Introduction
Transrectal ultrasound (TRUS)-guided biopsy is the
most widely used method for the histological diagnosis of prostate
cancer (PCa), which provides real-time imaging of the prostate at a
relatively low cost. However, clinical practice suggests that
systematic biopsy may be associated with a high false-negative rate
(1), and systematic repeat biopsy
does not give a satisfactory cancer detection rate (2), in addition to increasing the risk of
complications and discomfort to the patient. Additional targeted
biopsy in suspicious areas identified by TRUS may improve detection
rates, as the sensitivity of conventional TRUS for cancer lesions
is relatively low (3,4). Even new imaging techniques such as
sonoelastography and contrast-enhanced TRUS do not provide a
considerable benefit to the diagnosis of PCa (5,6).
It has been reported that T2-weighted (T2W) magnetic
resonance imaging (MRI) and diffusion-weighted imaging (DWI) are
useful for diagnosing PCa (7,8).
Therefore, there has been an increasing interest in the use of MRI
for the diagnosis of PCa. MRI has been used to guide prostatic
biopsy successfully, as first reported in 2000; however, MRI
guidance is time-consuming and requires specific biopsy equipment
(9). It has been hypothesized that
TRUS-guided targeted biopsy in suspicious areas identified by MRI
has the potential to obtain a high positive rate; however, few
patients have been enrolled in studies to investigate this
(10,11). The study by Singh et al
revealed that patient selection, specifically whether they had
undergone a negative TRUS-guided biopsy or not and the different
interval between two biopsies, influenced the PCa detection rate
(12). To study whether MRI is
able to increase the PCa detection rate generally, prospective
research is required to compare the detection rate between patients
undergoing conventional TRUS and those additionally examined by
MRI.
The present study was conducted to investigate
whether 3-Tesla (3-T) multiparametric MRI prior to biopsy improved
the PCa detection rate in patients at their first TRUS-guided
biopsies, and to investigate which subgroup had the most evident
improvement in PCa detection rate.
Materials and methods
Subjects
Between June 2008 and December 2013, 429 consecutive
patients (median age, 67 years; range, 45–91 years) underwent 3-T
multiparametric MRI prior to their first TRUS-guided prostate
biopsies. All patients presented as a result of abnormal digital
rectal examination (DRE) findings and/or persistently elevated
serum prostate-specific antigen (PSA) levels. Nine cases were
excluded due to DWI artifacts resulting from movement of the
patient during image acquirement. In the remaining 420 patients,
the median PSA level was 9.73 (2.43–35.65) ng/ml and the median
prostate volume was 44.82 (21.22–83.22) ml. There were 52 patients
with abnormal DRE findings. MRI was performed 2–14 (median 7) days
prior to biopsy. The study was approved by the Ethics Committee of
Medical College, Shanghai Jiaotong University (Shanghai, China).
Signed informed consent was obtained from all patients.
MRI examination
MRI was performed with a 3-T MRI system (Achieva;
Philips, Best, The Netherlands), using a pelvic phased-array coil.
First, conventional MRI was performed, including T1-weighted (T1W),
T2W and T2W spectral presaturation attenuated inversion recovery
(SPAIR). The repetition time/echo time (TR/TE) in T1W, T2W and T2W
SPAIR imaging were 353 msec/10 msec, 2,754 msec/80 msec and 2,879
msec/80 msec, respectively. The other main parameters were as
follows: thickness, 3 mm; spacing, 1 mm, field of view (FOV),
160×200 mm; matrix, 128×132; number of signal averages (NSA), 3
times. Then, a DWI sequence was performed. The main parameters were
as follows: b value, 0 and 1,000 sec/mm2; TR/TE 2,500
msec/60 msec; FOV,160×144 mm; matrix, 80×60; thickness, 6 mm; NSA 4
times. An apparent diffusion coefficient (ADC) map was obtained by
the computer automatically.
MRI imaging was evaluated by a radiologist who had
10 years’ experience of prostate MR imaging. It was considered
abnormal when there were low signal nodules with a mass-like
appearance on T2W or T2W SPAIR and high signal nodules on DWI, in
either the peripheral zone or the transition zone. The radiologist
recorded a confidence level for the probability of malignancy (1,
definitely absent; 2, probably absent; 3, undetermined; 4, probably
present; 5, definitely present) in different sectors, which was
applied as in our previous study (13). Areas of levels 3 to 5 in any MRI
imaging were regarded as suspicious. The distances from the
suspicious area to the tip, the exterior margin and the posterior
border of the prostate were recorded. The prostate volume was
calculated using the ellipsoid formula (length × height × width ×
0.52) (14). PSA density (PSAD)
was calculated by dividing the PSA level by the prostate
volume.
Biopsy protocol
Transperineal prostate biopsy was performed by two
operators, guided by transrectal ultrasound. Using a 16-gauge core
biopsy gun (Bard Magnum™; Bard Biopsy Systems, Tempe, AZ, USA), a
12-core systematic biopsy (10 cores distributed in a fan-shape from
the peripheral zone and 2 cores from the transition zone) was first
performed without knowledge of the location of suspicious MRI
findings. After two operators had reviewed the information
concerning the MRI-suspicious area recorded by the radiologist,
targeted biopsy was performed. One or two cores were taken from
each suspicious area. Whether the needle was in the correct site
was determined by measuring the distance of the needle to the
exterior margin and posterior border of prostate, which coincided
with the same distances on the corresponding axial MRI.
Statistical analysis
Differences were analyzed using the Student’s
t-test, Kruskal-Wallis test and Chi-square test. P<0.05 was
considered to indicate a significant difference. All statistical
analyses were performed using SAS software, version 9.13 (SAS
Institute, Inc., Cary, NC, USA).
Results
Clinical characteristics of the 420 patients are
presented in Table I. PCa was
detected in 173 patients (41.2%, 173/420). Among these 173
patients, 41 patients (23.7%, 41/173) were detected by targeted
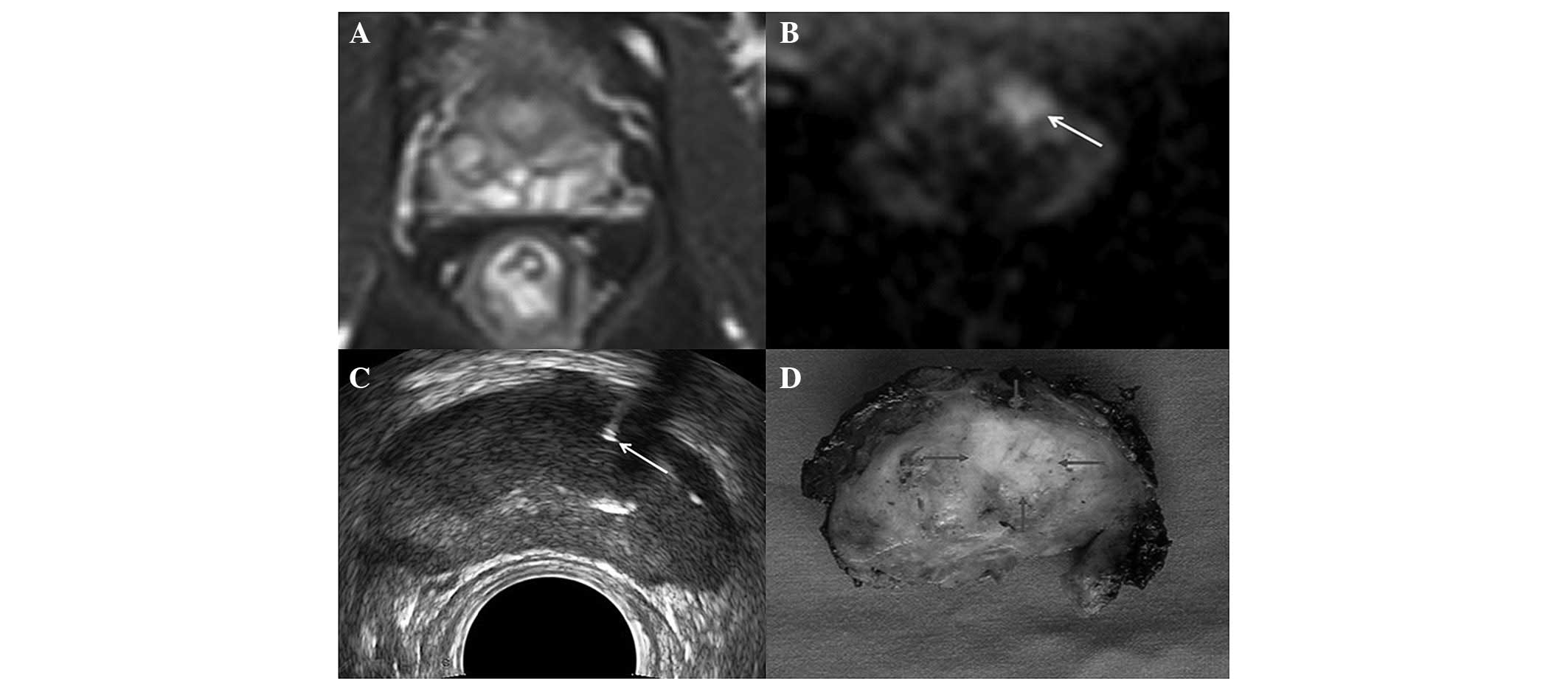
biopsy, but not by systematic biopsy (Fig. 1); 28 patients (16.2%, 28/173) were
detected by systematic biopsy, but not by targeted biopsy, and 104
patients (60.1%, 104/173) were detected by both systematic biopsy
and targeted biopsy. The increase in the cancer detection rate by
targeted biopsy identified by MRI was 9.8% (41/420; P=0.0033). As
shown in Table II, among the
three groups with PCa detected by different biopsy regimens there
were significant differences in serum PSA level, PSAD, prostate
volume, DRE findings and TRUS findings, but no differences in age
and the biopsy Gleason score.
 | Table ICharacteristics of all patients
enrolled in the study. |
Table I
Characteristics of all patients
enrolled in the study.
| Characteristics | Prostate cancer | Benign prostate
disease | P-value |
|---|
| No. of patients
(%) | 173 (41.2) | 247 (58.8) | |
| Age (years) | 71 (63–77) | 65 (58–78) | 0.041a |
| PSA level
(ng/ml) | 11.59
(4.78–19.50) | 8.42
(5.34–28.72) | 0.017a |
| Prostate volume
(ml) | 39.57
(25.25–56.01) | 48.52
(31.75–70.06) | 0.029a |
| PSAD
(ng/ml2) | 0.22 (0.14–0.38) | 0.13 (0.07–0.33) | 0.010a |
| No. of patients with
abnormal DRE (%) | 34 (19.7) | 18 (7.3) | 0.0002 |
| No. of patients with
abnormal TRUS (%) | 73 (42.2) | 45 (18.2) | <0.0001 |
 | Table IIComparison of the characteristics in
patients with prostate cancer detected by different biopsy
regimens. |
Table II
Comparison of the characteristics in
patients with prostate cancer detected by different biopsy
regimens.
| Characteristics | TB alone | SB alone | TB + SB | P-value |
|---|
| No. of patients | 41 (23.7) | 28 (16.2) | 104 (60.1) | |
| Age (years) | 68 (61–73) | 69 (66–73) | 72 (66–79) | 0.66a |
| PSA (ng/ml) | 7.55
(5.12–10.36) | 9.38
(6.21–14.16) | 13.78
(3.65–18.17) | 0.008a |
| Prostate volume
(ml) | 47.65
(30.65–62.35) | 35.15
(25.06–46.38) | 37.57
(24.46–53.93) | 0.020a |
| PSAD
(ng/ml2) | 0.13 (0.10–0.17) | 0.19 (0.12–0.34) | 0.26 (0.15–0.40) | 0.028a |
| DRE (No. of
patients) | | | | 0.028 |
| Normal | 37 (90.2) | 18 (64.3) | 84 (80.8) | |
| Abnormal | 4 (9.8) | 10 (35.7) | 20 (19.2) | |
| TRUS (No. of
patients) | | | | 0.002 |
| Normal | 33 (80.5) | 12 (42.9) | 55 (52.9) | |
| Abnormal | 8 (19.5) | 16 (57.1) | 49 (47.1) | |
| Biopsy Gleason
score (no. of patients) | | | | 0.261 |
| <7 | 23 (56.1) | 15 (53.6) | 61 (58.7) | |
| ≥7 | 18 (43.9) | 13 (46.4) | 43 (41.3) | |
The efficiency of additional targeted biopsy
identified by MRI on the cancer detection rate in different
subgroups of patients according to PSA level, PSAD, prostate
volume, TRUS findings, and DRE findings is summarized in Table III. The improvement of the cancer
detection rate was 9.8% in all cases. There was significant
increase in the cancer detection rate in the patient subgroup with
a PSA level of 4–10 ng/ml, PSAD of 0.12–0.20 ng/ml2,
prostate volume >50 ml, negative TRUS findings and negative DRE
findings, and the P-values were 0.0256, 0.0133, 0.0099, 0.0027 and
0.0037, respectively.
 | Table IIIEffect of additional targeted biopsy
identified by MRI on cancer detection rates. |
Table III
Effect of additional targeted biopsy
identified by MRI on cancer detection rates.
|
Characteristics | No. of
patients | No. of cancer
patients | Increase in the no.
of cancer patients | Increase in the
positive rate (%) |
|---|
| PSA (ng/ml) |
| <4 | 56 | 12 | 3 | 3/56 (5.4) |
| 4–10 | 218 | 84 | 22 | 22/218
(10.1)a |
| ≥10 | 146 | 77 | 16 | 16/146 (11.0) |
| Prostate volume
(ml) |
| <30 | 105 | 52 | 5 | 5/105 (4.8) |
| 30–50 | 172 | 68 | 16 | 16/172 (9.3) |
| ≥50 | 143 | 53 | 20 | 20/143
(14.0)b |
| PSAD
(ng/ml2) |
| <0.12 | 80 | 16 | 5 | 5/80 (6.3) |
| 0.12–0.20 | 185 | 80 | 23 | 23/185
(12.4)c |
| ≥0.20 | 155 | 77 | 13 | 13/155 (8.4) |
| TRUS |
| Normal | 302 | 100 | 33 | 33/302
(10.9)d |
| Abnormal | 118 | 73 | 8 | 8/118 (6.8) |
| DRE |
| Normal | 368 | 139 | 37 | 37/368
(10.1)e |
| Abnormal | 52 | 34 | 4 | 4/52 (7.7) |
| Overall | 420 | 173 | 41 | 41/420
(9.8)f |
Discussion
Since PCa is often multifocal and the volume of
prostate sampled by biopsy is relatively small, there is high
false-negative rate in conventional TRUS-guided systematic biopsy.
Various regimens have been devised to improve the diagnostic yield
of prostate biopsies, such as increasing the number of biopsy cores
and sampling from the suspicious areas of TRUS for example
(15,16). However, the ideal strategy for
prostate biopsy has not yet been identified. A study revealed that
even saturation biopsy did not significantly improve cancer
detection compared with standard biopsy, and was not able to rule
out the presence of PCa (17).
MRI has been increasingly used to detect and locate
lesions of PCa. 3-T MRI is considered to be superior to 1.5-T MRI
with a higher signal to noise ratio and greater spatial resolution
(18). Currently, the optimal MRI
techniques for PCa are the integrated use of multimodal MRIs, for
example, DWI and T2W. DWI has certain advantages, such as fast
imaging, without the need for injection of contrast agents. Cancer
lesions often show high signal intensity compared with benign
tissues on DWI, regardless of whether they are in the peripheral
zone or in the transition zone.
Previous studies have found that TRUS-guided repeat
biopsies alone result in positive rates of 10–41.1% (1,19–21).
When MRI data are added, positive rates for TRUS-guided repeat
biopsies of between 24.7 and 40.5% have been observed (10,22,23).
It has been suggested that the cancer detection rate might be
influenced by previous biopsy techniques and the interval between
biopsies (24,25). However, it remains unclear whether
additional MRI examination prior to biopsy is useful. Lattouf et
al observed that MRI prior to TRUS-guided repeat biopsy tended
to give higher cancer yields, but the difference was not
statistically significant (26).
Furthermore, to the best of our knowledge, there are no studies
evaluating the advantage of MRI in a large series of cases prior to
first biopsy in addition to the standard 12-core systematic
biopsy.
In the present study, the overall cancer detection
rate was 41.2%, and 41 out of the 173 prostate cancer patients were
detected only by targeted biopsy identified by MRI. The improvement
of cancer detection rate by targeted biopsy was 9.8% (41/420;
P=0.0033). Targeted biopsy identified by MRI may be useful to
improve the positive rate on first biopsy. This finding differs
from the results of Shigemura et al who reported that only
1.04% of cancers had positive cores in MRI targeted biopsy alone
(27). This difference may be
caused by differences in prostate volume (mean 44.82 vs. 31.9 ml)
and serum PSA level (mean 9.73 vs. 8.58 ng/ml) between the two
studies. Previous studies have identified patient subgroups with
high positive rates in repeat biopsy; there are high false negative
rates in patients with a large prostate volume and elevated serum
PSA levels (20,28).
In the subgroup analysis of the present study, the
improvement of the cancer detection rate by targeted biopsy was
10.1, 12.4, 14.0, 10.9 and 10.1%, respectively, in patients with a
PSA level of 4–10 ng/ml, PSAD of 0.12–0.20 ng/ml2,
prostate volume of >50 ml, negative TRUS findings and negative
DRE findings, with P-values of 0.0256, 0.0133, 0.0099, 0.0027 and
0.0037, respectively. A significant increase in the PCa detection
rate by targeted biopsy was found in the subgroup with a PSA level
of 4–10 ng/ml, while there was no significant difference in the
subgroups with a PSA level of <4 ng/ml or >10 ng/ml. This may
be explained by the fact that, as a sensitive marker for PCa, a PSA
level of <4 ng/ml presents a low incidence of PCa. Despite the
addition of MRI data, there is only a small chance of detecting
more cancer. By contrast, when the PSA level is >10 ng/ml, the
cancer lesions are often so evident that they are detected by
systematic biopsy. In patients with a PSA level of 4–10 ng/ml, a
considerable number of lesions may remain undetected by systematic
biopsy (29), which provides an
opportunity for MRI to identify suspicious areas due to its high
sensitivity. For similar reasons, the subgroup with a PSAD of
0.12–0.20 ng/ml2 obtained the most marked increase in
PCa detection rate compared with the other two subgroups. It has
previously been reported that a significantly increased prostate
volume is one of the important factors responsible for PCa being
missed by biopsy (30,31). Since PCa is a multifocal disease,
the biopsy technique only provides a limited sample volume of the
prostate. The results of the present study showed that the subgroup
with a prostate volume of >50 ml obtained the greatest increase
in the PCa detection rate. This was in accord with previous studies
(30–32). Due to the higher sensitivity of MRI
for prostate cancer, it is possible for the detection rate to be
improved more significantly in the subgroup of patients with
negative TRUS findings or negative DRE findings. For patients with
abnormal TRUS or DRE findings, the traditional TRUS-guided biopsy
was able to diagnose PCa, and additional targeted biopsies in
MRI-suspicious areas were not able to increase the detection rate
significantly.
One limitation of the present study was that the
accuracy of targeted biopsies may have been reduced since they were
not performed with real-time guided-MRI, which could not be used
routinely due to it being time-consuming and requiring specific
biopsy equipment. A promising imaging technique comprising a fusion
of MRI and TRUS may have the ability to improve the accuracy of
prostate biopsy (33).
Investigation of the value of this technique in our further studies
is planned. Another limitation of the present study was that the
MRI results were not compared with specimens of radical
prostatectomy. Too small a specimen may cause the pathologist to
draw a false negative diagnosis. Additionally, the judgment of
normal or suspicious MRI imaging was partly operator-dependent. The
confidence levels for the probability of malignancy were used to
minimize the subjectivity.
In conclusion, this study indicates that MRI may be
recommended particularly for the subgroup of patients with a PSA
level of 4–10 ng/ml, PSAD of 0.12–0.20 ng/ml2, prostate
volume of >50 ml, negative TRUS findings and negative DRE
findings. 3-T multiparametric MRI has the potential to improve the
prostate cancer detection rate on first biopsy.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation (30770562 and 81371574).
References
|
1
|
Mitterberger MJ, Aigner F, Horninger W, et
al: Comparative efficiency of contrast-enhanced colour Doppler
ultrasound targeted versus systematic biopsy for prostate cancer
detection. Eur Radiol. 20:2791–2796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ploussard G, Nicolaiew N, Marchand C, et
al: Risk of repeat biopsy and prostate cancer detection after an
initial extended negative biopsy: longitudinal follow-up from a
prospective trial. BJU Int. 111:988–996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nelson ED, Slotoroff CB, Gomella LG and
Halpern EJ: Targeted biopsy of the prostate: the impact of color
Doppler imaging and elastography on prostate cancer detection and
Gleason score. Urology. 70:1136–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen MK, Luo Y, Zhang H, et al:
Investigation of optimal prostate biopsy schemes for Chinese
patients with different clinical characteristics. Urol Int.
89:425–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamoi K, Okihara K, Ochiai A, et al: The
utility of transrectal real-time elastography in the diagnosis of
prostate cancer. Ultrasound Med Biol. 34:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puech P, Huglo D, Petyt G, Lemaitre L and
Villers A: Imaging of organ-confined prostate cancer: functional
ultrasound, MRI and PET/computed tomography. Curr Opin Urol.
19:168–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao H, Fukatsu H and Ishigaki T: Prostate
cancer detection with 3-T MRI: comparison of diffusion-weighted and
T2-weighted imaging. Eur J Radiol. 61:297–302. 2007. View Article : Google Scholar
|
|
8
|
Osugi K, Tanimoto A, Nakashima J, et al:
What is the most effective tool for detecting prostate cancer using
a standard MR scanner? Magn Reson Med Sci. 12:271–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D’Amico AV, Tempany CM, Cormack R, et al:
Transperineal magnetic resonance image guided prostate biopsy. J
Urol. 164:385–387. 2000. View Article : Google Scholar
|
|
10
|
Park BK, Lee HM, Kim CK, Choi HY and Park
JW: Lesion localization in patients with a previous negative
transrectal ultrasound biopsy and persistently elevated prostate
specific antigen level using diffusion-weighted imaging at three
Tesla before rebiopsy. Invest Radiol. 43:789–793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hambrock T, Somford DM, Hoeks C, et al:
Magnetic resonance imaging guided prostate biopsy in men with
repeat negative biopsies and increased prostate specific antigen. J
Urol. 183:520–527. 2010. View Article : Google Scholar
|
|
12
|
Singh AK, Krieger A, Lattouf JB, et al:
Patient selection determines the prostate cancer yield of dynamic
contrast-enhanced magnetic resonance imaging-guided transrectal
biopsies in a closed 3-Tesla scanner. BJU Int. 101:181–185.
2008.
|
|
13
|
Wang R, Chen JJ, Zhou YC, et al:
Evaluation of diffusion-weighted magnetic resonance imaging and
contrast-enhanced harmonic ultrasonography in detection and
location of prostate transition-zone cancer. J Int Med Res.
39:256–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eri LM, Thomassen H, Brennhovd B and
Håheim LL: Accuracy and repeatability of prostate volume
measurement by transrectal ultrasound. Prostate Cancer Prostatic
Dis. 5:273–278. 2002. View Article : Google Scholar
|
|
15
|
Abdollah F, Scattoni V, Raber M, et al:
The role of transrectal saturation biopsy in tumour localization:
pathological correlation after retropubic radical prostatectomy and
implication for focal ablative therapy. BJU Int. 108:366–371. 2011.
View Article : Google Scholar
|
|
16
|
Salomon G, Drews N, Autier P, et al:
Incremental detection rate of prostate cancer by real-time
elastography targeted biopsies in combination with a conventional
10-core biopsy in 1,024 consecutive patients. BJU Int. 113:548–553.
2014. View Article : Google Scholar
|
|
17
|
Jones JS, Patel A, Schoenfield L, et al:
Saturation technique does not improve cancer detection as an
initial prostate biopsy strategy. J Urol. 175:485–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rouvière O, Hartman RP and Lyonnet D:
Prostate MR imaging at high-field strength: evolution or
revolution? Eur Radiol. 16:276–284. 2006. View Article : Google Scholar
|
|
19
|
Busby JE and Evans CP: Determining
variables for repeat prostate biopsy. Prostate Cancer Prostatic
Dis. 7:93–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SJ, Miyake H, Hara I and Eto H:
Predictors of prostate cancer on repeat transrectal
ultrasound-guided systematic prostate biopsy. Int J Urol. 10:68–71.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chun FK, de la Taille A, van Poppel H, et
al: Prostate cancer gene 3 (PCA3): development and internal
validation of a novel biopsy nomogram. Eur Urol. 56:659–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheikh AB, Girouin N, Colombel M, et al:
Evaluation of T2-weighted and dynamic contrast-enhanced MRI in
localizing prostate cancer before repeat biopsy. Eur Radiol.
19:770–778. 2009. View Article : Google Scholar
|
|
23
|
Prando A, Kurhanewicz J, Borges AP,
Oliveira EM Jr and Figueiredo E: Prostatic biopsy directed with
endorectal MR spectroscopic imaging findings in patients with
elevated prostate specific antigen levels and prior negative biopsy
findings: early experience. Radiology. 236:903–910. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eskicorapci SY, Guliyev F, Islamoglu E,
Ergen A and Ozen H: The effect of prior biopsy scheme on prostate
cancer detection for repeat biopsy population: results of the
14-core prostate biopsy technique. Int Urol Nephrol. 39:189–195.
2007. View Article : Google Scholar
|
|
25
|
Scattoni V, Maccagnano C, Zanni G, et al:
Is extended and saturation biopsy necessary? Int J Urol.
17:432–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lattouf JB, Grubb RL 3rd, Lee SJ, et al:
Magnetic resonance imaging-directed transrectal
ultrasonography-guided biopsies in patients at risk of prostate
cancer. BJU Int. 99:1041–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shigemura K, Motoyama S and Yamashita M:
Do additional cores from MRI cancer-suspicious lesions to
systematic 12-core transrectal prostate biopsy give better cancer
detection? Urol Int. 88:145–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ceylan C, Doluoglu OG, Aglamis E and
Baytok O: Comparison of 8, 10, 12, 16, 20 cores prostate biopsies
in the determination of prostate cancer and the importance of
prostate volume. Can Urol Assoc J. 8:E81–E85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Djavan B, Zlotta A, Remzi M, et al:
Optimal predictors of prostate cancer on report prostate biopsy: a
prospective study of 1,051 men. L Urol. 163:1144–1148. 2000.
View Article : Google Scholar
|
|
30
|
Ankerst DP, Till C, Boeck A, et al:
Predicting risk of prostate cancer in men receiving finasteride:
effect of prostate volume, number of biopsy cores, and American
Urological Association symptom score. Urology. 82:1076–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elshafei A, Li YH, Hatem A, et al: The
utility of PSA velocity in prediction of prostate cancer and high
grade cancer after an initially negative prostatebiopsy. Prostate.
73:1796–1802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuligowaka E, Barish MA, Fenlon HM and
Blake M: Predictors of prostate carcinoma: accuracy of gray-scale
and color Doppler US and serum markers. Radiology. 220:757–764.
2001. View Article : Google Scholar
|
|
33
|
Ukimura O, Hirahara N, Fujihara A, et al:
Technique for a hybrid system of real-time transrectal ultrasound
with preoperative magnetic resonance imaging in the guidance of
targeted prostate biopsy. Int J Urol. 17:890–893. 2010. View Article : Google Scholar : PubMed/NCBI
|