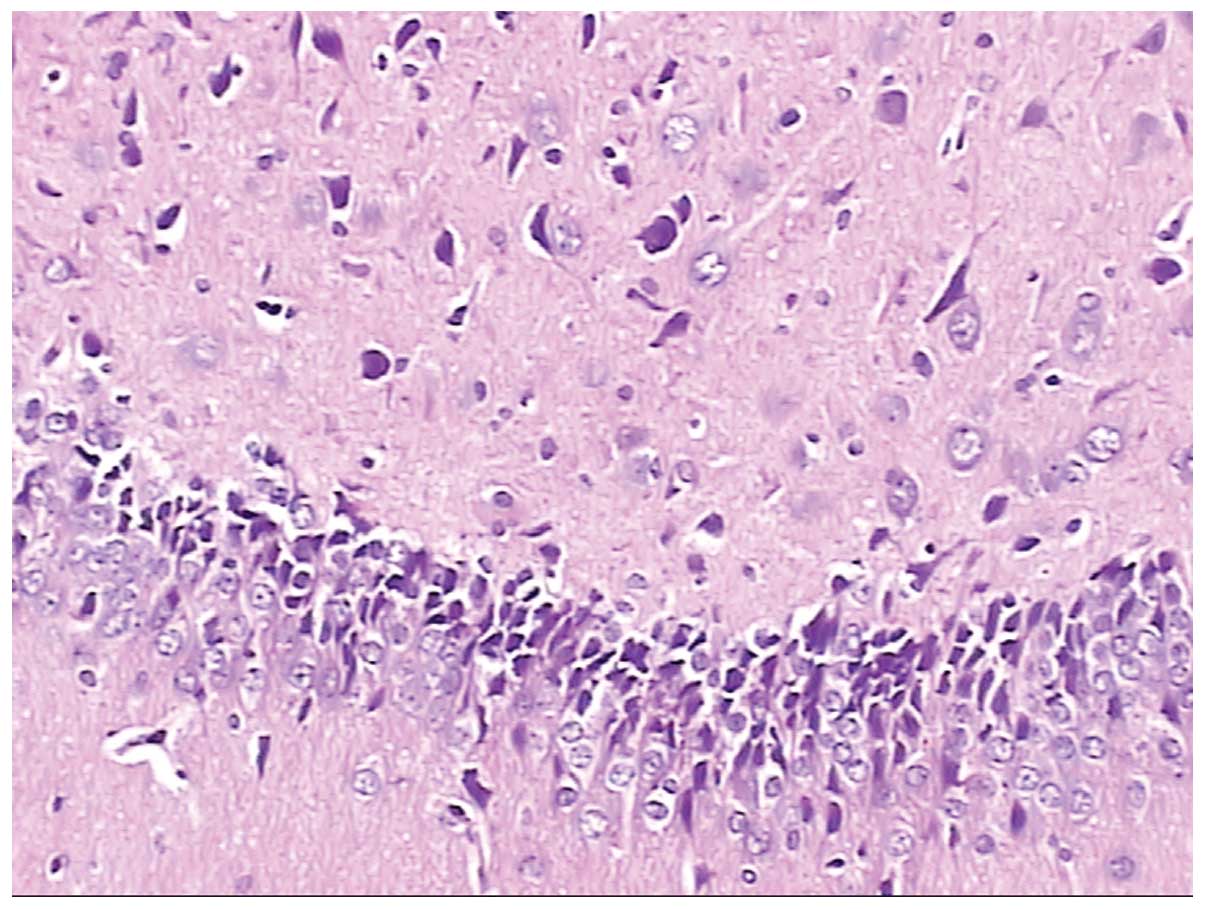
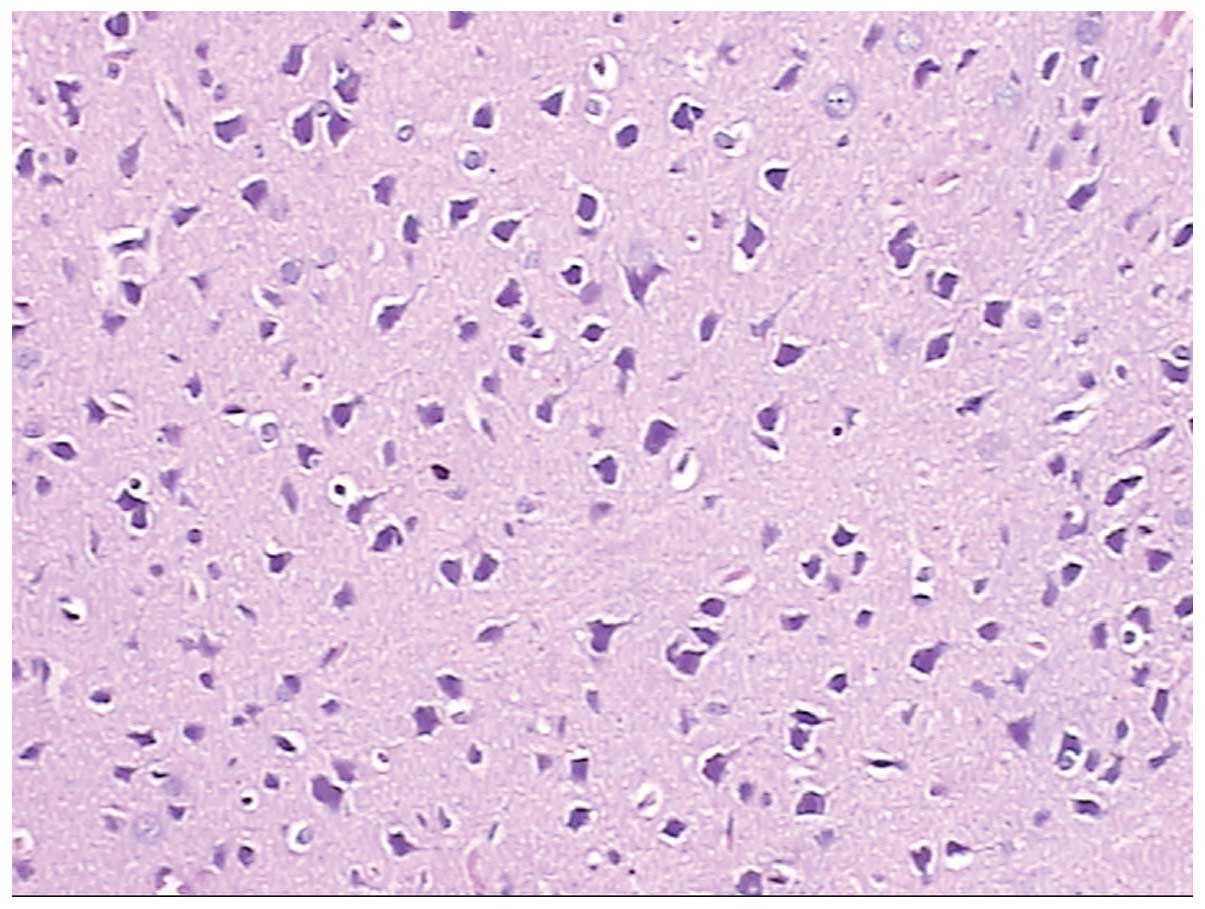
Furthermore, Figs. 8 and 9 are pathological brain neurons and
hippocampal tissues from rats that had returned to low altitude
from high altitude, where brain neurons were edematous, metamorphic
and karyopyknotic, and hippocampal neurons were metamorphic and
karyopyknotic.
Results of hematoxylin and eosin
staining of kidney tissue
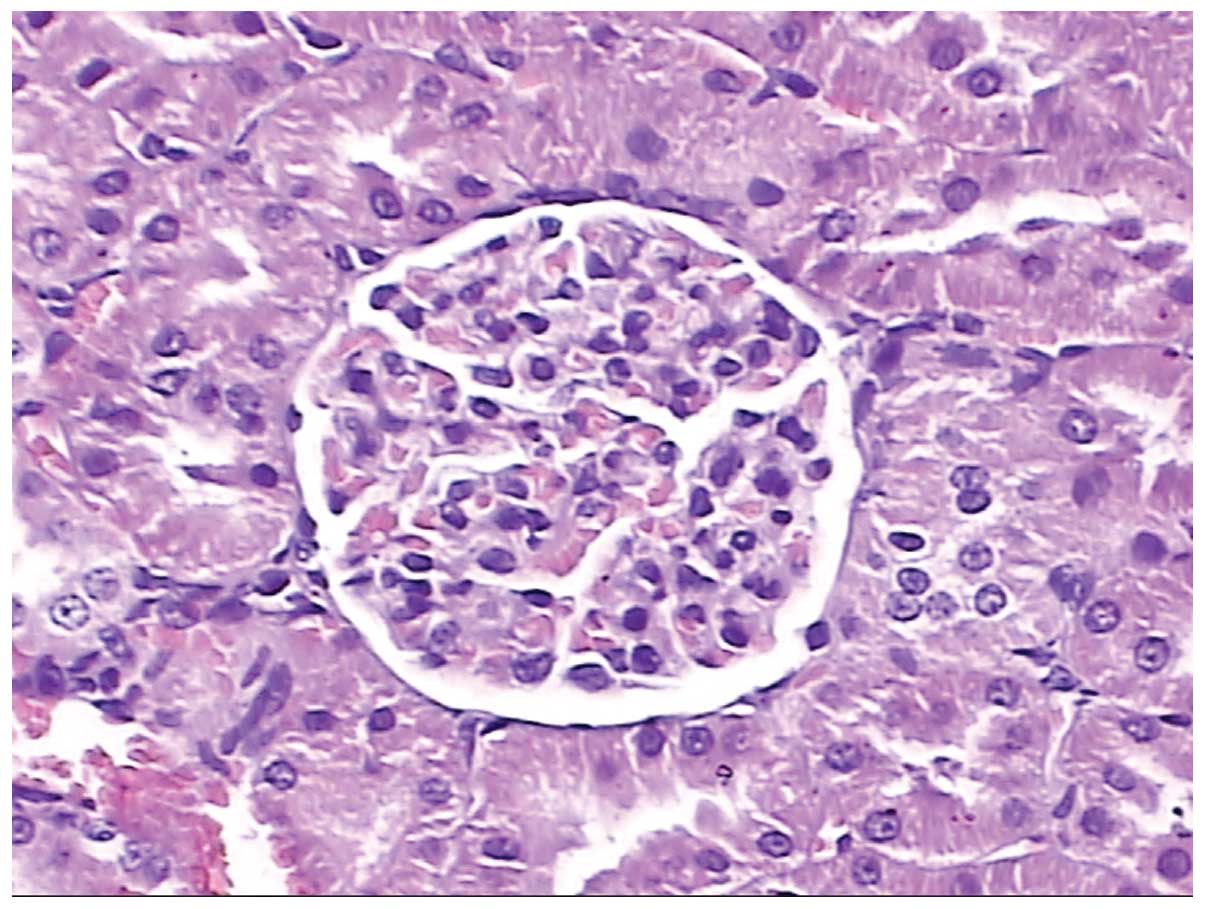
In Fig. 10, normal
kidney glomerulus tissue is shown in which the capillary nodes and
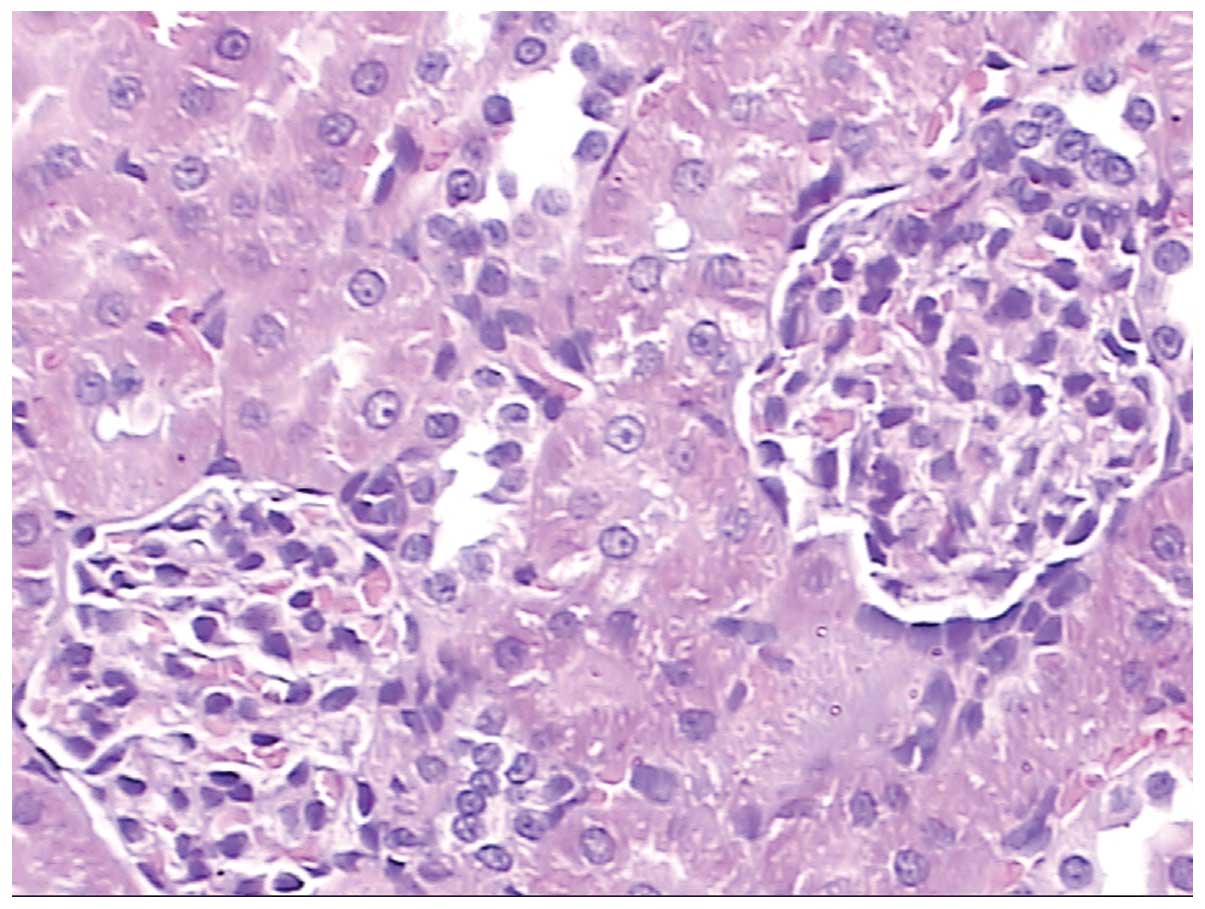
glomera are clear with regular capsular spaces. Fig. 11 shows pathological kidney tissue
following acute exposure to high altitude, in which hyperplastic
mesangial cells can be observed. Fig.
12 shows pathological kidney tissues from rats that had
returned to low altitude from high altitude. There were no evident
changes, with the exception that that the mesangial cells were
hyperplastic.
Discussion
Blood gas analysis indicates that the blood pH was
significantly decreased when the rats were acutely exposed to high
altitude and also when the rats returned to low altitude from high
altitude. When the blood pH is low, acidic drugs dissociate less
(8) and the levels of molecular
drugs, which have greater liposolubility and pass from the plasma
into cells more easily, are increased. As a result, the
distribution of acid drugs is likely to increase following acute
exposure to high altitude and also when returning to low altitude
from high altitude. Alkaline drugs are likely to be affected in a
reverse manner. Therefore, it is necessary and of great value to
investigate in greater detail the association between pH and the
dissociation of drugs at high altitude.
A previous study (9) reported that Hb levels in rats at high
altitude were higher than those in rats at low altitude. However,
there was no significant difference in Hb levels between groups A
and B in the present study. This may be attributable to the fact
that the time span after acute exposure to high altitude was not
long enough and it takes time for EPO levels to increase. In rats
that were acutely exposed to high altitude and then returned to low
altitude, the Hb level was lower than that in rats maintained at
low altitude or at high altitude, and thus the capacity to carry
oxygen was reduced. However, the pO2 and sO2
levels were similar to those in rats acutely exposed to high
altitude. This may due to deadaptation (10) when rats return to low altitude from
high altitude, or other reasons, which are worthy of further
research in the future.
Blood gas analysis also indicated that the
concentration of Na+ decreased at high altitude, which
suggests that Na+ flowed into cells abundantly under
anoxic conditions and this is likely to lead to cellular edema and
capillary occlusion. Hypoxic microcirculation is thus exacerbated
and would greatly affect drug disposition.
In the study by Gao et al (11), the K+ concentration in
rats at high altitude was increased compared with that in rats at
low altitude. K+ outflow causes the absence of
intracellular K+, which is indispensable for protein
synthesis and metabolism (including enzyme activity), which
seriously affects the metabolism and excretion of drugs. In the
present study, there was no significant difference in K+
concentration between the three groups. As for Cl−, in
rats that were exposed to high altitude and then returned to low
altitude, the Cl− concentration increased compared with
that of rats maintained at low altitude. Serum chloride plays a
part in the synthesis of gastric acid (gastric acid levels
increases following food intake, and serum chloride levels
decrease) (12). In addition,
chloride also takes part in renin secretion and adjustment (a
reduction in serum chloride in the macula densa of the
juxtaglomerular apparatus leads to inhibition of renin secretion,
and verse versa). Serum chloride levels increase with dysbolism of
sodium and acid base imbalance, which is in line with the present
study. The results of the present study suggest that changes in the
concentration of Cl− are likely to affect digestion and
absorption by the intestines and the functioning of kidneys, and
further affect the absorption and excretion of drugs.
The results of the pathological examinations
revealed that at high altitude, the alveolar walls were hyperemic,
edematous and incrassate while the alveolar epithelium was
hyperplastic with infiltrative neutrophilic granulocytes. The
alveolar septa were widened. This suggests that oxygen exchange in
the lungs becomes difficult, which is consistent with the blood gas
analysis results. These pathological changes did not recover after
the rats returned to low altitude from high altitude, which
explains why there is no significant difference in the results of
blood gas analysis, with the exception of K+ levels.
At high altitude, the concentration of serum
Na+ was significantly decreased, which suggests that
Na+ flowed into cells abundantly under anoxic conditions
and led to cellular edema, capillary occlusion, and hypoxia of the
microcirculation. As a result, pathological examination revealed
pulmonary edema and cerebral edema, which is consistent with the
blood gas analysis results. In conclusion, the blood gas index and
pathological features of the rats were changed significantly at
high altitude, which is likely to seriously affect drug absorption,
distribution, metabolism and excretion and ultimately the
therapeutic effects and adverse reactions of drugs.
Evident changes in the levels of LDH, ALP, TBIL and
Cl− were observed in the present study. The study
conducted by Mei et al (13) found that LDH levels were markedly
decreased in rats at high altitude. The study by Dai et al
(14) showed that urea,
creatinine, Cl− and ALP levels were clearly decreased
following acute exposure to high altitude, which is consistent with
the present study, which found that LHD and TP levels were
decreased by 58.44% and 26.82%, respectively, while TBIL and ALP
were increased by 338.00% and 24.94%, respectively. These results
suggest that the heart functions and hepatic functions of the rats
changed following acute exposure to high altitude. Heart function
has a great influence on blood rheology, blood pressure and
circulation, thus affecting drug absorption and distribution. As
the liver is the major organ responsible for drug metabolism,
changes of liver function seriously affect the formation of
metabolizing enzymes and their activities (15) thus altering drug distribution and
metabolism in a hypoxic environment.
The reduction of total protein levels in the blood
is likely to affect the protein binding rates of drugs, leading to
increased levels of free drug in the blood and as a result, the
drug concentration may be higher than the normal level in a hypoxic
environment. Total protein levels rebounded when the rats were
returned to low altitude, indicating that blood drug concentrations
may be reduced when returning from high to low altitude. TBIL
levels continued to rise while ALP and UA levels decreased upon
return to low altitude, which suggests that liver function
recovered to a certain extent, and its influence on drug metabolism
was reduced when the rats returned to low altitude from high
altitude. The study by Li (15)
found that UA levels increased at high altitude. However, in the
present study, the increase in UA levels at high altitude was not
significant, whereas an increase of urea levels was evident when
the rats returned to low altitude from high altitude, suggesting
that the kidney function did not change much under acute hypoxia;
however, changes were evident when the rats returned to low
altitude from high altitude, which may be attributable to
deadaptation (10,16). In the pathological observation,
only minor changes in the kidneys were identified. However, since
kidneys are the main path of drug excretion, although the results
indicate that there was little injury of the kidneys in a hypoxic
environment, drug excretion may be seriously affected in such an
environment.
In conclusion, the kidneys are the main path of drug
excretion, and have a great influence on the absorption,
distribution, metabolism and excretion of drugs. There are changes
in the elimination rate constant (Ke), peak time (Tmax), peak
concentration (Cmax), half life (t1/2), clearance (CL)
and area under the curve (AUC) associated with the drug metabolism
and excretion and are thought to have a significant effect of the
therapeutic and side effects of drugs (17). The degrees of influence require
study in future experiments. Changes in pharmacokinetics have a
close association with the therapeutic effects and adverse
reactions of drugs, making it necessary to adjust the dose and
usage of commonly used drugs at high altitude. This study may
provide a basis and new ideas for clinical pharmacy at high
altitude, for improving clinical medication and avoiding adverse
reactions, in order to achieve personalized medication at high
altitude.
Acknowledgements
The authors would like to express their sincere
gratitude to the Department of Pharmacy of the Second Military
Medical University and Maqu Huanghe Shouqu Yaoyuan Development Co.,
Ltd. for their support and housing at the investigation site to
facilitate this study. In addition, the authors would like to
express special thanks to Dr Zhou, Teacher Cao and Manager Zaxi for
technical assistance in the present study.
References
|
1
|
Zhao H, Chai W, Gao W, Xu L, Zhang H and
Yang Y: Hyperoxygenated solution: effects on acute hypobaric
hypoxia-induced oxidative damage in rabbits. High Alt Med Biol.
10:283–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donovan L, Welford SM, Haaga J, LaManna J
and Strohl KP: Hypoxia - implications for pharmaceutical
developments. Sleep Breath. 14:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner GM and Stevens CW: Pharmacology.
4th Edition. Elsevier; Philadelphia, PA, USA: 2012
|
|
4
|
Jun F, Jun Q, Chun-xia L, et al:
Establishment of normal reference range of blood hematological and
biochemical indicators of SPF wistar rats. Gonggong Weisheng Yu
Yufang Zazhi. 21:50–52. 2010.(In Chinese).
|
|
5
|
Grenache DG and Parker C: Integrated and
automatic mixing of whole blood: an evaluation of a novel blood gas
analyzer. Clin Chim Acta. 375:153–157. 2007. View Article : Google Scholar
|
|
6
|
Mahutte CK: On-line arterial blood gas
analysis with optodes: current status. Clinical Bioch. 31:119–130.
1998. View Article : Google Scholar
|
|
7
|
Venkatesh B and Boots RJ: Carbon dioxide
and oxygen partial pressure measurements in the cerebrospinal fluid
in a conventional blood gas analyzer: analysis of bias and
precision. J Neurol Sci. 147:5–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra A and Kesisoglou F: Impaired drug
absorption due to high stomach pH: a review of strategies for
mitigation of such effect to enable pharmaceutical product
development. Mol Pharm. 10:3970–3979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JF, Guo ZJ, Huang HQ, et al:
Correlation between hemoglobin and mountain response after rapidly
ascended to highlands in different periods. Journal of Qinghai
Medical College. 31:184–186. 2010.(In Chinese).
|
|
10
|
Karinen HM, Peltonen JE, Kähönen M and
Tikkanen HO: Prediction of acute mountain sickness by monitoring
arterial oxygen saturation during ascent. High Alt Med Biol.
11:325–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao F, Feng XY and Du FM: Effects of blood
glucose, lipid and dielectric on SMZCo pharmacokinetics after
short- and long-time exposure to high-altitude. Chinese Journal of
Modern Applied Pharmacy. 27:239–242. 2010.(In Chinese).
|
|
12
|
Liu RW: Modern Clinical Laboratary
Diagnostics. Chemical Industry Press; Beijing: pp. 99–123. 2009
|
|
13
|
Mei D, Xu B, Sun K, Wang LH and Zhang W:
Changes of serum biochemical parameters during hypothermia and
hypoxia in rats. Space Med Med Eng (Beijing). 12:274–276. 1999.(In
Chinese).
|
|
14
|
Dai Y, Dai DJ, Wang Z and Ren Q: Effect of
acute hypobaric hypoxia on renal function and structure in rats.
Space Med Med Eng (Beijing). 3:215–217. 2000.(In Chinese).
|
|
15
|
Li SZ: Hypoxia height dependability
disease in high altitude and discussion of new mountain sickness.
Medical Journal of National Defending Forces in Southwest China.
21:336–338. 2011.(In Chinese).
|
|
16
|
Grocott M, Montgomery H and Vercueil A:
High-altitude physiology and pathophysiology: implications and
relevance for intensive care medicine. Crit Care. 11:2032007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bito LZ: Prostaglandins and other
eicosanoids: their ocular transport, pharmacokinetics, and
therapeutic effects. Trans Ophthalmol Soc U K. 105:162–170.
1986.PubMed/NCBI
|