Introduction
Massive blood transfusion is commonly defined as the
administration of ≥10 units of packed red blood cells (pRBCs) to an
individual patient (1,2) or the transfusion of more than one
blood volume in 24 h (1,3–5).
Alternative definitions include a ≥50% loss in blood volume within
3 h or a rate of loss of 150 ml blood/min in the severe traumatic
and emergent situations (3).
Massive blood transfusion is often provided to those who are
injured during military operations, who have multiple injuries due
to other causes, and who undergo complex surgery. A rational blood
transfusion can improve the outcome of surgery, whereas
unreasonable transfusion can increase mortality in patients.
Transfusion plays a key role in saving the lives of
patients who have suffered massive blood loss. However, studies
have found that mortality remains high for trauma patients who have
received massive blood transfusion and suggest that there is a
certain correlation between RBC transfusion volume and the
mortality of patients (6–8). Stanworth et al (9) found that the mortality of patients
who had received a pRBC transfusion was 9% for 0–5 units, 22% for
6–9 units and 42% for ≥10 units. Thus, it is necessary to maintain
a balance between the advantages and disadvantages of RBC
transfusion during massive blood transfusion.
Therefore, a multicenter retrospective study was
conducted on cases of massive blood transfusion in 20 comprehensive
hospitals from different regions of China to explore the
correlation between RBC volume and the mortality of surgical
inpatients with massive blood transfusion.
Materials and methods
Study protocol
This study was retrospective in nature. Data were
collected from the medical records of surgical inpatients who
received massive transfusion at 20 large-scale hospitals between
January 2009 and December 2010. Between June 2010 and January 2011,
2,000 copies of the Massive Transfusion Survey Table (hereafter
referred to as the Survey Table) were distributed to 20 Class III
comprehensive hospitals in the northwest, southwest, central south,
north and northeast regions of China. Members of the National
Massive Transfusion Current Status Investigation Coordination Group
(hereafter referred to as the Coordination Group) were responsible
for collecting the data from these hospitals using the Survey
Table. The data analysis was conducted at Shaanxi Provincial
People’s Hospital, which is the Third Affiliated Hospital of the
Medical College of Xi’an Jiaotong University (Xi’an, China). The
present study was approved by the ethics committee of Xi’an
Jiaotong University.
Study population
Patients who received a transfusion of ≥10 units of
pRBCs over a period of ≤24 h for trauma, cardiac surgery, obstetric
conditions or other common surgeries (for example, orthopedic,
thoracic, general, urinary, hepatobiliary and neurological surgery)
were included in the study. By contrast, patients with coagulation
disorders, hepatic failure due to medical causes, and
coagulopathies were excluded from the analysis. Patients who
received transfusions of <10 units for ≤24 h were assigned to
the control group. Informed consent was obtained from the patients
or the patients’ families prior to their inclusion in the current
study.
Survey table
The directors of the transfusion departments of the
20 participating hospitals discussed the topic, consulted experts
and designed the Survey Table with reference to several
international and domestic sources, in accordance with the
principles of equality, voluntariness and mutual benefits. A
meeting of the Coordination Group was then held, where 35 experts
of clinical transfusion, surgery, anesthesia, gynecology and
obstetrics, hematology and medical statistics discussed the study
protocol and mode of data collection and also perfected and added
supplements to the Survey Table. Suitable training was then offered
to the investigating staff.
Components of the survey table
The survey Table comprised four sections, as
follows: i) Clinical and demographic characteristics of the
patient, including name, gender, age, body weight, blood type,
ethnicity, admission number, admission department, primary
diagnosis, secondary diagnosis, pathologic diagnosis, nature of
surgery and vital signs on admission. ii) Details regarding the
perioperative complications, clinical condition within 24 h and
after 24 h of the transfusion, and the total amount of blood
transfused. iii) The results of the following blood tests performed
before, within 24 h and after 24 h of transfusion: routine blood
test, coagulation tests, liver function test, kidney function test,
and arterial blood gas analysis. iv) Adverse events due to massive
transfusion.
Quality control
The Survey Table was first subjected to a
small-scale preliminary test at Shaanxi Provincial People’s
Hospital so that revisions could be made on the basis of the
results and comments by experts to further improve the Table. One
unit of pRBCs was derived from 200 ml whole blood and had a volume
of 140–172 ml. One unit of fresh frozen plasma (FFP) was derived
from 200 ml whole blood and had a volume of 100 ml. One bag of
apheresis platelet was 10 units, and had a volume of 150–250 ml.
One unit of platelet concentrate was derived from 200 ml whole
blood and had a volume of 20–30 ml. The pRBCs were stored at 2–6°C.
FFP was stored at ≤−18°C and thawed in a 37°C water bath, for
~10–15 min. Platelets were stored at 20–24°C in a platelet
shaker.
Data collected with devices and
reagents
The main test devices and reagents used were as
follows: Sysmex XE-2100 or XT-1800i hematology analyzer (Sysmex
Corporation, Kobe, Japan); Coulter LH780 Coulter Hematology
Analyzer (Beckman Coulter, Brea, CA, USA); Hitachi 7170A or 7180
Biochemical Analyzer (Hitachi, Tokyo, Japan); Roche Modular DP
Automatic Biochemical Analyzer (Roche Diagnostics, Indianapolis,
IN, USA); Olympus AU640 Biochemical Analyzer, (Olympus Corporation,
Tokyo, Japan); Radiometer ABL-77 Blood Gas Analyzer (Radiometer,
Copenhagen, Denmark); Roche Cobas- B123 Blood Gas Analyzer (Roche
Diagnostics); and Sysmex CA1500/CA7000 Automatic Blood Coagulation
Analyzer (Sysmex Corporation). All test reagents used were
device-supporting reagents.
Data on the blood tests performed were collected
from the laboratory records. These included: blood routine,
coagulation tests, liver function test, kidney function and blood
gas analysis. The data were collected for the blood tests performed
prior to transfusion and at 16 different units during the 24-h
transfusion (2U, 4U, 6U, 8U, 10U, 12U, 14U, 16U, 18U, 20U, 22U,
24U, 26U, 28U, 30U and 40U) and subjected to statistical analysis.
The tests were conducted at the laboratory of each participating
hospital, each of which undergoes internal quality control and an
external quality assessment conducted by the Clinical Test Center
of the Ministry of Health.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA). Epidata
version 3.01 (Epidata Association, Odense, Denmark) was used for
double data entry verification and database construction. The data
on the demographic characteristics and clinical features were
expressed as means with standard deviations or as absolute numbers.
Categorical variables were analyzed by χ2 test, while
continuous variables with normal distribution were analyzed by the
Shapiro-Wilk test, analysis of variance, or the Kruskall-Wallis
test, as appropriate. The Bonferroni method was applied for
post-hoc tests to determine the significance of the differences
between the group that received massive transfusion and the control
group that did not. Linear regression was used to describe the
relation between the number of units of pRBCs transfused and the
platelet count. P<0.05 was considered to indicate a
statistically significant result.
Results
Survey results
In total, 1,753 copies of the Survey Table were
received from the 20 hospitals and the recovery rate was 87.65%
(1,753/2,000). Of these, 1,601 copies were qualified tables without
missing items and the qualification rate was 91.33% (1,601/1,753).
The demographics and clinical data for the various RBC transfusion
volume groups are shown in Table
I. Among the 1,601 massive blood transfusion patients, 268
patients had undergone trauma (mortality, 34; survival, 234;
mortality rate, 12.69%), 383 patients had undergone cardiac surgery
(mortality, 53; survival, 330; mortality rate, 13.84%), 876
patients had undergone general surgery (mortality, 42; survival,
834; mortality rate, 4.79%) and 74 patients were obstetric patients
(mortality, 3; survival, 71; mortality rate, 4.05%).
 | Table IDemographics and clinical data on
various RBC transfusion volume groups. |
Table I
Demographics and clinical data on
various RBC transfusion volume groups.
| Variable | 0–4 units | 5–9 units | 10–14 units | 15–19 units | 20–24 units | 25–29 units | 30–39 units | ≥40 units | P-value |
|---|
| Number of patients, n
(%) | 96 (6.0) | 457 (28.5) | 662 (41.3) | 174 (10.9) | 114 (7.1) | 46 (2.9) | 34 (2.1) | 18 (1.1) | <0.001a |
| Age, years (±
SD) | 48.4 (23.2) | 46.1 (17) | 45.1 (16.4) | 44.7 (17) | 43 (17.8) | 48.2 (16.8) | 45.4 (17.8) | 42.2 (16.7) | 0.329a |
| Males, n (%) | 43 (4.8) | 210 (23.4) | 390 (43.4) | 109 (12.1) | 79 (8.8) | 31 (3.4) | 27 (3) | 10 (1.1) | <0.001a |
| Weight, kg (±
SD) | 53.9 (20) | 57 (12.6) | 58.4 (11.4) | 58.3 (11.9) | 59.2 (11.4) | 59.1 (7.5) | 59.9 (10.8) | 56.8 (14.8) | 0.158a |
| Number of patients
(1)c, n (%) | 14 (5.2) | 67 (25.0) | 109 (40.7) | 34 (12.7) | 30 (11.2) | 7 (2.6) | 4 (1.5) | 3 (1.1) | <0.001a |
| Number of patients
(2)d, n (%) | 12 (3.1) | 104 (27.2) | 158 (41.3) | 44 (11.5) | 32 (8.4) | 16 (4.2) | 10 (2.6) | 7 (1.8) | <0.001a |
| Number of patients
(3)e, n (%) | 68 (7.8) | 267 (30.5) | 358 (40.9) | 91 (10.4) | 47 (5.4) | 21 (2.4) | 17 (1.9) | 7 (0.8) | <0.001a |
| Number of patients
(4)f, n (%) | 2 (2.7) | 19 (25.7) | 37 (50) | 5 (6.8) | 5 (6.8) | 2 (2.7) | 3 (4.1) | 1 (1.4) | <0.001a |
| Clinical data (before
transfusion) |
| R, n/min (± SD) | 20.3 (3.5) | 20.2 (3.5) | 20.2 (3) | 20.5 (3.6) | 21.9 (6) | 21 (3.1) | 19.9 (2.2) | 19.9 (2.4) | 0.012a |
| P, n/min (± SD) | 90.1 (17.7) | 94.9 (76) | 93.1 (58.6) | 87.5 (18.2) | 90.7 (20.9) | 93.7 (23) | 118.8 (143.1) | 81.1 (21.5) | 0.437a |
| RP, mmHg (± SD) | 112.6 (19.5) | 113.6 (25.6) | 113.5 (29.6) | 111.3 (32.7) | 109.4 (31.6) | 111.3 (29.7) | 114.2 (22.5) | 122.5 (33.1) | 0.770a |
| T,°C (± SD) | 36.7 (0.7) | 36.6 (1) | 36.6 (0.4) | 36.5 (1) | 36.6 (0.6) | 36.2 (1.6) | 36.7 (0.5) | 36.5 (0.3) | 0.128a |
| RBC,
×1012/l (± SD) | 3.6 (1.1) | 3.9 (0.9) | 3.9 (1.1) | 3.8 (1) | 3.6 (1.2) | 3.7 (1.1) | 4.2 (1) | 4.1 (1.1) | 0.023a |
| Hb, g/l (± SD) | 104.6 (32.6) | 116.3 (29.2) | 117.1 (33.1) | 120.1 (77.7) | 111.8 (31) | 113.9 (32.3) | 127.8 (25) | 128.1 (31.1) | 0.059a |
| PLT,
×109/l (± SD) | 152.2 (88.7) | 184.1 (91.7) | 181.9 (102.7) | 165.1 (95.1) | 156.8 (86.3) | 177 (99.2) | 180.7 (73.8) | 165.1 (104.5) | 0.036a |
| PT, s (± SD) | 15 (9.8) | 13.8 (6.4) | 13.7 (4.5) | 15.5 (9.7) | 13.8 (3.8) | 15.5 (7.5) | 13.4 (2.8) | 15.9 (6.3) | 0.058a |
| APTT, sec (±
SD) | 34.3 (11.2) | 33.4 (11.8) | 36.2 (26.8) | 35.3 (14.3) | 38.2 (27.8) | 35.3 (12.4) | 34.7 (6.7) | 44.1 (21.7) | 0.457a |
| TT, sec (± SD) | 16 (4.3) | 17.3 (13.8) | 17.1 (5.6) | 17.3 (6.6) | 20.4 (13.7) | 17.9 (4.8) | 17.1 (2.9) | 18.1 (8.1) | 0.311a |
| INR (± SD) | 1.4 (1) | 1.4 (2.2) | 1.2 (1) | 1.2 (0.7) | 1.4 (1.7) | 1.2 (0.6) | 1.1 (0.2) | 1.3 (0.5) | 0.957a |
| FIB, g/l (±
SD) | 6.4 (22.9) | 12.2 (47.1) | 11.7 (52.6) | 6.4 (20.4) | 6.3 (10.6) | 21.6 (48.6) | 23.1 (83.5) | 2.9 (1.5) | 0.574a |
| Clinical data
(after transfusion) |
| Days of stay (±
SD) | 24.5 (13.4) | 25 (14.5) | 28.8 (22) | 29.7 (23.3) | 33 (32.1) | 29.8 (19) | 28 (18.3) | 47.6 (44.6) | <0.001a |
| Stay in ICU, days
(± SD) | 2.9 (3.4) | 3.9 (3.5) | 6.6 (10.7) | 16 (53.5) | 7.8 (9) | 7.1 (7.3) | 7.6 (12.6) | 20.3 (27.5) | 0.021a |
| Surgery time,
h | 1.4 (1.7) | 2.8 (3.4) | 3.5 (3.7) | 3.2 (3.7) | 5.2 (4.5) | 3.2 (4.2) | 5.7 (5.5) | 4.8 (4.9) | <0.001a |
| pRBC in 24 h
(units) | 4 | 8 | 12 | 16 | 22 | 26 | 30 | 40 | <0.001b |
| FFP in 24 h
(units) | 3 | 6 | 10 | 14 | 20 | 20 | 22 | 20 | <0.001b |
| PLT in 24 h
(units) | 20 | 10 | 10 | 10 | 10 | 10 | 15 | 10 | 0.773b |
| pRBC in 72h
(units) | 10 | 8 | 10 | 16 | 20 | 22 | 26 | 26 | <0.001b |
| FFP in 72h
(units) | 6 | 6 | 8 | 12 | 14 | 15 | 16 | 18 | <0.001b |
| PLT in 72h
(units) | 16 | 15 | 10 | 10 | 20 | 10 | 10 | 13 | 0.592b |
Patient mortality
The mortality of the patients increased with the
increase in the volume of RBC transfusion when the total RBC
transfusion was >10 units, regardless of whether this was within
24 or 72 h. Within 24 h, as the volume of transfused RBCs increased
from 10 to 40 units, the mortality rate rose from 6.0 to 38.9%.
Within 72 h, as the volume of RBCs increased from 10 to 40 units,
the mortality rate rose from 5.2% to 28.0%. When the volume of
transfused RBCs was 5–9 units within 24 and 72 h, the mortality
rate was the lowest, which was 3.7 and 2.3%, respectively. For
transfusion with 0–4 units, the mortality rates were 7.3 and 9.7%,
respectively (Table II).
 | Table IIMortality and survival rates in
various RBC transfusion volume groups [n (%)]. |
Table II
Mortality and survival rates in
various RBC transfusion volume groups [n (%)].
| Duration | Outcome | 0–4 units | 5–9 units | 10–14 units | 15–19 units | 20–24 units | 25–29 units | 30–39 units | ≥40 units | Total | P-value |
|---|
| 24 h | Mortality | 7 (7.3) | 17 (3.7) | 40 (6.0) | 19 (10.9) | 20 (17.5) | 14 (30.4) | 8 (23.5) | 7 (38.9) | 132 (8.2) | 0.001 |
| Survival | 89 (92.7) | 440 (96.3) | 622 (94.0) | 155 (89.1) | 94 (82.5) | 32 (69.6) | 26 (76.5) | 11 (61.1) | 1469 (91.8) | |
| 72 h | Mortality | 21 (9.7) | 7 (2.3) | 29 (5.2) | 20 (9.1) | 16 (13.4) | 11 (15.5) | 14 (21.5) | 14 (28.0) | 132 (8.2) | 0.001 |
| Survival | 195 (90.3) | 299 (97.7) | 525 (94.8) | 200 (90.9) | 103 (86.6) | 60 (84.5) | 51 (78.5) | 36 (72.0) | 1469 (91.8) | |
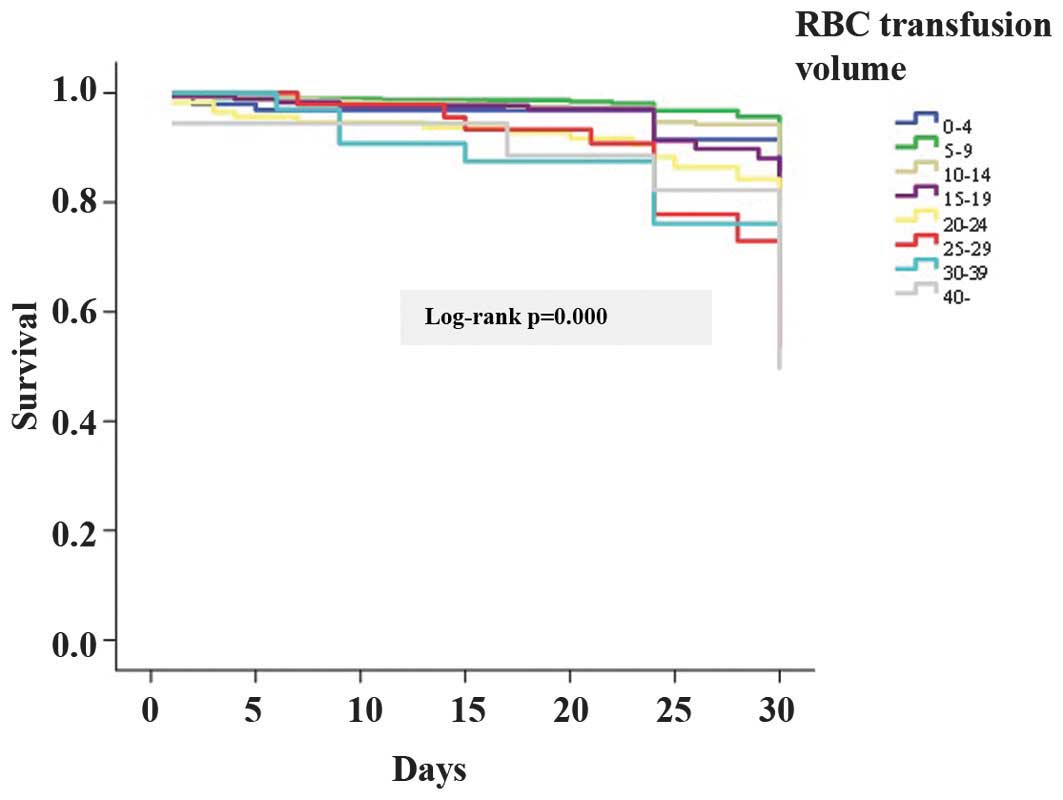
Survival analysis showed that there were significant
differences in mortality among the patients according to the RBC
transfusion volume (χ2=72.857, P<0.001; Fig 1).
Logistic regression analysis
Multivariate logistic regression analysis was
performed with hospital mortality as the dependent variable. The
following variables were considered as independent predictors: i)
age, ii) gender, iii) surgery duration, iv) weight, v) length of
stay in hospital, vi) intensive care unit (ICU) stay, vii) RBC
volume (in 24 h) and viii) FFP volume (in 24 h). The results are
presented as odds ratios (ORs) with 95% confidence intervals (95%
CI; Table III). The factors that
were identified to be significantly correlated with mortality were
RBC volume (OR = 0.52, 95% CI: 0.43–0.64; P<0.001), length of
stay in hospital (OR = 2.79; 95% CI: 1.31–5.92; P=0.01), and ICU
stay (OR = 0.43; 95% CI; 0.21–0.88; P=0.02).
 | Table IIIResults of multivariate logistic
regression analysis. |
Table III
Results of multivariate logistic
regression analysis.
| Variable | β | SE | P-value | Odds ratio | 95.0% CI |
|---|
| RBC volume | −0.65 | 0.10 | <0.001 | 0.52 | 0.43–0.64 |
| Length of stay | 1.02 | 0.38 | 0.01 | 2.79 | 1.31–5.92 |
| ICU stay | −0.84 | 0.36 | 0.02 | 0.43 | 0.21–0.88 |
| Constant | 2.14 | 1.00 | 0.03 | 8.48 | |
Discussion
Transfusion plays an important role in saving the
lives of patients in emergency and danger. Timely and sufficient
blood transfusion is critical for the survival of patients who have
suffered massive blood loss. Survival rates following massive
transfusion have significantly increased in recent years. However,
massive transfusion protocols have not always been associated with
improved mortality (10). Long
et al (11) examined the
impact of postoperative hematocrit as an indicator of survival
following massive transfusion in the trauma patient. They found
that transfusion to hematocrits between 29.1 and 39% conveyed a
survival benefit, whereas resuscitation to supraphysiologic
hematocrits ≥39% conveyed no additional survival benefit. Sharpe
et al (12) evaluated the
effect of the number of RBC units transfused on the plasma:RBC and
platelet:RBC ratios and their association with mortality in
patients receiving massive transfusion. The authors found that
patients receiving relatively higher quantities of RBCs were more
likely to have a lower plasma:RBC ratio and were more likely to
die.
The present study found that among 1,048 patients
who received ≥10 units RBC transfusion volume within 24 h, the
mortality rate was 10.31%, which is lower than the rate observed in
related studies (9,13). This may be because the 20 medical
institutions that participated in the present study are large
general hospitals with better conditions, or because fewer trauma
cases and a greater proportion of general surgery cases with good
pre-operation preparation were included in this study, or due to
the immediate application of fresh frozen plasma with a high
percentage accompanying the RBC transfusion to correct coagulation
at an initial stage; further research is required to clarify this.
The present study also found that if the patients were classified
by clinical department, the mortality of cardiac surgery and trauma
patients receiving massive blood transfusion was 13.84 and 12.69%
respectively, which is higher than the 4.79 and 4.05% mortality of
general surgery and obstetrics patients, respectively.
According to previous studies, there is some
correlation between RBC transfusion volume and the mortality of
patients. The study conducted by Como et al (8) demonstrated that the mortality rate of
147 patients with massive blood transfusion was up to 39% and the
mortality rate was 51% for patients receiving >50 units of blood
products transfused within 24 h. Stanworth et al (9) indicated that the mortality rate was
9% when patients receiving 0–5 units pRBC transfusion, 22% for
those receiving 6–9 units pRBCs and 42% for those receiving ≥10
units pBRCs. Surgenor et al (13) reported that RBC transfusion during
or following cardiac surgery showed a certain correlation with the
increase in the mortality of patients. The long-term risk of
mortality was 16%, which was higher than that of patients
undergoing transfusion with 1 or 2 units of pRBCs. The results of
the present study for 1,601 surgical inpatients with transfusion
were consistent with these previous studies. The present study also
identified that a correlation existed between RBC transfusion
volume and patient mortality. The multivariate logistic regression
analysis results indicated that RBC transfusion volume (in 24 h),
length of stay and ICU stay constitute independent risk factors for
patient mortality.
However, certain limitations existed in this study.
It was a large multicenter retrospective study, with a review of
registry data in which a variable proportion of records may have
been missing data. This is inevitable to a degree in analyses of
multiple registries.
In summary, the present study highlights the
correlation between RBC transfusion volume and patient mortality
for surgical inpatients with massive blood transfusion. The
mortality rate increased with as the volume of RBC transfusion
increased. RBC transfusion volume, length of stay and ICU stay
constitute independent risk factors for patient mortality during
massive blood transfusion.
Acknowledgements
This study was supported by a grant from Johnson
& Johnson (China) Medical Equipment Co., Ltd (Shanghai, China).
The authors would like to thank the other 19 centers participating
in this study: Shi-Jie Mu, Ai-Jun Xia and Xian-Qin Zhang from
Xijing Hospital, the Fourth Military Medical University (Xi’an,
China); Dai-Yu Li from Affiliated Hospital of Luzhou Medical
College (Luzhou, China); Shu-Min Zhao from Southwest Hospital, the
Third Military Medical University (Chongqing, China); Wei Jiao from
the People’s Hospital of Zhuang Autonomous Region (Nanning, China);
Li Tong from the First Affiliated Hospital of Kunming Medical
University (Kunming, China); Qing-Bao Meng from Shenzhen People’s
Hospital (Shenzhen, China); Jie Li from the Fourth Clinical Medical
College of Hebei Medical University (Shijiazhuang, China); Shi-Ming
Yang from Tangdu Hospital, the Fourth Military Medical University
(Xi’an, China); Suo-Liang Yao from Xi’an Hong Hui Hospital (Xi’an,
China); Bi-Juan Li from Xiangya Hospital of Center-South University
(Changsha, China); Qiu-Shi Wang from Shengjing Hospital of China
Medical University (Shenyang, China); Cui-Ying Li from General
Hospital of Chengdu Military Region (Chengdu, China); Mei-Ning Han
from the Second Affiliated Hospital of Medical College of Xi’an
Jiaotong University (Xi’an, China); Zhi-Xi Hu from Yan’an
University Affiliated Hospital (Yan’an, China); Jin-Shan Jiao from
the First Affiliated Hospital of Shanxi Medical University
(Taiyuan, China); Xian-Ping Lv from the First Affiliated Hospital
of Zhengzhou University (Zhengzhou, China); Yan-Li Bai from Xi’an
Central Hospital (Xi’an, China); Xiao-Xia Shi from Xianyang 215
Hospital (Xianyang, China); and Fang-Xiang Chen from Daping
Hospital, the Third Military Medical University (Chongqing,
China).
References
|
1
|
Malone DL, Hess JR and Fingerhut A:
Massive blood transfusion practices around the globe and a
suggestion for a common massive blood transfusion protocol. J
Trauma. 60(6 Suppl): S91–S96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuster KM, Davis KA, Lui FY, Maerz LL
and Kaplan LJ: The status of massive blood transfusion protocols in
United States trauma centers: Massive transfusion or massive
confusion? Transfusion. 50:15452010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
British Committee for Standards in
Haematology. Stainsby D, MacLennan S, Thomas D, Isaac J and
Hamilton PJ: Guidelines on the management of massive blood loss. Br
J Haematol. 135:634–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hewitt PE and Machin SJ: ABC of
transfusion. Massive blood transfusion. BMJ. 300:1071990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozek-Langenecker S: Management of massive
operative blood loss. Minerva Anestesiol. 73:401–415.
2007.PubMed/NCBI
|
|
6
|
Cinat ME, Wallace WC, Nastanski F, West J,
Sloan S, Ocariz J and Wilson SE: Improved survival following
massive transfusion in patients who have undergone trauma. Arch
Surg. 134:964–970. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riskin DJ, Tsai TC, Riskin L,
Hernandez-Boussard T, Purtill M, Maggio PM, Spain DA and Brundage
SI: Massive transfusion protocols: The role of aggressive
resuscitation versus product ratio in mortality reduction. J Am
Coll Surg. 209:198–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Como JJ, Dutton RP, Scalea TM, Edelman BB
and Hess JR: Blood transfusion rates in the care of acute trauma.
Transfusion. 44:809–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanworth SJ, Morris TP, Gaarder C,
Goslings JC, Maegele M, Cohen MJ, König TC, Davenport RA, Pittet
JF, Johansson PI, Allard S, Johnson T and Brohi K: Reappraising the
concept of massive transfusion in trauma. Crit Care. 14:R2392010.
View Article : Google Scholar
|
|
10
|
Mitra B, O’Reilly G, Cameron PA, Zatta A
and Gruen RL: Effectiveness of massive transfusion protocols on
mortality in trauma: a systematic review and meta-analysis. ANZ J
Surg. 83:918–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Long K, Heaney JB, Simms ER, McSwain NE
and Duchesne JC: When enough is enough: impact of packed red blood
cells in massive transfusion outcomes. Am Surg. 79:810–814.
2013.PubMed/NCBI
|
|
12
|
Sharpe JP, Weinberg JA, Magnotti LJ,
Maclennan PA, Schroeppel TJ, Fabian TC and Croce MA: Accounting for
differences in transfusion volume: Are all massive transfusions
created equal? J Trauma Acute Care Surg. 72:1536–1540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Surgenor SD, Kramer RS, Olmstead EM, Ross
CS, Sellke FW, Likosky DS, Marrin CA, Helm RE Jr, Leavitt BJ,
Morton JR, Charlesworth DC, Clough RA, Hernandez F, Frumiento C,
Benak A, DioData C and O’Connor GT; Northern New England
Cardiovascular Disease Study Group. The association of
perioperative red blood cell transfusions and decreased long-term
survival after cardiac surgery. Anesth Analg. 108:1741–1746. 2009.
View Article : Google Scholar : PubMed/NCBI
|