Introduction
Unlike other tissues, articular cartilage is
avascular and has a poor healing potential following defects
(1,2). Autologous chondrocyte implantation
(ACI) is highly recommended for articular cartilage repair
(3). During the process of ACI,
the isolation of chondrocytes from the donor tissue and expansion
of the cells in vitro are necessary; however, this approach
is confronted with several problems, including the limited number
of isolated chondrocytes and the loss of chondrocyte phenotypes. An
alternative is the use of growth factors but their popularization
and application in the clinic is limited for a number of reasons:
i) Growth factors may induce the formation of osteophytes,
resulting in the degeneration of articular cartilage (4); ii) tumorigenesis may occur (5–7); and
iii) growth factors are generally expensive. The identification of
another molecular substance to substitute for growth factors in the
restoration of defects is, therefore, important.
The possible beneficial health effects of green tea
have received considerable attention. The polyphenols in green tea,
catechins, which include (−)-epigallocatechin-3-gallate (EGCG),
(−)-epigallocatechin, (−)-epicatechin-3-gallate and
(−)-epicatechin, are major constituents in brewed green tea
(8). It is known that these green
tea polyphenols are effective free radical scavengers and potent
antioxidants (9,10), and there is considerable
epidemiological evidence suggesting that there is an inverse
correlation between green tea consumption and cancer development
(11–14). Other polyphenols with strong
antioxidant properties, found in foods or beverages such as tea,
grape and turmeric, have shown both cancer chemopreventative and
chemotherapeutic effects in numerous cell culture systems and
animal tumor bioassays (15–17).
Among various nutraceutical ingredients, EGCG, the most abundant
and most active catechin derivative in green tea, is predominantly
accountable for the biological effects of green tea (15).
EGCG is the most active and widely found polyphenol
in green tea and is well known to be a primary contributor to the
potential benefits of green tea to human health (18–20).
The main mechanisms underlying the action of EGCG have been
suggested to involve its potent antioxidant activity, which allows
neuro- and cardioprotection (21,22).
Other favored mechanisms entail the chemopreventative,
anti-inflammatory, antithrombotic and cytoprotective effects of
EGCG (23–25). The protective effect of extracts of
EGCG on the metabolism of human chondrocytes in cartilage
alteration has also been demonstrated (26,27).
Given that EGCG has beneficial health effects, it was hypothesized
that it could be a potential chondroprotective agent to replace
growth factors when applied in ACI. In this study, the effect of
EGCG on chondrocytes and their growth in vitro was
investigated. Examination of the cell proliferation, morphology,
glycosaminoglycan (GAG) synthesis and cartilage-specific gene
expression following treatment with EGCG was performed. The present
study may provide a basis for the development of a novel agent that
can replace growth factors in the treatment of articular cartilage
defects.
Materials and methods
Materials and chemicals
EGCG (purity ≥98%, high-performance liquid
chromatography) was purchased from Shanghai Yuanye Bio-Technology
Co., Ltd. (Shanghai, China) and stored at 4°C. Prior to the
experiments, EGCG was dissolved in dimethylsulfoxide (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) and kept
at −20°C until ready for use.
Articular chondrocyte culture
Articular chondrocytes were harvested from knee
joint cartilage slices of one-week-old New Zealand rabbits (Animal
Center of Guangxi Medical University, Nanning, China) by enzymatic
digestion. In brief, cartilage slices from two rabbits were
dissociated enzymatically with 0.25% trypsin (Beijing Solarbio
Science and Technology Co., Ltd.) for 30 min and then with 2 mg/ml
collagenase type II (Gibco-BRL, Carlsbad, CA, USA) in α-modified
Eagle’s medium (α-MEM; Gibco-BRL) for 3 h. Following
centrifugation, the chondrocytes were resuspended. The cells were
cultured with α-MEM containing 20% (v/v) fetal bovine serum
(Gibco-BRL) and 1% (v/v) penicillin/streptomycin (Beijing Solarbio
Science and Technology Co., Ltd.) in a 5% CO2 humidified
incubator at 37°C with the culture medium replaced every other day
after plating. Articular chondrocytes at passage 2, with a cell
density of 2×104/ml, were used for further studies.
Cells were treated with taurine at a final concentration of 15, 30
and 60 μg/ml, and a group without taurine-treatment served as a
control. This study was approved by the Institutional Ethical
Committee of Guangxi Medical University (approval no 20140121).
Cell proliferation analysis and
biochemical assay
Subsequent to being cultured for 2, 4 and 6 days,
the cells were washed three times with phosphate-buffered saline
(PBS). Cell precipitations were collected following digestion with
Proteinase K solution (Sigma, St Louis, MO, USA) for 16 h at 60°C.
The DNA production was measured by a spectrofluorometer (UV-1700,
Shimadzu Company, Kyoto, Japan) using Hoechst 33258 (Sigma) dye at
460 nm with the absorbance value of Hoechst 33258 dye alone as the
baseline. The total secretion of GAG was quantified by absorbance
value employing a 1,9-dimethylmethylene blue (Sigma)
spectrophotometric assay at 525 nm with chondroitin sulfate as the
standard sample. The total GAG secretion in each well was
calculated according to the standard curve. The secretion of GAGs
in each chondrocyte was normalized to the total DNA production of
the chondrocytes, which indicated the biosynthetic activity of the
cells in various culture media.
Morphological examination
Subsequent to being cultured for 6 days, the cells
were washed three times with PBS and fixed in 95% alcohol for 30
min. The cells were then washed with PBS solution and stained with
a hematoxylin and eosin kit (Jiancheng Institute of Biotechnology,
Nanjing, China). Finally, the cells were observed and photographed
using an inverted phase contrast microscope (Axiovert200; Zeiss
Corporation, Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The gene expression of types I, II and X collagen,
aggrecan and Sox9 was analyzed by RT-qPCR detection. Total RNA was
sequentially extracted with an additional purification step
employing an RNA isolation kit [Tiangen Biotech (Beijing) Co.,
Ltd., Beijing, China] according to the manufacturer’s instructions.
An equal quantity of RNA (300 ng) was used as a template and
reverse transcribed into cDNA using an RT kit (Fermentas,
Burlington, ON, Canada), and then amplified using a SYBR Green
Realtime PCR Master Mix kit (Roche Diagnostics, Mannheim, Germany)
on a real-time fluorescence quantitative instrument (RealPlex 4;
Eppendorf Corporation, New York, NY, USA). The primers (from
Parkson Company, Shanghai, China) used for PCR are shown in
(Table I). The dissociation curve
of each primer pair was analyzed to confirm the primer specificity.
Marker gene expression levels of the chondrocytes were analyzed by
the 2−ΔΔCT method using GAPDH as the internal control.
Each sample was repeated three times for each gene.
 | Table IPrimer sequences used in the
quantitative polymerase chain reaction experiments. |
Table I
Primer sequences used in the
quantitative polymerase chain reaction experiments.
| mRNA | Forward primer | Reverse primer |
|---|
| GAPDH |
5′-CTATAAATTGAGCCCGCAGC-3′ |
5′-ACCAAATCCGTTGACTCCG-3′ |
| Aggrecan |
5′-CTACACGCTACACCCTCGAC-3′ |
5′-ACGTCCTCACACCAGGAAAC-3′ |
| Type I
collagen |
5′-GTTCAGCTTTGTGGACCTCCG-3′ |
5′-GCAGTTCTTGGTCTCGTCAC-3′ |
| Type II
collagen |
5′-AAGCTGGTGAGAAGGGACTG-3′ |
5′-GGAAACCTCGTTCACCCCTG-3′ |
| Type X
collagen |
5′-CGCTGAACGATACCAAATGCC-3′ |
5′-TTCCCTACAGCTGATGGTCC-3′ |
| Sox9 |
5′-AAGCTCTGGAGACTTCTGAACG-3′ |
5′-CGTTCTTCACCGACTTCCTCC-3′ |
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was determined using one-way analysis of
variance followed by Dunnett’s post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
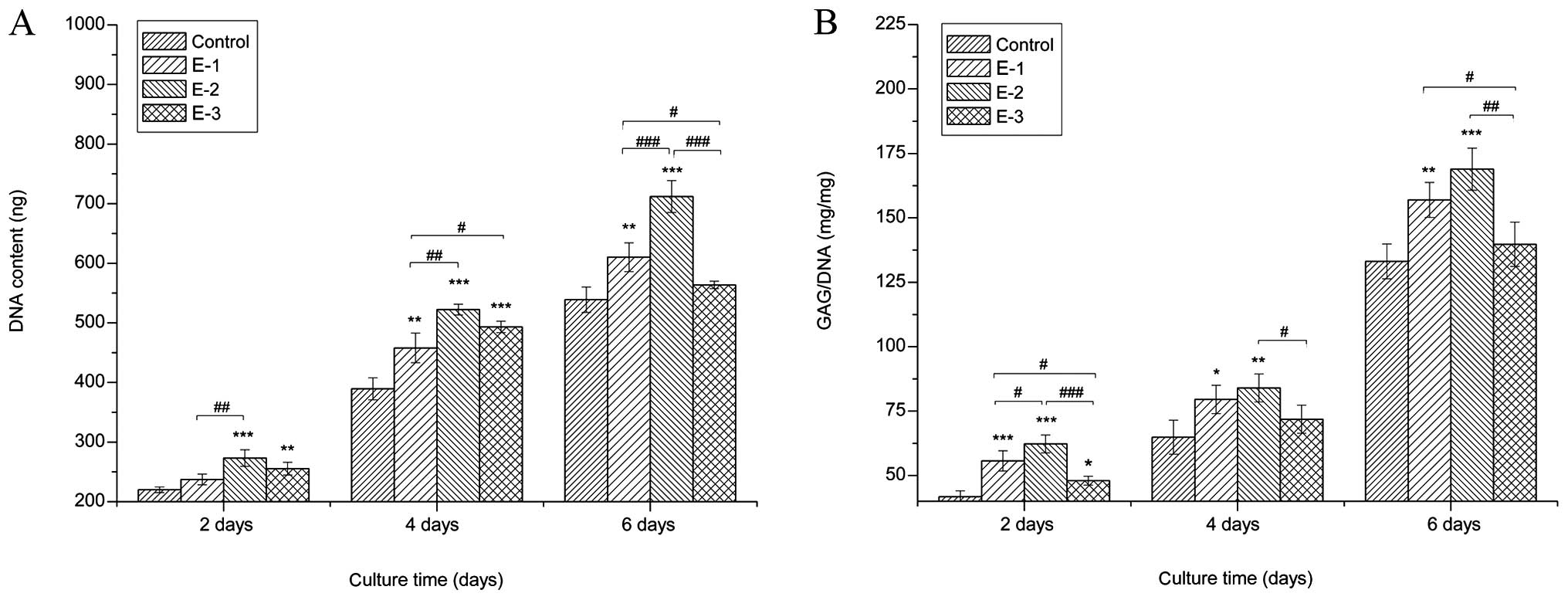
Cell proliferation
The proliferation of chondrocytes cultured with
various concentrations of EGCG (0, 5, 10 and 20 μM) was detected by
the DNA content measurements. Cells cultured with EGCG grew faster
than those in the control group (P<0.05), as proved by
distinctly higher DNA values than the control in the same culture
period. Among the groups, the greatest cell proliferation was
achieved with 10 μM EGCG. The results indicated that EGCG
facilitated chondrocyte growth, particularly at the concentration
of 10 μM (Fig. 1A).
Secretion of GAGs
To determine if extracellular GAG production was
affected by EGCG, biochemical assays were performed after 2, 4 and
6 days of culture. The results of intracellular GAG production
following the treatment of chondrocytes with different
concentrations of EGCG (Fig. 1B)
showed that GAG production in culture media treated with EGCG was
significantly improved over that in the control at the same
time-point. In particular, EGCG at a concentration of 10 μM
exhibited the strongest promotion of GAG synthesis among the three
concentrations.
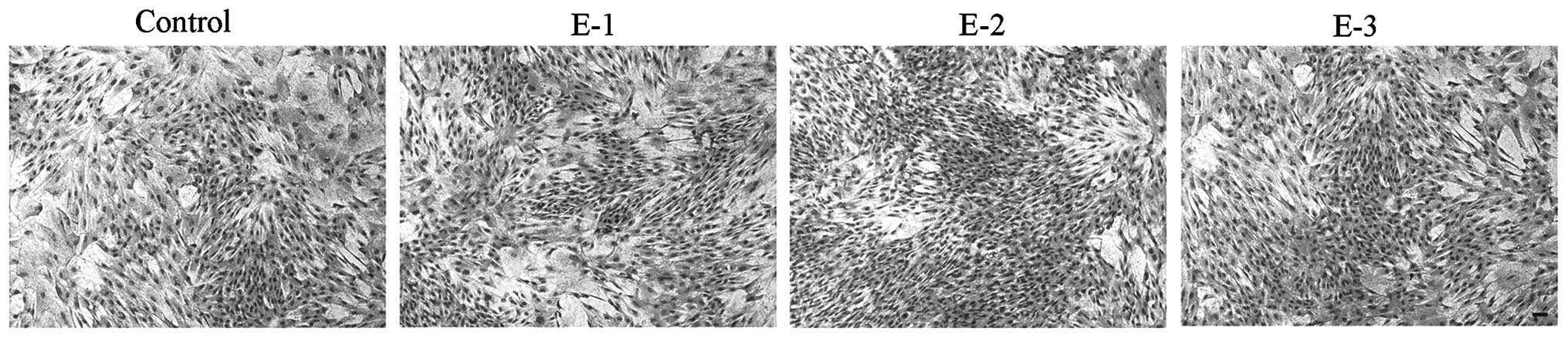
Cell morphology
The morphology of articular chondrocytes following
treatment with EGCG at various concentrations (0, 5, 10 and 20 μM)
is shown in Fig. 2. No evident
difference in cell morphology was observed between cells with and
without EGCG after 6 days in culture. Compared with the control,
the chondrocytes in the presence of EGCG grew better and appeared
to have a superior proliferation tendency with the gradually
increasing days. At a concentration of 10 μM, EGCG could better
facilitate the proliferation of chondrocytes than at other
concentrations.
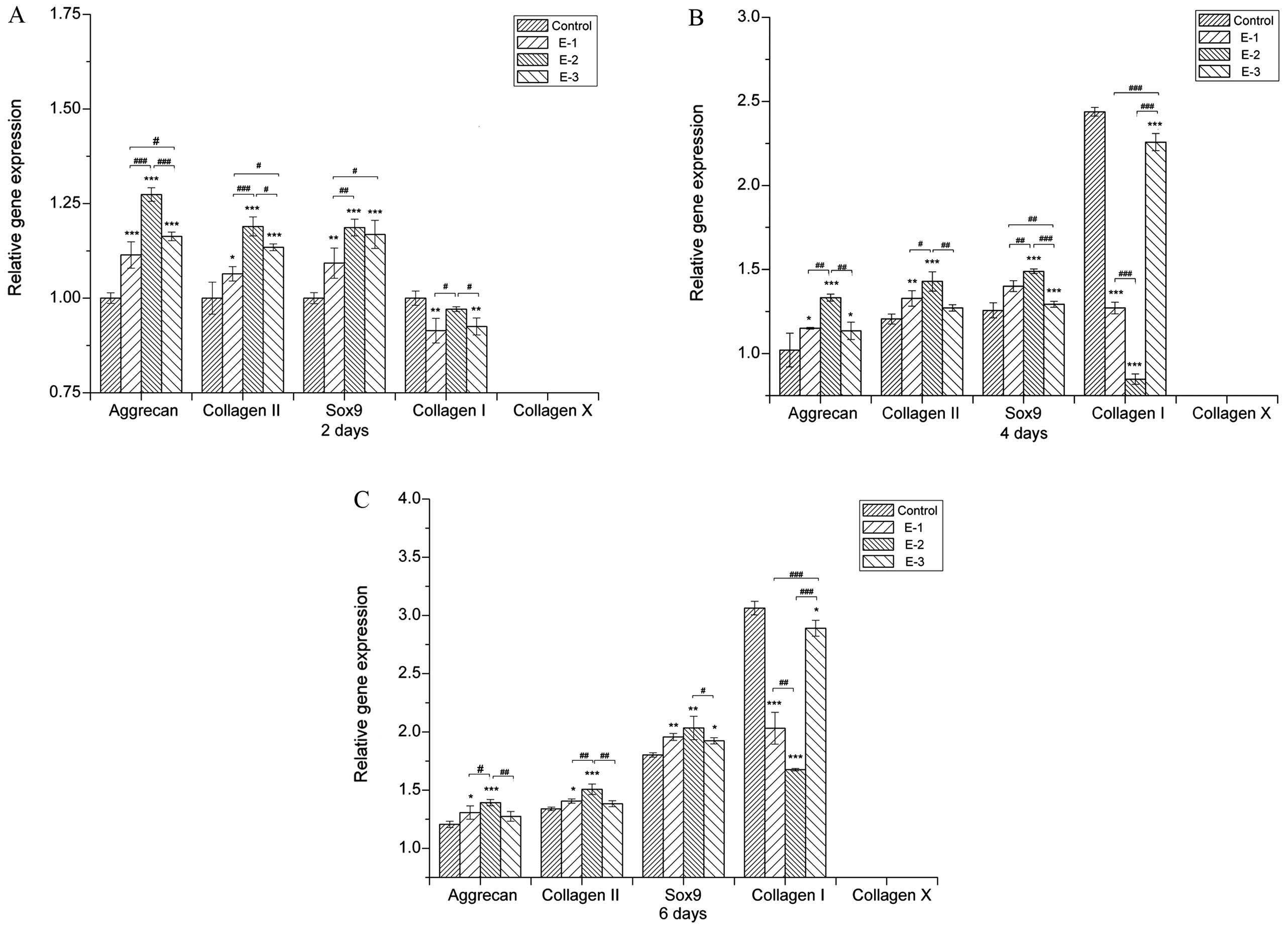
Gene expression
The effect of EGCG on the extracellular matrix (ECM)
synthesis by chondrocytes was further examined through the gene
expression of collagen I, collagen II, collagen X, Sox9 and
aggrecan [a proteoglycan (PG) composed of GAGs] after 2, 4 and 6
days of culture. As shown in Fig.
3, cartilage-specific gene expression, such as aggrecan,
collagen II and Sox9, was significantly enhanced by EGCG at
concentrations of 5–20 μM with the 10 μM group showing the highest
aggrecan, collagen II and Sox9 expression. The presence of EGCG
upregulated aggrecan, collagen II and Sox9 expression, suggesting
that EGCG either delayed or prevented the chondrocytes from
dedifferentiating into a hypertrophic phenotype. At the same time,
collagen X expression was downregulated in all groups, indicating
that cell dedifferentiation and hypertrophy were not
significant.
EGCG at various concentrations led to lower collagen
I expression when compared with the control group subsequent to
being in culture for 2, 4 and 6 days. In addition, the levels of
collagen I in the 10 μM group were lower than those in other
groups. These results further suggested that EGCG could inhibit the
dedifferentiation of chondrocytes.
Concentrations of EGCG ranging from 5 to 20 μM
therefore upregulated the synthesis of cartilage markers. Among the
groups, EGCG at a concentration of 10 μM produced the highest
aggrecan and collagen II expression, which was consistent with the
results of GAG production (Fig.
1B).
Discussion
Anti-inflammatory agents may provide anti-arthritic
effects to facilitate the resolution of cartilage inflammation
following injury. EGCG is reported to have a role in the treatment
of osteoarthritis (26,28). The present study focused on the
effects of EGCG on primary rabbit chondrocytes to demonstrate EGCG
as a potential pro-chondrogenic agent that can replace growth
factors in cell-based therapies for cartilage repair.
The present study showed that EGCG, which is a novel
antioxidant, could well support the growth of chondrocytes. As
demonstrated by cell proliferation assays and morphological
examination, EGCG could significantly promote chondrocyte growth
compared with the control. Furthermore, EGCG could markedly promote
GAG deposition in chondrocytes, as shown by biochemical assay
(Fig. 1B). PGs are important
components of ECMs (29). For all
PGs, GAGs constitute a major component of their molecular mass;
furthermore, GAGs and a large number of water molecules generate
expansion pressure and make the cartilage flexible, which plays an
important role in maintaining cartilage load-bearing capacity
(30). Consistent with the
increase in GAG production, EGCG could upregulate the gene
expression of cartilage-specific aggrecan, collagen II and Sox9
(Fig. 3). The chondrogenic
transcription factor Sox9 plays a major role in an increased level
of chondrogenesis (31,32), in particular activating
co-expression with collagen type II (33–35).
In addition, extensive gene therapy approaches using viral methods
to overexpress Sox9 have resulted in marked improvements in the
secretion of cartilaginous matrix by articular chondrocytes, bone
marrow-derived stem cells and nucleus pulposus cells (36–38).
These data indicated that EGCG could facilitate chondrocyte
proliferation and stimulate exuberant cartilage matrix
secretion.
The expression of collagen type I, which marks the
dedifferentiation of chondrocytes, was effectively inhibited by
EGCG. Dedifferentiation occurs when the differentiated phenotype of
chondrocytes, primarily composed of type II collagen and
cartilage-specific PGs, is lost and replaced by a complex collagen
phenotype consisting of a majority of type I collagen and a low
level of PG synthesis (39–41).
Furthermore, collagen type X, which is specifically associated with
hypertrophic chondrocytes and precedes the onset of endochondral
ossification (42), was nearly
undetectable in the EGCG groups, indicating that the hypertrophy of
chondrocytes would not be induced by EGCG. As a consequence, the
decreasing collagen I expression and the inconspicuous expression
of collagen X could suggest that EGCG prevents the
dedifferentiation and hypertrophy of chondrocytes.
EGCG, which is the ester of epigallocatechin and
gallic acid, has been found to inhibit the degradation of human
cartilage PG and type II collagen, and selectively inhibits a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)-1, ADAMTS-4, and ADAMTS-5 (43,44).
Further research has revealed that EGCG ameliorates the
interleukin-1β-mediated suppression of transforming growth factor-β
synthesis, and enhances type II collagen and aggrecan core protein
synthesis in human articular chondrocytes (45).
With regard to the recommended dose of EGCG, the
present results demonstrated that DNA synthesis of rabbit articular
chondrocytes was increased in a dose-dependent manner when
chondrocytes were cultured in medium containing EGCG at
concentrations of 5–20 μM; EGCG at 10 μM could support the
strongest cell proliferation and stimulate the greatest matrix
secretion.
Acknowledgements
This study has been financially supported by the
National Science and Technology Pillar Program of China (grant no.
2012BAI42G00), the Guangxi Scientific Research and Technological
Development Foundation (grant no. Guikehe 14125008-2-14), the
Guangxi Science Fund for Distinguished Young Scholars (grant no.
2014GXNSFGA118006) and the Guangxi Natural and Open Project of
Guangxi Key Laboratory of Traditional Chinese Medicine Quality
Standards (grant no. Guizhongzhongkai 201304).
References
|
1
|
Carranza-Bencano A, García-Paino L, Armas
Padrón JR and Cayuela Dominguez A: Neochondrogenesis in repair of
full-thickness articular cartilage defects using free autogenous
periosteal grafts in the rabbit. A follow-up in six months.
Osteoarthritis Cartilage. 8:351–358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sánchez M, Anitua E, Azofra J, Andía I,
Padilla S and Mujika I: Comparison of surgically repaired Achilles
tendon tears using platelet-rich fibrin matrices. Am J Sports Med.
35:245–251. 2007. View Article : Google Scholar
|
|
3
|
Chung C and Burdick JA: Engineering
cartilage tissue. Adv Drug Deliv Rev. 60:243–262. 2008. View Article : Google Scholar
|
|
4
|
Hsieh PC, Thanapipatsiri S, Anderson PC,
Wang GJ and Balian G: Repair of full-thickness cartilage defects in
rabbit knees with free periosteal graft preincubated with
transforming growth factor. Orthopedics. 26:393–402.
2003.PubMed/NCBI
|
|
5
|
Waterfield MD, Scrace GT, Whittle N, et
al: Platelet-derived growth factor is structurally related to the
putative transforming protein p28sis of simian sarcoma virus.
Nature. 304:35–39. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Josephs SF, Guo C, Ratner L and Wong-Staal
F: Human-proto-oncogene nucleotide sequences corresponding to the
transforming region of simian sarcoma virus. Science. 223:487–491.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Downward J, Yarden Y, Mayes E, et al:
Close similarity of epidermal growth factor receptor and v-erb-B
oncogene protein sequences. Nature. 307:521–527. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
del Peso L, González-García M, Page C,
Herrera R and Nuñez G: Interleukin-3-induced phosphorylation of BAD
through the protein kinase Akt. Science. 278:687–689. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salah N, Miller NJ, Paganga G, Tijburg L,
Bolwell GP and Rice-Evans C: Polyphenolic flavanols as scavengers
of aqueous phase radicals and as chain-breaking antioxidants. Arch
Biochem Biophys. 322:339–346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang CS and Landau JM: Effects of tea
consumption on nutrition and health. J Nutr. 130:2409–2412.
2000.PubMed/NCBI
|
|
12
|
Blot WJ, McLaughlin JK and Chow WH: Cancer
rates among drinkers of black tea. Crit Rev Food Sci Nutr.
37:739–760. 1997. View Article : Google Scholar
|
|
13
|
Buschman JL: Green tea and cancer in
humans: a review of the literature. Nutr Cancer. 31:151–159. 1998.
View Article : Google Scholar
|
|
14
|
Kohlmeier L, Weterings KG, Steck S and Kok
FJ: Tea and cancer prevention: an evaluation of the epidemiologic
literature. Nutr Cancer. 27:1–13. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Na HK and Surh YJ: Intracellular signaling
network as a prime chemopreventive target of (−)-epigallocatechin
gallate. Mol Nutr Food Res. 50:152–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anti-cancer agent: review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Kong D, Wang Z and Sarkar FH:
Regulation of microRNAs by natural agents: An emerging field in
chemoprevention and chemotherapy research. Pharm Res. 27:1027–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mak JC: Potential role of green tea
catechins in various disease therapies: progress and promise. Clin
Exp Pharmacol Physiol. 39:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed S: Green tea polyphenol
epigallocatechin 3-gallate in arthritis: progress and promise.
Arthritis Res Ther. 12:2082010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pandey KB and Rizvi SI: Plant polyphenols
as dietary antioxidants in human health and disease. Oxid Med Cell
Longev. 2:270–278. 2009. View Article : Google Scholar
|
|
21
|
Mandel SA, Amit T, Weinreb O and Youdim
MB: Understanding the broad-spectrum neuroprotective action profile
of green tea polyphenols in aging and neurodegenerative diseases. J
Alzheimers Dis. 25:187–208. 2011.PubMed/NCBI
|
|
22
|
Stangl V, Lorenz M and Stangl K: The role
of tea and tea flavonoids in cardiovascular health. Mol Nutr Food
Res. 50:218–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shanmugam MK, Kannaiyan R and Sethi G:
Targeting cell signaling and apoptotic pathways by dietary agents:
role in the prevention and treatment of cancer. Nutr Cancer.
63:161–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan MH, Lai CS, Dushenkov S and Ho CT:
Modulation of inflammatory genes by natural dietary bioactive
compounds. J Agric Food Chem. 57:4467–4477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae JY, Kanamune J, Han DW, Matsumura K
and Hyon SH: Reversible regulation of cell cycle-related genes by
epigallocatechin gallate for hibernation of neonatal human tarsal
fibroblasts. Cell Transplant. 18:459–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akhtar N and Haqqi TM:
Epigallocatechin-3-gallate suppresses the global
interleukin-1beta-induced inflammatory response in human
chondrocytes. Arthritis Res Ther. 13:R932011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasheed Z, Anbazhagan AN, Akhtar N,
Ramamurthy S, Voss FR and Haqqi TM: Green tea polyphenol
epigallocatechin-3-gallate inhibits advanced glycation end
product-induced expression of tumor necrosis factor-alpha and
matrix metalloproteinase-13 in human chondrocytes. Arthritis Res
Ther. 11:R712009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed S, Wang N, Lalonde M, Goldberg VM
and Haqqi TM: Green tea polyphenol epigallocatechin-3-gallate
(EGCG) differentially inhibits interleukin-1 beta-induced
expression of matrix metalloproteinase-1 and -13 in human
chondrocytes. J Pharmacol Exp Ther. 308:767–773. 2004. View Article : Google Scholar
|
|
29
|
Buschmann MD and Grodzinsky AJ: A
molecular model of proteoglycan-associated electrostatic forces in
cartilage mechanics. J Biomech Eng. 117:179–192. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson D, Ash H, Yayon A, Nevo Z and
Aviezer D: Characteristics of cartilage biopsies used for
autologous chondrocytes transplantation. Cell Transplant.
10:203–208. 2001.PubMed/NCBI
|
|
31
|
Akiyama H: Transcriptional regulation in
chondrogenesis by Sox9. Clin Calcium. 21:845–851. 2011.(In
Japanese). PubMed/NCBI
|
|
32
|
Tew SR and Clegg PD: Analysis of post
transcriptional regulation of SOX9 mRNA during in vitro
chondrogenesis. Tissue Eng Part A. 17:1801–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng LJ, Wheatley S, Muscat GE, et al: SOX9
binds DNA, activates transcription, and coexpresses with type II
collagen during chondrogenesis in the mouse. Dev Biol. 183:108–121.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marshall OJ and Harley VR: Molecular
mechanisms of SOX9 action. Mol Genet Metab. 71:455–462. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davies SR, Chang LW, Patra D, et al:
Computational identification and functional validation of
regulatory motifs in cartilage-expressed genes. Genome Res.
17:1438–1447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tew SR, Li Y, Pothacharoen P, Tweats LM,
Hawkins RE and Hardingham TE: Retroviral transduction with SOX9
enhances re-expression of the chondrocyte phenotype in passaged
osteoarthritic human articular chondrocytes. Osteoarthritis
Cartilage. 13:80–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paul R, Haydon RC, Cheng H, et al:
Potential use of Sox9 gene therapy for intervertebral degenerative
disc disease. Spine (Phila Pa 1976). 28:755–763. 2003. View Article : Google Scholar
|
|
38
|
Tsuchiya H, Kitoh H, Sugiura F and
Ishiguro N: Chondrogenesis enhanced by overexpression of sox9 gene
in mouse bone marrow-derived mesenchymal stem cells. Biochem
Biophys Res Commun. 301:338–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benya PD and Shaffer JD: Dedifferentiated
chondrocytes reexpress the differentiated collagen phenotype when
cultured in agarose gels. Cell. 30:215–224. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schnabel M, Marlovits S, Eckhoff G,
Fichtel I, Gotzen L, Vécsei V and Schlegel J:
Dedifferentiation-associated changes in morphology and gene
expression in primary human articular chondrocytes in cell culture.
Osteoarthritis Cartilage. 10:62–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karlsen TA, Shahdadfar A and Brinchmann
JE: Human primary articular chondrocytes, chondroblasts-like cells,
and dedifferentiated chondrocytes: differences in gene, microRNA,
and protein expression and phenotype. Tissue Eng Part C Methods.
17:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwan KM, Pang MK, Zhou S, et al: Abnormal
compartmentalization of cartilage matrix components in mice lacking
collagen X: implications for function. J Cell Biol. 136:459–471.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adcocks C, Collin P and Buttle DJ:
Catechins from green tea (Camellia sinensis) inhibit bovine and
human cartilage proteoglycan and type II collagen degradation in
vitro. J Nutr. 132:341–346. 2002.PubMed/NCBI
|
|
44
|
Vankemmelbeke MN, Jones GC, Fowles C, et
al: Selective inhibition of ADAMTS-1, -4 and -5 by catechin gallate
esters. Eur J Biochem. 270:2394–2403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andriamanalijaona R, Kypriotou M, Baugé C,
et al: Comparative effects of 2 antioxidants, selenomethionine and
epigallocatechin-gallate, on catabolic and anabolic gene expression
of articular chondrocytes. J Rheumatol. 32:1958–1967.
2005.PubMed/NCBI
|