Introduction
Digestive system cancers, including esophageal,
gastric, hepatocellular, bowel, pancreatic, gallbladder and anal
cancers, are the most common types of cancer worldwide. There are
an estimated 3.4 million new cases diagnosed worldwide each year
and the mortality rates have increased over the past decade
(1). Although the exact mechanism
of carcinogenesis remains to be fully understood, accumulating
evidence has confirmed that certain risk factors (such as dietary,
ethnic and socioeconomic factors) and interactions between genetic
and environmental factors may play important roles in the
pathogenesis of these types of cancer (2,3).
Serine/threonine kinase 15 (STK15, also known as
Aurora-A or AURKA) is a centrosome-localized serine/threonine
kinase that is involved in cell cycle regulation, particularly the
passage from G2 to M, through the formation of mitotic
spindles (4). The STK15 gene,
which consists of nine exons, is located on chromosome 20q13.2, a
region frequently amplified and overexpressed in various types of
human cancer (5). STK15 has been
reported to be overexpressed in numerous types of malignancies,
including colorectal and pancreatic cancers (6,7).
Considerable evidence indicates that overexpression of the STK15
gene results in centrosome amplification, chromosomal instability,
aneuploidy and transformation (8).
Two non-synonymous polymorphisms, 91T>A (rs2273535) and
169G>A (rs1047972), have been identified in the STK15 gene. A
thymine (T)/adenine (A) polymorphism located at nucleotide position
91 encodes a phenylalanine (Phe)-to-isoleucine (Ile) substitution
at amino acid position 31. A guanine (G)/A polymorphism at
nucleotide 169 encodes a valine (Val)-to-Ile substitution at amino
acid position 57. The two polymorphisms are located within two
conserved motifs in the N-terminus region of the STK15 gene
(9). It has been revealed that the
A allele of the 91T>A (31Ile>Phe) polymorphism is
preferentially amplified and more potent than the T allele in
leading to aneuploidy and transformation (8). Furthermore, the 169G>A
(57Val>Ile) polymorphism was found to affect the kinase activity
of aurora kinase A (10).
Studies have suggested the presence of an
association between the two coding polymorphisms in the STK15 gene
and an increased risk of digestive system cancers (10–23);
however, the results have been inconsistent. The aim of the present
study was therefore to conduct a meta-analysis to evaluate the
association between the two STK15 polymorphisms and susceptibility
to digestive system cancers.
Materials and methods
Search strategy
The electronic literature databases of PubMed, Web
of Science, China National Knowledge Infrastructure (CNKI), WanFang
and VIP were searched for all relevant articles. The last search
update was February 18, 2014, using the search terms:
‘Serine/threonine kinase 15 or STK15 or Aurora-A or AURKA’ and
‘genetic polymorphism or polymorphisms or variant’ and ‘digestive
system cancer or gastric cancer or colorectal cancer or
hepatocellular carcinoma or pancreatic cancer or esophageal
cancer’. The search was restricted to humans without language
exclusions. Additional studies were identified by a manual search
of the references from the original or review articles on this
topic.
Inclusion and exclusion criteria
Studies included in this meta-analysis were selected
according to the following criteria: i) Studies that evaluated the
association between the STK15 polymorphisms (91T>A or 169G>A)
and digestive system cancers; ii) studies that had a case-control
design; and iii) studies that had a detailed genotype frequency of
cases and controls or that had presented sufficient data for this
to be calculated from the article text. The major exclusion
criteria were i) case-only studies, case reports and review
articles; ii) studies without raw data of the STK15 genotype; and
iii) repetitive publications.
Data extraction
For each study, the following data were extracted
independently by two investigators: The name of the first author,
age and gender of the subjects, year of study publication, country
of origin, ethnicity, source of controls, genotype methods, number
of cases and controls, and the Hardy-Weinberg equilibrium (HWE) in
the controls (P-value). The results were compared and disagreements
were discussed among all authors and resolved with consensus.
Statistical analysis
The HWE was evaluated for each study using an
internet-based HWE calculator (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl) (24). The risk of digestive system cancers
associated with the STK15 polymorphisms was estimated for each
study by the odds ratio (OR) and 95% confidence interval (CI). Four
different ORs were calculated: The dominant model (variant
homozygote + heterozygote versus wild-type homozygote), the
recessive model (variant homozygote versus heterozygote + wild-type
homozygote), heterozygote comparison (heterozygote versus wild-type
homozygote) and homozygote comparison (variant homozygote versus
wild-type homozygote). A χ2-test-based Q statistic test
was performed to assess the heterogeneity between studies (25). The effect of heterogeneity was also
quantified by the I2 test. When a significant Q test
(P>0.05) or I2 value <50% indicated homogeneity
across the studies, the fixed effects model was used (26); otherwise, the random effects model
was used (27). Stratification
analyses on ethnicity and tumor type were subsequently performed.
Analysis of sensitivity was performed to evaluate the stability of
the results. Finally, potential publication bias was investigated
using Begg’s funnel plot and Egger’s regression test (28,29).
P<0.05 was considered to indicate a statistically significant
difference.
All analyses were performed using the Cochrane
Collaboration RevMan 5.2 (The Nordic Cochrane Centre, The Cochrane
Collaboration, Copenhagen, 2012) and STATA package version 12.0
(Stata Corporation, College Station, TX, USA).
Results
Study characteristics
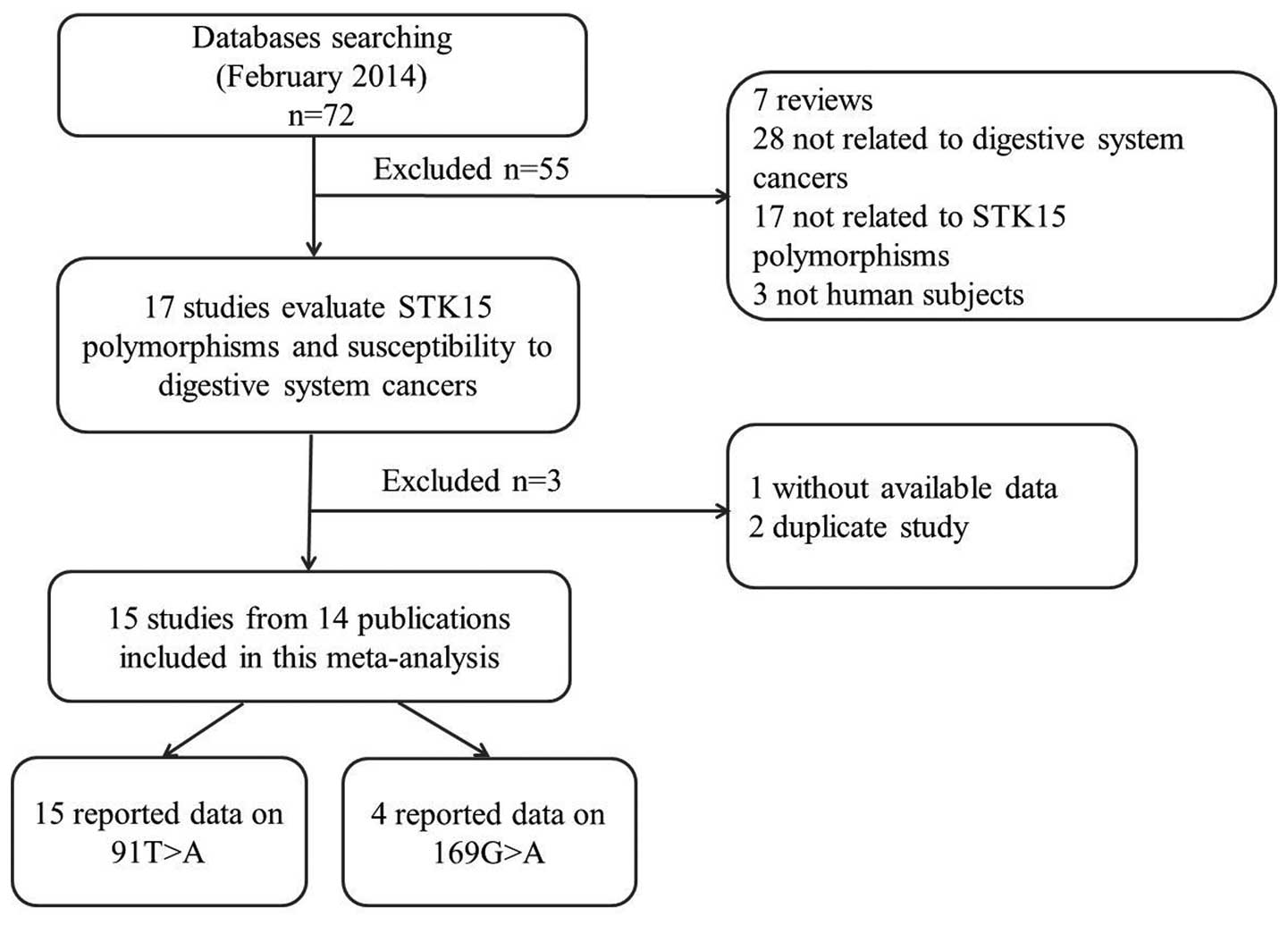
The search strategy retrieved 72 potentially
relevant studies. According to the inclusion criteria, 14 studies
(10–23) with full-text were included in the
present meta-analysis and 58 studies were excluded. The flow chart
of the study selection is summarized in Fig. 1. Since the study by Ewart-Toland
et al (16) included two
populations, these populations were treated separately in the
current meta-analysis (Tables I
and II); as such, there were 15
case-control studies from 14 publications with 7,619 cases and
7,196 controls concerning the 91T>A polymorphism and four
studies with 826 cases and 713 controls concerning the 169G>A
polymorphism. Of the 15 eligible studies, 10 studies (11,12,14,10,16–19,22)
were written in English and five studies (13,15,20,21,23)
in Chinese; nine studies (12,13,15,10,18–21,23)
were conducted on Asian populations, five studies (11,14,16,17,22)
on Caucasian populations and one study (16) on a mixed population. Four tumor
types were addressed: Six studies (12,15,10,19–21)
focused on esophageal cancer; six studies (14,16,17,22,23)
on colorectal cancer; two studies (13,18)
on gastric cancer and one study (11) on hepatocellular carcinoma. The
distribution of genotypes among the controls was consistent with
the HWE for all selected studies, with the exception of three
(12,10,21).
 | Table ICharacteristics of studies included in
the meta-analysis. |
Table I
Characteristics of studies included in
the meta-analysis.
| A, Studies on the
91T>A polymorphism |
|---|
|
|---|
| First author
(ref.) | Year | Country | Ethnicity | Tumor type | Source of
controls | Genotype methods |
|---|
| Akkiz (11) | 2010 | Turkey | Caucasian | Hepatocellular | HB | PCR-RFLP |
| Chava (12) | 2011 | India | Asian | Esophageal | NR | PCR |
| Chen L (13) | 2005 | China | Asian | Gastric | HB | PCR-RFLP |
| Chen JY (14) | 2007 | USA | Caucasian | Colorectal | HB | Direct
sequencing |
| Chen XB (15) | 2009 | China | Asian | Esophageal | PB | PCR-RFLP |
| Ewart-Toland
(16) | 2005a | USA | Mixed | Colorectal | PB | PCR-RFLP |
| Ewart-Toland
(16) | 2005b | Scotland | Caucasian | Colorectal | PB | PCR-RFLP |
| Hienonen (17) | 2006 | Finland | Caucasian | Colorectal | PB | Direct
sequencing |
| Ju (18) | 2006 | South Korea | Asian | Gastric | HB | Mass ARRAY |
| Kimura (10) | 2005 | Japan | Asian | Esophageal | HB | PCR |
| Miao (19) | 2004 | China | Asian | Esophageal | PB | PCR-RFLP |
| Sang (20) | 2012 | China | Asian | Esophageal | HB | MALDI-TOF MS |
| Wang (21) | 2007 | China | Asian | Esophageal | PB | PCR-RFLP |
| Webb (22) | 2006 | UK | Caucasian | Colorectal | PB | Illuminasentric
bead array |
| Zhang (23) | 2006 | China | Asian | Colorectal | PB | PCR-RFLP |
|
| B, Studies on the
169G>A polymorphism |
|
| First author
(ref.) | Year | Country | Ethnicity | Tumor type | Source of
controls | Genotype
methods |
|
| Chen L (12) | 2005 | China | Asian | Gastric | HB | PCR-RFLP |
| Chen JY (13) | 2007 | USA | Caucasian | Colorectal | HB | Direct
sequencing |
| Ju (17) | 2006 | South Korea | Asian | Gastric | HB | Mass ARRAY |
| Kimura (18) | 2005 | Japan | Asian | Esophageal | HB | PCR |
 | Table IIPatient data for studies included in
the meta-analysis. |
Table II
Patient data for studies included in
the meta-analysis.
| A, Studies on the
91T>A polymorphism |
|---|
|
|---|
| Age (years) | Gender
(male/female) | Genotype
(case/control) | |
|---|
|
|
|
| |
|---|
| First author
(ref.) | Case | Control | Case | Control | Total | WT Ho (TT) | Ht (TA) | VR Ho (AA) |
P-valueHWE |
|---|
| Akkiz (11) | 58 (20–81)a | 58 (20–81)a | 106/22 | 106/22 | 128/128 | 77/99 | 47/27 | 4/2 | 0.919 |
| Chava (12) | 56.03 | NR | NR | NR | 50/150 | 22/81 | 28/66 | 0/3 | 0.012 |
| Chen L (13) | 49.04±12.89b | 51.66±16.12b | 45/23 | 38/37 | 68/75 | 5/10 | 27/32 | 36/33 | 0.615 |
| Chen JY (14) | 43.0±12.7b | 44.8±12.0b | 36/24 | 27/38 | 60/65 | 44/38 | 13/21 | 3/6 | 0.236 |
| Chen XB (15) | NR | NR | 178/10 | 307/17 | 188/324 | 43/38 | 79/168 | 66/118 | 0.060 |
| Ewart-Toland
(16) | NR | NR | NR | NR | 344/448 | 200/279 | 121/148 | 23/21 | 0.809 |
| Ewart-Toland
(16) | NR | NR | NR | NR | 1675/1038 | 1031/630 | 558/368 | 86/40 | 0.126 |
| Hienonen (17) | 68 (32–90)a | NR | 109/126 | NR | 235/94 | 122/46 | 94/43 | 19/5 | 0.208 |
| Ju (18) | 57.7±12.6b | 52.4±8.7b | 339/162 | 289/138 | 501/427 | 75/58 | 215/190 | 211/179 | 0.504 |
| Kimura (10) | NR | NR | NR | NR | 197/146 | 29/12 | 103/82 | 65/52 | 0.010 |
| Miao (19) | 58.3±9.6b | 57.5±9.5b | 460/196 | 443/213 | 656/656 | 58/91 | 290/316 | 308/249 | 0.560 |
| Sang (20) | NR | NR | NR | NR | 380/380 | 173/153 | 161/188 | 46/39 | 0.089 |
| Wang (21) | 59.8±9.7b | 58.8±7.9b | 202/94 | 202/100 | 296/302 | 34/36 | 103/111 | 159/155 | 0.026 |
| Webb (22) | 61±11.4b | 59±10.9b | 1471/1087 | 836/1844 | 2558/2680 | 1564/1667 | 880/888 | 114/125 | 0.628 |
| Zhang (23) | 57.0±11.0b | 57.4±9.6b | 171/112 | 170/113 | 283/283 | 30/42 | 111/137 | 142/104 | 0.775 |
|
| B, Studies on the
169G>A polymorphism |
|
| Age (years) | Gender
(male/female) | Genotype
(case/control) | |
|
|
|
| |
| First author
(ref.) | Case | Control | Case | Control | Total | WT Ho (GG) | Ht (GA) | VR Ho (AA) |
P-valueHWE |
|
| Chen L (13) | 49.04±12.89b | 51.66±16.12b | 45/23 | 38/37 | 68/75 | 49/61 | 19/11 | 0/3 | 0.019 |
| Chen JY (14) | 43.0±12.7b | 44.8±12.0b | 36/24 | 27/38 | 60/65 | 39/43 | 20/20 | 1/2 | 0.859 |
| Ju (18) | 57.7±12.6b | 52.4±8.7b | 339/162 | 289/138 | 501/427 | 387/414 | 100/104 | 14/9 | 0.409 |
| Kimura (10) | NR | NR | NR | NR | 197/146 | 118/99 | 65/47 | 14/0 | 0.020 |
Quantitative data synthesis
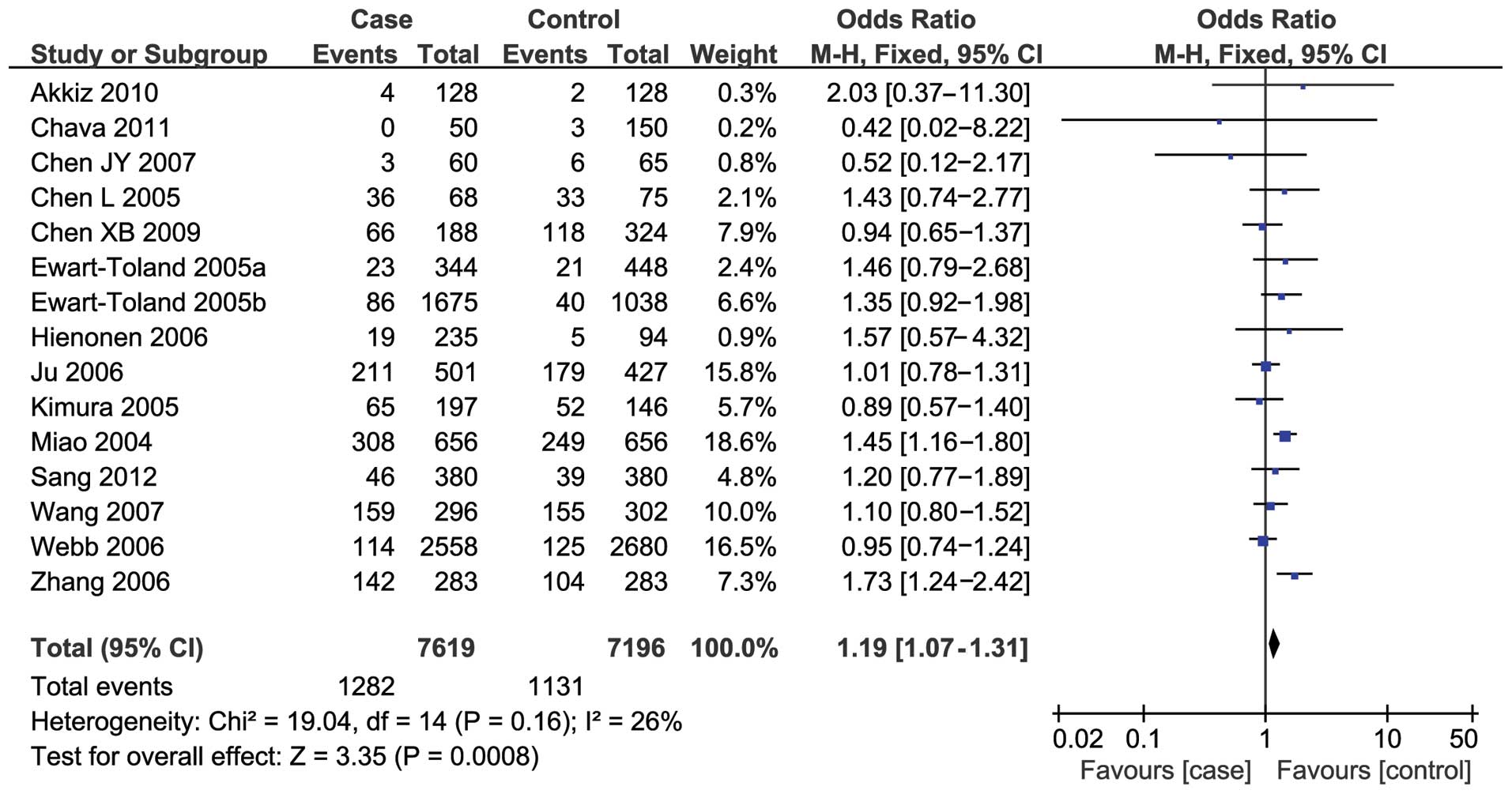
Fifteen studies reported an association between the
91T>A polymorphism and susceptibility to digestive system
cancers. Overall, a significantly increased risk was found under
the recessive model (OR, 1.19; 95% CI, 1.07–1.31) (Fig. 2), while no notable associations
were observed under the three other models (dominant model: OR,
1.02; 95% CI, 0.87–1.21; TA versus TT: OR, 0.97; 95% CI, 0.83–1.14;
AA versus TT: OR, 1.12; 95% CI, 0.89–1.42).
In the subgroup analysis by ethnicity, a significant
association was detected in the Asian population under the
recessive model (OR, 1.21; 95% CI, 1.08–1.36) but under the other
three models. No association was observed in the Caucasian or mixed
populations.
Stratification by tumor type indicated that the
91T>A polymorphism was associated with an increased risk of
esophageal and colorectal cancers under the recessive model (OR,
1.19; 95% CI, 1.03–1.38; and OR, 1.24; 95% CI, 1.04–1.46;
respectively); however, no significant association was detected for
gastric cancer. Only one study focused on hepatocellular cancer and
the results showed that the STK15 91T>A polymorphism may be a
genetic susceptibility factor for hepatocellular carcinoma
(Table III).
 | Table IIISummary of the ORs of the
serine/threonine kinase 15 polymorphisms and risk of digestive
system cancers. |
Table III
Summary of the ORs of the
serine/threonine kinase 15 polymorphisms and risk of digestive
system cancers.
| A, Studies on the
91T>A polymorphism |
|---|
|
|---|
| | Dominant model | Recessive
model | Ht versus WT
Ho | VR versus WT
Ho |
|---|
| |
|
|
|
|
|---|
| Variables | Na | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 |
|---|
| Total | 15 | 1.02
(0.87–1.21) | <0.0001 | 68 | 1.19
(1.07–1.31) | 0.16 | 26 | 0.97
(0.83–1.14) | 0.0005 | 63 | 1.12
(0.89–1.42) | 0.002 | 58 |
| Ethnicity |
| Asian | 9 | 0.99
(0.73–1.35) | 0.0002 | 73 | 1.21
(1.08–1.36) | 0.10 | 40 | 0.92
(0.69–1.22) | 0.003 | 66 | 1.07
(0.74–1.55) | 0.0005 | 71 |
| Caucasian | 5 | 1.04
(0.83–1.29) | 0.01 | 68 | 1.08
(0.88–1.32) | 0.37 | 6 | 1.02
(0.81–1.28) | 0.01 | 68 | 1.08
(0.88–1.33) | 0.36 | 9 |
| Mixed | 1 | 1.19
(0.89–1.58) | NA | NA | 1.46
(0.79–2.68) | NA | NA | 1.14
(0.84–1.54) | NA | NA | 1.12
(0.99–1.28) | NA | NA |
| Tumor type |
| Esophageal | 6 | 0.90
(0.59–1.37) | 0.0001 | 80 | 1.19
(1.03–1.38) | 0.24 | 26 | 0.85
(0.57–1.28) | 0.0007 | 77 | 0.91
(0.55–1.53) | 0.0006 | 77 |
| Colorectal | 6 | 1.03
(0.95–1.12) | 0.20 | 31 | 1.24
(1.04–1.46) | 0.08 | 49 | 1.01
(0.93–1.10) | 0.37 | 8 | 1.18
(0.98–1.42) | 0.15 | 39 |
| Gastric | 2 | 0.97
(0.68–1.37) | 0.20 | 39 | 1.06
(0.83–1.35) | 0.33 | 0 | 0.94
(0.65–1.36) | 0.30 | 5 | 1.00
(0.69–1.45) | 0.17 | 48 |
|
Hepatocellular | 1 | 2.26
(1.31–3.90) | NA | NA | 2.03
(0.37–11.30) | NA | NA | 2.24
(1.28–3.92) | NA | NA | 2.57
(0.46–14.41) | NA | NA |
|
| B, Studies on the
169G>A polymorphism |
|
| | Dominant model | Recessive
model | Ht versus WT
Ho | VR versus WT
Ho |
| |
|
|
|
|
| Variables | Na | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 | OR (95% CI) | P-valueb | I2 |
|
| Total | 4 | 1.02
(0.82–1.28) | 0.13 | 47 | 1.27
(0.25–6.49) | 0.07 | 58 | 1.13
(0.90–1.43) | 0.45 | 0 | 1.45
(0.29–7.22) | 0.08 | 56 |
| Ethnicity |
| Asian | 3 | 0.98
(0.77–1.24) | 0.14 | 49 | 2.04
(0.32–13.00) | 0.07 | 62 | 1.07
(0.84–1.36) | 0.91 | 0 | 2.29
(0.38–13.69) | 0.09 | 59 |
| Caucasian | 1 | 1.69
(0.77–3.71) | NA | NA | 0.15
(0.01–2.98) | NA | NA | 2.15
(0.94–4.94) | NA | NA | 0.18
(0.01–3.52) | NA | NA |
Four studies reported an association between the
169G>A polymorphism and the risk of digestive system cancers.
The combined results based on all the studies revealed no
significant associations among the studies with any of the genetic
models (dominant model: OR, 1.02; 95% CI, 0.82–1.28; recessive
model: OR, 1.27; 95% CI, 0.25–6.49; GA versus GG: OR, 1.13; 95% CI,
0.90–1.43; AA versus GG: OR, 1.45; 95% CI, 0.29–7.22). In the
subgroup analysis by ethnicity, similar results were demonstrated
in the Asian and Caucasian populations (Table III).
Heterogeneity and sensitivity
analyses
Substantial heterogeneities were observed among the
studies for the association between the risk of digestive system
cancers and the 91T>A (dominant model: I2=68%,
P<0.0001; TA versus TT: I2=63%, P=0.0005; AA versus
TT: I2=58%, P=0.002) and 169G>A (recessive model:
I2=58%, P=0.07; AA versus TT: I2=56%, P=0.08)
STK19 polymorphisms. The source of the heterogeneity for the
genetic model comparisons by ethnicity and tumor site was
subsequently analyzed. For the 91T>A polymorphism, the
heterogeneity was partially decreased or removed in colorectal and
gastric cancers and Caucasian populations; however, significant
heterogeneity remained for esophageal cancer and Asian populations.
For the 169G>A polymorphism, the heterogeneity significantly
decreased when the study by Kimura et al (10) was excluded from the analysis. A
sensitivity analysis was performed to evaluate the stability of the
results. Since the statistical significance of the results did not
change when any single study was omitted, the stability of the
results was confirmed.
Publication bias
Begg’s funnel plot and Egger’s tests were used to
address potential publication bias in the available literature. The
shape of the funnel plots did not show any evidence of funnel plot
asymmetry (data not shown). Egger’s test also demonstrated that
there was no statistical significance in the evaluation of
publication bias (dominant model, P=0.991; TA versus TT, P=0.721;
AA versus TT, P=0.925; recessive model, P=0.835).
Discussion
STK15, a member of the Aurora family, plays a vital
role in bipolar mitotic spindle formation and regulates chromosome
segregation in mammalian cells (30). It has been reported that STK15 is
overexpressed in numerous types of cancer, including colorectal,
pancreatic, breast and prostate (6,7,31,32).
Although the mechanism remains unclear, it is believed that the
polymorphism may partially affect STK15 expression and therefore
modify its function. Ewart-Toland et al (8) suggested that the STK15 91T>A
polymorphism (T→A) variant changed the activity of the STK15 box 1,
leading to an inhibition of p53 binding and the decreased
degradation of STK15. It was further suggested that the stabilized
overexpression of STK15 led to centrosome amplification, improper
cytokinesis, chromosomal instability and the promotion of
tumorigenesis (8). To date, a
number of studies have investigated the association between STK15
polymorphisms and the risk of cancers, particularly cancers of the
digestive system (10–23); however, the results have been
inconsistent. In a study from Turkey, Akkiz et al (11) reported that the STK15 91T>A
polymorphism may be a genetic susceptibility factor for
hepatocellular carcinoma. Similarly, Hienonen et al
(17) observed that the STK15
91T>A polymorphism was a low penetrance colorectal cancer
susceptibility factor in Finnish populations; however, Webb et
al (22) suggested that there
was no association between the polymorphism and colorectal cancer
susceptibility based on their results. With regard to the 169G>A
polymorphism, Ju et al (18) reported that the 169G>A
polymorphism in the STK15 gene was associated with the progression
of gastric cancer; however, in a study from China, Chen (13) failed to detect any association
between the 169G>A polymorphism and the risk of gastric
cancer.
Recently, two meta-analyses (33,34)
evaluated the association between the STK15 91T>A polymorphism
and risk of cancer, and reported that the STK15 91T>A
polymorphism may be a risk factor for cancer. In comparison, the
present study conducted a comprehensive literature search of
different databases and included several additional studies.
Furthermore, the association between the 169G>A polymorphism and
the risk of digestive system cancers was explored. In the current
meta-analysis, 15 studies were pooled to examine the association
between the two STK15 polymorphisms and risk of digestive system
cancers. The results demonstrated that there was a significant
association between the STK15 91T>A polymorphism and the risk of
digestive system cancers.
In the subgroup analysis by ethnicity, there was a
significant association in Asian descent, but not in Caucasian and
mixed populations. Different genetic backgrounds and environmental
exposures among the different ethnic groups may contribute to this
discrepancy (35). When stratified
by tumor type, the 91T>A polymorphism was associated with an
increased risk of esophageal and colorectal cancers, but not
gastric cancer. Only one study focused on hepatocellular carcinoma
and the results revealed that the STK15 91T>A polymorphism may
be a genetic susceptibility factor for hepatocellular carcinoma;
however, since only a few studies on gastric cancer and
hepatocellular carcinoma were included, these results should be
interpreted with caution, and further studies are required.
No significant association was found between the
169G>A polymorphism and the risk of digestive system cancers in
any of the genetic models. When stratified according to ethnicity,
similar results were observed in Asian and Caucasian populations.
This lack of association may have been due to the limited
literature (only four studies) in the present meta-analysis. The
conclusions should therefore be considered sensibly. Furthermore,
cancer is a multi-factorial disease that results from complex
interactions between a number of environmental and genetic factors
(gene-gene or gene-environment). Not all of the studies included,
however, analyzed the same environmental or genetic factors and,
due to lack of individual data in the present review, more detailed
analyses, such as analyses of joint effects with other risk factors
or gene-gene or gene-environment interactions, were not able to be
performed.
Heterogeneity is a potential problem when
interpreting the results of all meta-analyses (36). In the current meta-analysis,
heterogeneity was observed in the overall comparison for certain
genetic models. When stratified by ethnicity and tumor site, the
heterogeneity was partially decreased or removed in colorectal and
gastric cancers and Caucasian populations; however, heterogeneity
remained for esophageal cancer and Asian populations. For the
169G>A polymorphism, the heterogeneity significantly decreased
when the study by Kimura et al (10) was excluded from analysis. These
results suggest that the ethnic difference, different tumor types
and particular study type may be the source of heterogeneity in the
present meta-analysis. When sensitivity analyses were conducted by
successively excluding one study, the estimated pooled OR changed
little, strengthening the results from the meta-analysis.
Furthermore, no publication bias was observed, highlighting the
possibility of true results.
The current meta-analysis has limitations that
require acknowledgement. Firstly, due to incomplete raw data or
publication limitations, certain relevant studies were unable to be
included in the present analysis. Secondly, the results were based
on unadjusted estimates, which may cause serious confounding bias.
Thirdly, the data from the European populations were relatively
small and significant heterogeneity was observed in certain models,
which may have resulted in failure to confirm marginal
associations.
In conclusion, the present meta-analysis suggests
that the STK15 gene 91T>A polymorphism, but not the 169G>A
polymorphism, may be a risk factor for digestive system cancers,
particularly for esophageal and colorectal cancers.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pharoah PD, Dunning AM, Ponder BA and
Easton DF: Association studies for finding cancer-susceptibility
genetic variants. Nat Rev Cancer. 4:850–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Risch N: The genetic epidemiology of
cancer: interpreting family and twin studies and their implications
for molecular genetic approaches. Cancer Epidemiol Biomarkers Prev.
10:733–741. 2001.PubMed/NCBI
|
|
4
|
Nigg EA: Centrosome aberrations: cause or
consequence of cancer progression? Nat Rev Cancer. 2:815–825. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou H, Kuang J, Zhong L, et al: Tumour
amplified kinase STK15/BTAK induces centrosome amplification,
aneuploidy and transformation. Nat Genet. 20:189–193. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bischoff JR, Anderson L, Zhu Y, et al: A
homologue of Drosophila aurora kinase is oncogenic and amplified in
human colorectal cancers. EMBO J. 17:3052–3065. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li D, Zhu J, Firozi PF, et al:
Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human
pancreatic cancer. Clin Cancer Res. 9:991–997. 2003.PubMed/NCBI
|
|
8
|
Ewart-Toland A, Briassouli P, de Koning
JP, et al: Identification of Stk6/STK15 as a candidate
low-penetrance tumor-susceptibility gene in mouse and human. Nat
Genet. 34:403–412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venter JC, Adams MD, Myers EW, et al: The
sequence of the human genome. Science. 291:1304–1351. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimura MT, Mori T, Conroy J, Nowak NJ,
Satomi S, Tamai K and Nagase H: Two functional coding single
nucleotide polymorphisms in STK15 (Aurora A) coordinately increase
esophageal cancer risk. Cancer Res. 65:3548–3554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akkiz H, Bayram S, Bekar A, Akgöllü E and
Ozdil B: Relationship between functional polymorphism in the Aurora
A gene and susceptibility of hepatocellular carcinoma. J Viral
Hepat. 17:668–674. 2010.
|
|
12
|
Chava S, Mohan V, Pasupuleti N, et al:
Evaluation of Aurora-A gene polymorphism and esophageal cancer risk
in a South Indian population. Genet Test Mol Biomarkers.
15:185–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L: Genetic polymorphisms and disease
risks: STK15 gene and risk for gastric cancer, MMP9 gene and risk
for thoracic aortic aneurysm and thoracic aortic dissection
(unpublished PhD thesis). China Medical University; 2005, (In
Chinese).
|
|
14
|
Chen J, Sen S, Amos CI, Wei C, Jones JS,
Lynch P and Frazier ML: Association between Aurora-A kinase
polymorphisms and age of onset of hereditary nonpolyposis
colorectal cancer in a Caucasian population. Mol Carcinog.
46:249–256. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XB, Chen GL, Liu JN, Yang JZ, Yu DK,
Lin DX and Tan W: Genetic polymorphisms in STK15 and MMP-2
associated susceptibility to esophageal cancer in Mongolian
population. Zhonghua Yu Fang Yi Xue Za Zhi. 43:559–564. 2009.(In
Chinese). PubMed/NCBI
|
|
16
|
Ewart-Toland A, Dai Q, Gao YT, et al:
Aurora-A/STK15 T+91A is a general low penetrance cancer
susceptibility gene: a meta-analysis of multiple cancer types.
Carcinogenesis. 26:1368–1373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hienonen T, Salovaara R, Mecklin JP,
Järvinen H, Karhu A and Aaltonen LA: Preferential amplification of
AURKA 91A (Ile31) in familial colorectal cancers. Int J Cancer.
118:505–508. 2006. View Article : Google Scholar
|
|
18
|
Ju H, Cho H, Kim YS, et al: Functional
polymorphism 57Val>Ile of aurora kinase A associated with
increased risk of gastric cancer progression. Cancer Lett.
242:273–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao X, Sun T, Wang Y, Zhang X, Tan W and
Lin D: Functional STK15 Phe31Ile polymorphism is associated with
the occurrence and advanced disease status of esophageal squamous
cell carcinoma. Cancer Res. 64:2680–2683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sang YH, Gu HY, Ding GW, Yang WT, Chen SC
and Chen YB: Association between the esophageal cancer
susceptibility and the STK15 T+91A polymorphism. Zhonghua Shi Yan
Wai Ke Za Zhi. 29:12202012.(In Chinese).
|
|
21
|
Wang N, Wang GY, Guo W, Dong XJ and Li Y:
Study on the association between STK15 Phe31Ile polymorphisms and
esophageal squamous cell carcinoma. Zhonghua Liu Xing Bing Xue Za
Zhi. 28:394–397. 2007.(In Chinese). PubMed/NCBI
|
|
22
|
Webb EL, Rudd MF and Houlston RS:
Case-control, kin-cohort and meta-analyses provide no support for
STK15 F31I as a low penetrance colorectal cancer allele. Br J
Cancer. 95:1047–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang WJ, Miao XP, Sun T, et al:
Association between genetic polymorphism in STK15 and risk of
colorectal cancer in a Chinese population. Zhonghua Zhong Liu Za
Zhi. 28:43–46. 2006.(In Chinese). PubMed/NCBI
|
|
24
|
Institute of Human Genetics. HWE
calculator. http://ihg.gsf.de/cgi-bin/hw/hwa1.pluri.
Accessed February 20, 2014
|
|
25
|
Lau J, Ioannidis JP and Schmid CH:
Quantitative synthesis in systematic reviews. Ann Intern Med.
127:820–826. 1997. View Article : Google Scholar
|
|
26
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
27
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egan KM, Newcomb PA, Ambrosone CB, et al:
STK15 polymorphism and breast cancer risk in a population-based
study. Carcinogenesis. 25:2149–2153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka T, Kimura M, Matsunaga K, Fukada D,
Mori H and Okano Y: Centrosomal kinase AIK1 is overexpressed in
invasive ductal carcinoma of the breast. Cancer Res. 59:2041–2044.
1999.PubMed/NCBI
|
|
32
|
Buschhorn HM, Klein RR, Chambers SM, Hardy
MC, Green S, Bearss D and Nagle RB: Aurora-A over-expression in
high-grade PIN lesions and prostate cancer. Prostate. 64:341–346.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang W, Qiu H, Ding H, Sun B, Wang L, Yin
J and Gu H: Association between the STK15 F31I polymorphism and
cancer susceptibility: a meta-analysis involving 43,626 subjects.
PLoS One. 8:e827902013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu L, Zhou X, Jiang F, Xu L and Yin R:
STK15 rs2273535 polymorphism and cancer risk: a meta-analysis of
74,896 subjects. Cancer Epidemiol. 38:111–117. 2014. View Article : Google Scholar
|
|
35
|
Hirschhorn JN, Lohmueller K, Byrne E and
Hirschhorn K: A comprehensive review of genetic association
studies. Genet Med. 4:45–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boccia S, De Feo E, Gallì P, Gianfagna F,
Amore R and Ricciardi G: A systematic review evaluating the
methodological aspects of meta-analyses of genetic association
studies in cancer research. Eur J Epidemiol. 25:765–775. 2010.
View Article : Google Scholar : PubMed/NCBI
|