Introduction
Myocardial ischemia/reperfusion (MI/R) injury is an
injury that is induced when blood returns to transiently ischemic
myocardial tissues. It is associated with microvascular dysfunction
including impaired endothelium-dependent dilation in arterioles,
enhanced fluid filtration and leukocyte plugging in capillaries
(1). MI/R is a complicated
pathological process, the mechanism of which remains unclear. MI/R
injury poses great harm to patients. It may lead to a decline in
cardiac function and necrosis of myocardial tissue in ischemic
areas, particularly in hypertensive patients (2). Furthermore, endoplasmic reticulum
(ER) stress is coupled with MI/R injury. ER stress is a process in
which the abnormal accumulation of unfolded and misfolded proteins
in the ER damages ER functions and induces a number of pathological
processes. Apoptosis may be induced by ER stress as a method of
clearing damaged cells. The C/EBP homologous protein (CHOP) pathway
is the main pathway involved in regulating the apoptosis induced by
ER stress (3). CHOP belongs to the
C/EBP transcription factor family. ER stress induces the expression
of CHOP and leads to cell apoptosis; however, the targets of CHOP
remain unknown (4).
Glucose-regulated protein 78 (GRP78) is a significant protein
involved in the CHOP pathway. GRP78, also known as immunoglobulin
heavy chain-binding protein (Bip), is an important
glucose-regulated molecular chaperone (5). Thus, whether the CHOP pathway is
induced may be determined through detecting the expression levels
of CHOP and GRP78 proteins.
ER stress also activates a highly conserved unfolded
protein response (UPR). This response enhances the ability of the
ER to process and refold proteins, helping to remove damaged
proteins from the ER and to maintain homeostasis in cells. The UPR
is mainly mediated by three ER transmembrane proteins, which are
PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and
activating transcription factor 6 (ATF6) (6,7).
Overexpression of GRP78 is induced when an excess of misfolded and
unfolded proteins accumulate in the ER, activating the expression
of PERK1, IRE1 and ATF6 (8). The
activated, phosphorylated form of PERK (P-PERK) widely suppresses
the synthesis of functional proteins in cells by phosphorylating
its target protein, the α-subunit of eukaryotic initiation factor 2
(eIF2α). This process promotes the translation of certain other
mRNAs, such as activating transcription factor 2 (ATF2) (9). ATF2 is a transcription factor and a
member of the leucine zipper family of DNA-binding proteins
(10). ATF2 is essential for amino
acid responsiveness during the induction of CHOP expression
(11). Upon dissociation from
GRP78, ATF6 is transported into the nucleus and digested into its
50kDa activated form by restriction enzymes (12). These activated peptides are
transported into the nucleus and bind to the ER, which activates
related ER chaperones, such as GRP78, GRP94, protein disulfide
isomerase and certain transcription factors such as CHOP (12).
In the present study PERK, P-PERK, eIF2α,
phosphorylated eIF2α (P-eIF2α) and activating transcription factor
2 (ATF2) were selected as five biochemical markers to investigate
whether MI/R activates the expression of CHOP through a
PERK-eIF2α-ATF2 pathway. The study also explored whether the
induction pathway of ER stress was changed under the effects of
hypertension.
Materials and methods
Animals
The current study was performed in adherence with
Chinese National Regulations for the Administration of Affairs
concerning Experimental Animals. The animal models used were 16
male spontaneously hypersensitive rats (SHRs), weighing between 300
and 400 g and aged between 6 and 8 months, provided by the Animal
Center in South Campus of Suzhou University (Jiangsu, China). SHRs
were assigned to four groups: Group 1, sham surgery group; group 2,
myocardial ischemia reperfusion group; group 3, myocardial ischemia
reperfusion + captopril (a drug treating hypertension) group; and
group 4, sham + captopril group. Groups 1 and 4 were sham surgery
control groups under conditions of hypertension and
non-hypertension, respectively. Groups 2 and 3 were experimental
groups that underwent MI/R injury under conditions of hypertension
and non-hypertension respectively. SHRs were randomly divided into
the four groups. Captopril at a dose of 40 mg/kg was administered
orally once per day to the rats in groups 3 and 4 rats for 7 weeks
to lower their blood pressure to a normal level. The blood pressure
and body weights of the rats were measured prior to surgery to
ensure that captopril eliminated the symptoms of hypertension
without affecting other body condition parameters. During surgery,
animal preparation and the MI/R process were performed as described
previously (13). Monitoring of
blood pressure and heart rate was carried out using a non-invasive
blood pressure meter (BP-98A; Softron Co. Ltd, Tokyo, Japan).
Monitoring was initiated from immediately prior to anesthesia and
continued until the end of the experiment, when the animals were
humanely sacrificed under anesthesia. Samples of heart tissue were
collected immediately for further analysis, including hematoxylin
and eosin (H&E) staining, immunohistochemical staining and
western blotting.
H&E staining
Fresh heart tissues were placed into Bouin solution
(4% formaldehyde) for perfusion fixation. Following this, they were
dehydrated using alcohol and vitrified in dimethylbenzene. Samples
were embedded in paraffin, sectioned and stained with H&E
(SBT10001; Sunteambio Biotechnology, Shanghai, China). H&E
staining was conducted according to previously described methods
(14).
Immunohistochemical staining and
statistical analysis
Immunohistochemical staining was also performed on
the formaldehyde-fixed and paraffin-embedded tissue samples. CHOP
antibody (1:200; #2895; Cell Signaling Technology, Inc., Danvers,
MA, USA) and GRP78 antibody (1:200; ab21685; Abcam, Cambridge, MA,
USA) were used respectively as the primary antibodies.
Peroxidase-conjugated goat anti-rabbit IgG (1:500; 111-035-003;
Jackson ImmunoResearch, West Grove, PA, USA) was the secondary
antibody. The procedures of immunohistochemical staining were based
on previous protocols (15). When
observing the slides under a microscope, cell nuclei were colored
purple-blue and positive products were tan or yellow. Three
photographs of each slide were selected randomly and analyzed using
Image-Pro Plus 6.0 image analysis software (Media Cybernetics,
Inc., Rockville, MD, USA). The software calculated the area of the
positive regions, integrated optical density (IOD), number (n) of
positive cells and IOD/n. Based on the IOD/n of each group, the
mean ± standard deviation (SD) was calculated. Statistical analysis
was carried out using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) and P-values were calculated to compare the differences among
groups. When P<0.05, the difference was considered statistically
significant.
Western blotting and statistical
analysis
The expression of five important proteins involved
in the PERK-eIF2α-ATF2 pathway was examined in a western blot
assay. The primary antibodies were against PERK (1:1,000; #3192;
Cell Signaling Technology, Inc., CST), P-PERK (1:1,000; #3179; Cell
Signaling Technology, Inc.), eIF2α (1:1,000; #2103; Cell Signaling
Technology, Inc.), P-eIF2α (1:1,000; #9721; Cell Signaling
Technology, Inc.) and ATF2 (1:1,000; #9226; Cell Signaling
Technology, Inc.). The secondary antibody was horseradish
peroxidase (HRP)-labeled anti-rabbit and mouse IgG (H+L), which is
polyvalent. The internal reference was a GADPH antibody (1:5,000;
KC-5G5; Kandchen Bio-tech Inc., Shanghai, China). The whole
procedure was based on a previous protocol (16). Based on the intensity of the
protein bands, IOD was calculated using Gel-Pro Analyzer 4 software
(Media Cybernetics, Inc.) for statistical study. Data was analyzed
using the SPSS 17.0 statistics software package. Quantitative data
were presented as the mean ± SD, and qualitative data as a
percentage. One-way analysis of variance (ANOVA) was applied to
make comparisons within one group and ANOVA was used to make mean
comparisons in groups. A two-sided test was used to check
statistics.
Results
Establishment of animal models
Data on the body weights and blood pressures of rats
prior to and following captopril administration and surgery are
displayed in Tables I–III. No significant differences in body
weight (P>0.05) were identified among the four groups of rats
prior to captopril administration on day 0. Following captopril
administration to the rats in groups 3 and 4, the body weights of
all the rats were measured weekly until surgery on day 49. During
this time, no significant differences (P>0.05) were identified
among the four groups. The blood pressure parameters in Table II showed no significant
differences among the groups prior to captopril administration
(P>0.05). Sixty days following the initiation of captopril
administration to the rats in groups 3 and 4, a significant
difference was observed between the blood pressure values in the
rats of groups 1 and 2 and those in groups 3 and 4, which is
demonstrated in Table III.
 | Table IBody weight (g) of rats following
captopril administration and prior to surgery. |
Table I
Body weight (g) of rats following
captopril administration and prior to surgery.
| Day |
|---|
|
|
|---|
| Group | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
|---|
| Group 1 |
| Rat 1 | 336.0 | 345.0 | 351.5 | 365.0 | 372.0 | 372.0 | 382.0 | 388.0 |
| Rat 2 | 312.0 | 319.0 | 323.5 | 333.5 | 339.0 | 338.0 | 341.0 | 349.0 |
| Rat 3 | 310.0 | 318.0 | 325.0 | 337.0 | 340.0 | 348.0 | 353.0 | 355.0 |
| Rat 4 | 319.0 | 323.0 | 329.0 | 341.0 | 346.0 | 349.0 | 350.0 | 356.0 |
| Group 2 |
| Rat 1 | 306.0 | 307.0 | 310.5 | 319.0 | 326.0 | 334.0 | 338.0 | 342.0 |
| Rat 2 | 301.0 | 317.0 | 323.0 | 333.0 | 336.0 | 342.0 | 342.0 | 343.5 |
| Rat 3 | 315.0 | 329.0 | 335.0 | 342.0 | 350.0 | 355.0 | 360.0 | 363.0 |
| Rat 4 | 343.0 | 352.0 | 366.5 | 376.0 | 380.0 | 384.0 | 392.0 | 390.5 |
| Group 3 |
| Rat 1 | 329.0 | 335.0 | 347.0 | 351.5 | 345.0 | 345.0 | 365.0 | 363.0 |
| Rat 2 | 301.0 | 303.0 | 309.0 | 285.0 | 307.5 | 316.0 | 328.0 | 331.0 |
| Rat 3 | 285.0 | 288.0 | 294.5 | 304.0 | 309.0 | 318.5 | 325.0 | 329.0 |
| Rat 4 | 356.0 | 365.0 | 367.0 | 383.5 | 386.0 | 392.0 | 395.0 | 397.0 |
| Group 4 |
| Rat 1 | 357.0 | 358.5 | 366.5 | 372.0 | 383.0 | 390.0 | 394.0 | 396.0 |
| Rat 2 | 345.0 | 349.0 | 356.5 | 362.0 | 372.0 | 382.0 | 388.0 | 388.5 |
| Rat 3 | 304.0 | 305.0 | 306.0 | 305.0 | 307.5 | 310.0 | 320.0 | 318.5 |
| Rat 4 | 353.0 | 361.0 | 369.0 | 379.0 | 387.0 | 391.0 | 396.0 | 397.0 |
 | Table IIIBlood pressure parameters of rats 60
days after the initiation of captopril treatment, according to
group assignment. |
Table III
Blood pressure parameters of rats 60
days after the initiation of captopril treatment, according to
group assignment.
| Group | SBP | HR | MBP | DBP |
|---|
| Group 1 |
| Rat 1 | 211,199,208 | 399,412,413 | 176,181,188 | 159,172,178 |
| Rat 2 | 157,152,154 | 386,382,382 | 133,129,133 | 121,118,123 |
| Rat 3 | 197,193,202 | 290,288,297 | 173,166,170 | 161,153,153 |
| Rat 4 | 171,170,174 | 303,301,302 | 135,130,130 | 117,110,108 |
| Group 2 |
| Rat 1 | 185,183,173 | 411,410,403 | 157,163,148 | 143,153,136 |
| Rat 2 | 169,172,168 | 436,403,401 | 149,151,144 | 139,141,132 |
| Rat 3 | 171,164,180 | 407,382,389 | 134,127,139 | 116,108,119 |
| Rat 4 | 180,171,170 | 473,459,470 | 145,133,136 | 128,114,119 |
| Group 3 |
| Rat 1 | 118,115,120 | 483,495,472 | 106,102,108 | 100,96,102 |
| Rat 2 | 145,149,148 | 324,314,304 | 127,115,122 | 118,97,109 |
| Rat 3 | 144,139,142 | 399,388,390 | 106,113,109 | 87,100,93 |
| Rat 4 | 149,138,145 | 330,346,331 | 128,121,111 | 118,113,94 |
| Group 4 |
| Rat 1 | 156,139,130 | 321,338,369 | 126,107,110 | 111,91,100 |
| Rat 2 | 123,128,115 | 337,331,353 | 103,100,91 | 93,86,79 |
| Rat 3 | 126,136,136 | 354,370,359 | 103,120,109 | 92,112,96 |
| Rat 4 | 153,149,154 | 434,446,426 | 119,112,127 | 102,94,114 |
 | Table IIBlood pressure parameters of rats
prior to captopril administration (day 0). |
Table II
Blood pressure parameters of rats
prior to captopril administration (day 0).
| Group. | SBP | HR | MBP | DBP |
|---|
| Group 1 |
| Rat 1 | 213,221,241 | 426,417,427 | 172,178,212 | 152,157,198 |
| Rat 2 | 142,156,183 | 475,467,481 | 125,138,176 | 117,129,173 |
| Rat 3 | 149,160,150 | 409,420,387 | 123,123,118 | 120,105,102 |
| Rat 4 | 173,210,192 | 372,354,337 | 150,185,166 | 139,172,153 |
| Group 2 |
| Rat 1 | 233,236,242 | 384,389,415 | 181,180,213 | 155,152,199 |
| Rat 2 | 166,186,181 | 405,408,465 | 150,152,147 | 142,135,130 |
| Rat 3 | 158,166,160 | 418,399,392 | 143,129,124 | 136,110,106 |
| Rat 4 | 179,178,144 | 376,461,428 | 143,142,126 | 125,124,117 |
| Group 3 |
| Rat 1 | 179,210,205 | 389,378,359 | 159,167,177 | 149,145,163 |
| Rat 2 | 169,172,162 | 421,398,382 | 140,136,121 | 126,118,101 |
| Rat 3 | 148,148,160 | 490,466,463 | 124,118,129 | 112,103,114 |
| Rat 4 | 192,221,172 | 428,457,450 | 159,181,138 | 143,161,121 |
| Group 4 |
| Rat 1 | 212,160,192 | 489,476,498 | 185,132,162 | 172,118,147 |
| Rat 2 | 191,163,177 | 488,478,434 | 157,132,152 | 140,117,140 |
| Rat 3 | 196,142,155 | 356,360,378 | 161,129,129 | 143,123,115 |
| Rat 4 | 193,223,236 | 485,460,478 | 148,181,185 | 126,160,160 |
Injury caused by MI/R
Following H&E staining, the nuclei in the heart
tissue were stained blue and cytoplasms were stained red. Collagen
fibers were a varying red color. A comparison of the heart tissue
from experimental groups 2 and 3 with that from sham surgery groups
1 and 4 (Fig. 1) revealed severe
injuries in the experimental groups, including obvious infarcts,
inflammatory cell infiltration and the rupture and necrosis of
myocardial cells that had lost their normal ordered structure.
Extensive fibrous scar tissue had formed in the infarction zone and
infarcted border zone. Due to necrosis and injury in groups 2 and
3, it is evident that MI/R causes severe myocardial necrosis,
regardless of hypertension.
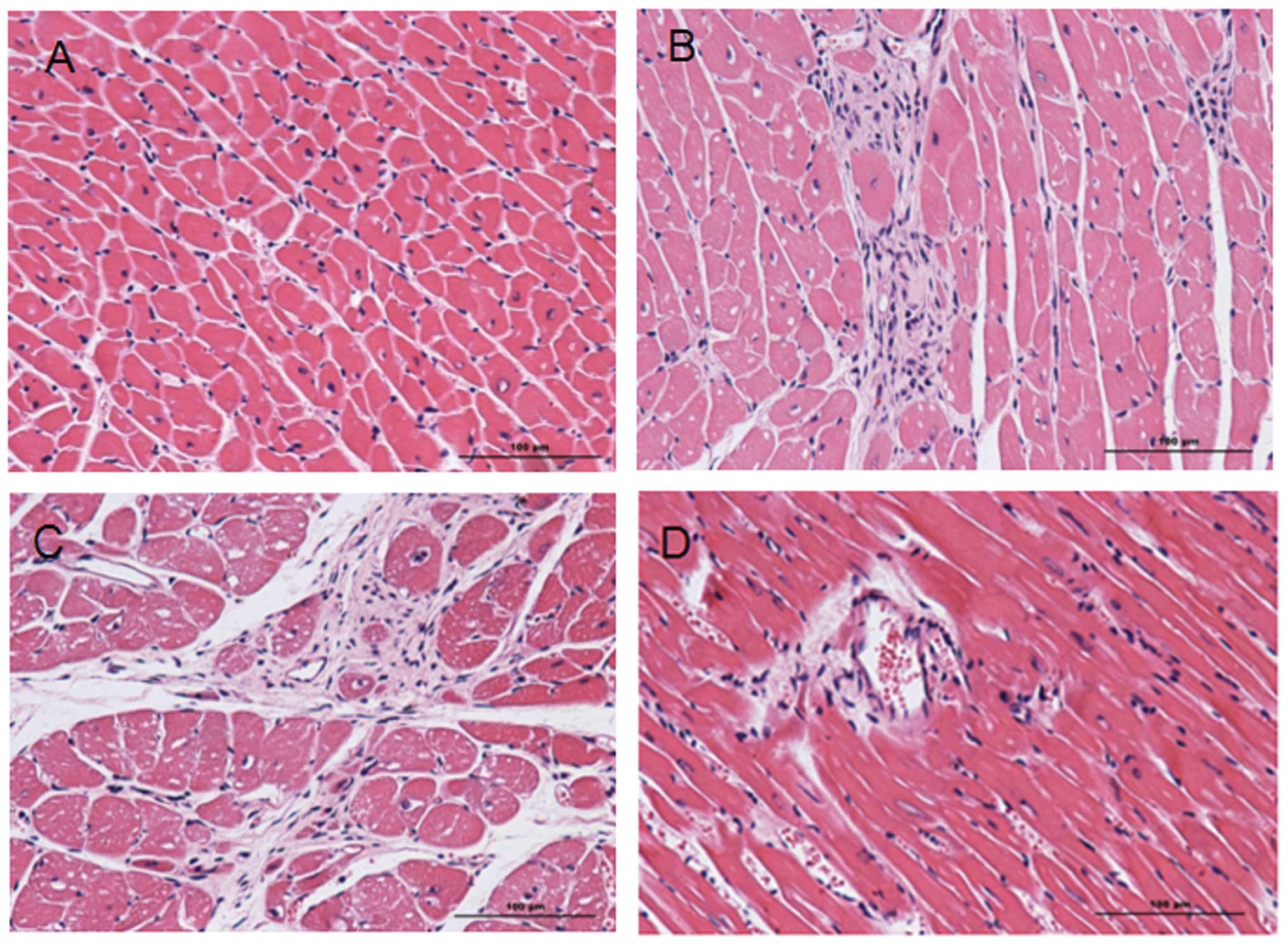 | Figure 1Hematoxylin and eosin (H&E)
staining images of heart tissue samples from groups 1–4. H&E
staining of (A) group 1, (B) group 2, (C) group 3 and (D) group 4.
In the H&E staining images, the nucleus is blue and the
cytoplasm is red. Collagen fibers show a varying red color.
Comparing the experimental groups 2 and 3 with sham surgery groups
1 and 4 (B and C with A and D), severe injuries can be identified
in the experimental groups, including obvious infarcts,
inflammatory cell infiltration and the rupture and necrosis of
myocardial cells that have lost their normal ordered structure. A
large amount of fibrous scar tissue is visible in the infarction
zone and infarcted border zone. Group 1, sham surgery; group 2,
myocardial ischemia reperfusion; group 3, myocardial ischemia
reperfusion and captopril treatment; group 4, sham surgery and
captopril treatment. |
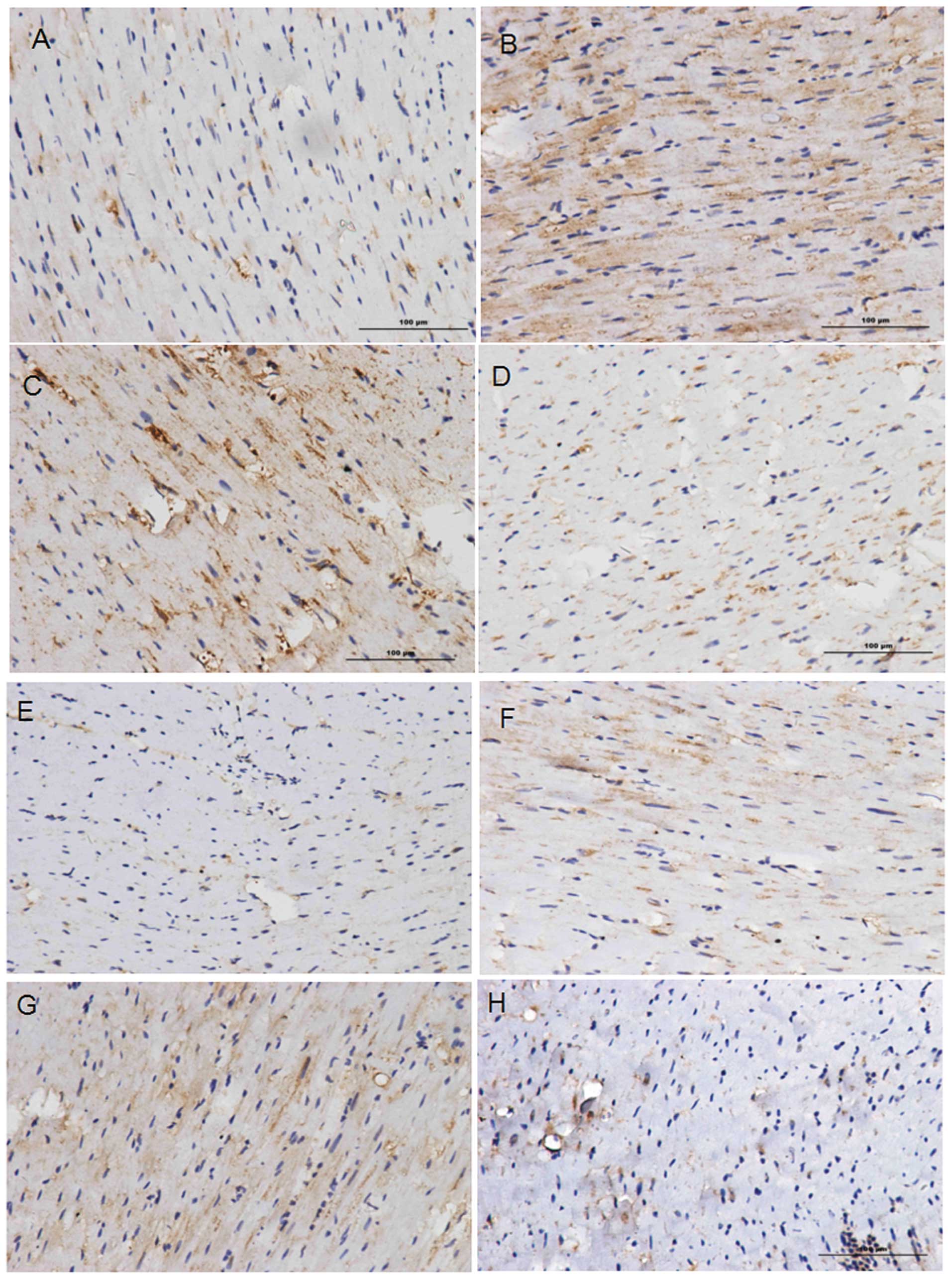
CHOP and GRP78 expression
Statistical analysis of the images of the
immunohistochemical staining (Fig.
2) was carried out using Image-Pro Plus 6.0 software. The
expression levels of CHOP and GRP78 in groups 2 and 3 were higher
than those in groups 1 and 4, thus suggesting that these proteins
accumulate in areas where tissue injury and cell necrosis are the
most severe. Comparison of the IODs for the CHOP-stained tissues
(Table IV) revealed significant
differences (P<0.05) between groups 1 and 3, and between groups
3 and 4. Comparison of the IODs for the GRP78-stained tissues
revealed significant differences (P<0.05) between groups 1 and
2, and between groups 3 and 4 (Table
V). No significant differences were identified among the other
pairs. This comparison confirms that CHOP and GRP78 are involved in
MI/R injury responses, indicating that MI/R injury induces ER
stress through the CHOP pathway. The fact that no marked
differences in the expression levels of CHOP or GRP78 were observed
between groups 2 and 3 indicates that hypertension does not have a
significant impact on ER stress induced by the CHOP pathway.
 | Table IVP-values for comparisons of
integrated optical density (IOD) values for C/EBP homologous
protein (CHOP) staining between groups. |
Table IV
P-values for comparisons of
integrated optical density (IOD) values for C/EBP homologous
protein (CHOP) staining between groups.
| Group No. | 1 | 2 | 3 | 4 |
|---|
| 1 | | 0.116 | 0.007 | 0.539 |
| 2 | 0.116 | | 0.148 | 0.310 |
| 3 | 0.007 | 0.148 | | 0.023 |
| 4 | 0.539 | 0.310 | 0.023 | |
 | Table VP-value for comparisons of integrated
optical density (IOD) for glucose-regulated protein78 (GRP78)
staining between groups. |
Table V
P-value for comparisons of integrated
optical density (IOD) for glucose-regulated protein78 (GRP78)
staining between groups.
| Group No. | 1 | 2 | 3 | 4 |
|---|
| 1 | | 0.037 | 0.187 | 0.858 |
| 2 | 0.037 | | 0.069 | 0.052 |
| 3 | 0.187 | 0.069 | | 0.025 |
| 4 | 0.858 | 0.052 | 0.025 | |
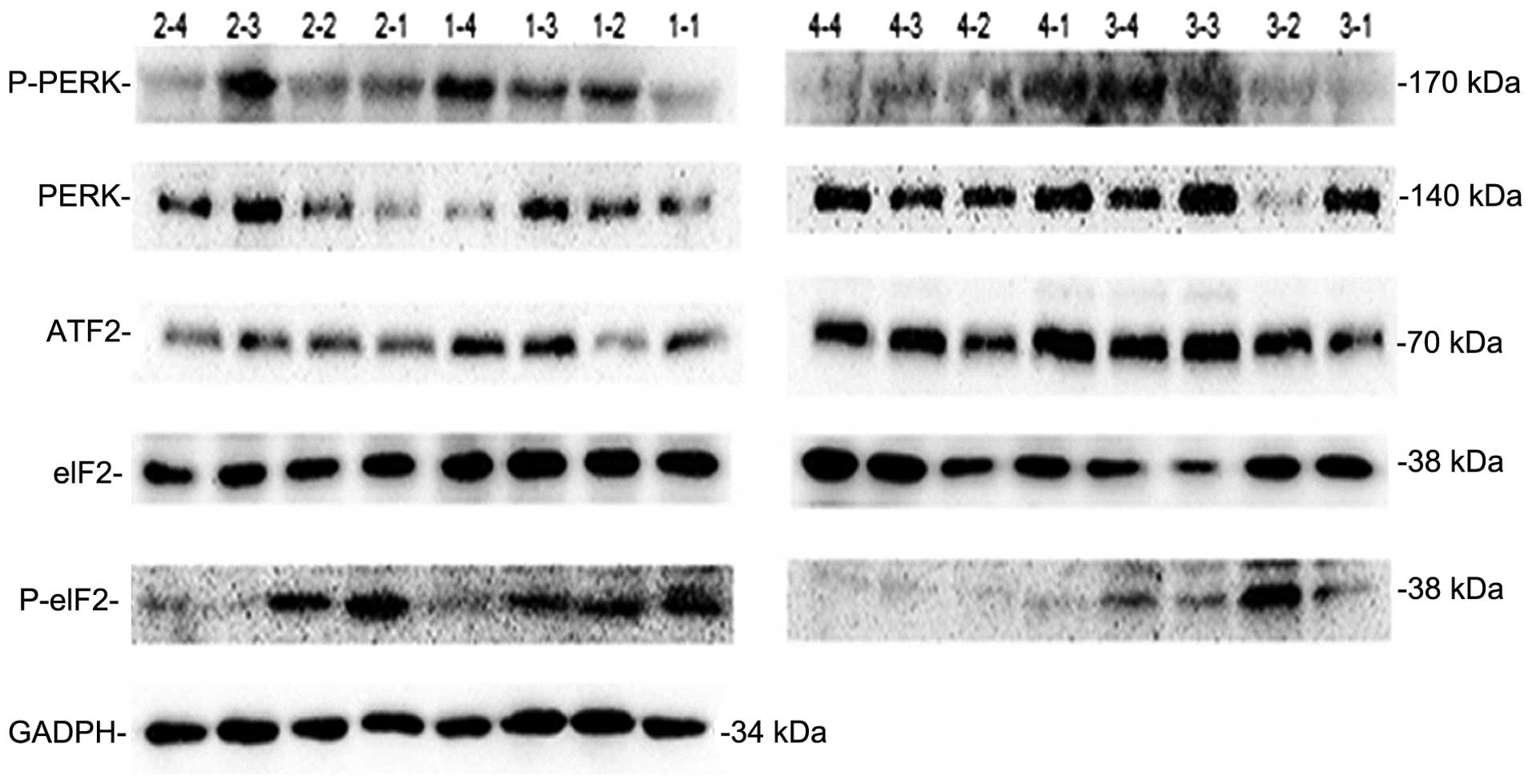
Expression of five important proteins
involved in the CHOP pathway
As aforementioned, PERK, P-PERK, eIF2α, p-eIF2α and
ATF2 were selected as five biochemical markers to investigate
whether MI/R activates the expression of CHOP through a
hypothetical PERK-eIF2α-ATF2 pathway. An SDS-PAGE image from the
western blot analysis (Fig. 3) was
processed using Gel-Pro Analyzer 4 software (data not shown). This
revealed that the expression level of the five signaling proteins
was higher as a whole in groups 2 and 3, than in groups 1 and 4.
Although the differences on an average level were not marked, due
to poor parallelism in the same group; this does not affect the
conclusion that the PERK-eIF2α-ATF2 pathway was induced by MI/R
injury.
No clear differences were observed between groups 2
and 3, indicating that hypertension is unlikely to affect the
PERK-eIF2α-ATF2 pathway as the main pathway inducing ER stress.
Discussion
According to World Health Organization (WHO)
statistics, ischemic heart disease ranks as the greatest cause of
mortality in humans. The most effective method of treating ischemic
heart disease is myocardial reperfusion, using either thrombolytic
therapy or primary percutaneous coronary intervention (PPCI)
(17). However, the process of
myocardial reperfusion may itself induce further cardiomyocyte
death, a phenomenon known as myocardial ischemia/reperfusion (MI/R)
injury (18–20). Although surgical techniques for the
avoidance of MI/R are being developed, MI/R injury remains a severe
problem.
The symptoms and severity of MI/R injury depend on
numerous factors, including age, gender, duration and hypertension.
The present study investigated the impact of hypertension on MI/R
injury. The spontaneous hypertension rat (SHR), which has a
syndrome similar to human spontaneous hypertension, was used as an
animal model. This was considered appropriate for the investigation
of MI/R injury combined with the factor of hypertension. Due to
differences in genetic background and health conditions, common
non-hypertensive rats were not suitable for use as controls. Thus,
the corresponding control group was constructed by the
administration of captopril to the SHR rats, so that their blood
pressure was reduced to normal levels. According to current
findings, captopril is a hypertension inhibitor that does not
interfere with the signaling pathway under investigation in the
present study. Captopril works either directly, by inhibiting the
angiotensin converting enzyme (ACE), which blocks the renin
angiotensin aldosterone (RAA) system, or indirectly, by inhibiting
the sympathetic nervous system (SNS) at different levels (21). The 16 male SHRs were randomly
divided into four groups. According to the weight and blood
pressure measurements taken prior to surgery (Tables I and II), no significant differences existed
among the groups. On day 60 after the initiation of drug
administration, the blood pressures in the groups that were
administered captopril had returned to normal (Table III) and were markedly lower than
those in the SHR groups. In this way, experimental groups 1 and 2
of hypertensive rats and control groups 3 and 4 of non-hypertensive
rats were successfully constructed. Following this, the rats in
groups 2 and 3 underwent surgery to construct the MI/R experimental
animal model group.
H&E staining verified the successful
construction of the animal model (Fig.
1). In sham surgery groups 1 and 4, the area of necrosis and
number of apoptotic cells was lower than in groups 2 and 3. ER
stress, coupled with MI/R injury, usually results in the excessive
accumulation of unfolded and misfolded proteins in the ER, which
induces the unfolded protein response (UPR). Thus, signaling
molecules involved in the UPR are typically used to indicate
whether ER stress occurs. Under conditions of no ER stress, GRP78
binds to three transmembrane proteins, namely PERK, IRE1 and ATF6,
to maintain the inactivated status of signal transduction factors.
When the UPR process is activated, the activated IRE1α promotes the
expression of UPR target molecules, which include endoplasmic
reticulum stress responsive elements (ERSE) such as GRP78, to
protect and recover homeostasis by reducing or terminating ER
stress reactions. A number of studies have found that ER stress
modulates not only the expression of apoptosis-inducing molecules
such as CHOP and caspase-12, but also the expression/activation of
certain survival molecules such as the growth arrest and DNA
damage-inducible protein GADD34 and GRP78. Therefore, in the
current study, one example of an apoptosis-inducing molecule and
one of a survival molecule, namely CHOP and GRP78, were selected as
markers to verify the induction of the ER stress regulatory pathway
subsequent to MI/R injury.
Immunohistochemical staining results revealed the
expression levels of CHOP and GRP78. When directly observing the
images (Fig. 2), it is clear that
the expression levels of the CHOP and GPR78 proteins in the MI/R
injury experimental groups (groups 2 and 3) were higher than those
in the sham surgery groups (groups 1 and 4). In addition, the
expression of these proteins was concentrated in certain areas,
where tissue necrosis and cell injury was the most severe.
According to analysis of IOD values, CHOP immunohistochemical
staining revealed that differences existed only between groups 1
and 3 and between groups 3 and 4, and the GRP78 staining revealed
that differences existed only between groups 1 and 2 and between
groups 3 and 4. These results confirm that MI/R injury induces ER
stress through the CHOP pathway.
Following this, five signal proteins involved in the
CHOP and GRP78 signaling pathway were selected for analysis to
further explore whether MI/R injury induces the expression of CHOP
through a PERK-eIF2α-ATF2 pathway. These proteins were PERK,
P-PERK, eIF2α, P-eIF2α and ATF2,
Western blot results revealed that, overall, the
expression levels of these five signal proteins were higher in
groups 2 and 3 than in groups 1 and 4, although this difference was
not evident at an average level due to a lack of parallelism. These
results indicate that MI/R injury induces the expression of CHOP
through a PERK-eIF2α-ATF2 pathway. A lack of parallelism existed in
the western blot experiment results, which resulted in the
differences exhibited being unclear. As an example, in the
detection of PERK in group 3, a high expression level of PERK was
detected in samples 3–1, 3–3 and 3–4, but no PERK was detected in
sample 3–2. This may be due to the sampling location used in each
rat, as only some of the cells in the necrotic tissue would undergo
severe ER stress. In addition, individual differences among the
rats would also be a major concern.
Hypertension did not demonstrate a significant
impact on MI/R injury in the present study. Data analysis did not
reveal any clear differences in protein expression levels between
the rats in groups 2 and 3 in all experimental results, including
those from H&E staining, immunohistochemical staining and
western blotting. This indicates that under hypertension
conditions, the main signaling pathway of MI/R injury does not
change The PERK-eIF2α-ATF2 pathway remains the main pathway by
which MI/R injury induces the expression of CHOP.
Acknowledgements
This study was supported by the Jiangsu Provincial
Outstanding Medical Academic Leader program (No. LJ201140) and the
National Natural Science Foundation of China (No. 81070139).
References
|
1
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Han Y, Meng G, Bai W, et al:
Direct renin inhibition with aliskiren protects against myocardial
ischemia/reperfusion injury by activating nitric oxide synthase
signaling in spontaneously hypertensive rats. J Am Heart Assoc.
3:e0006062014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong M, Cho J, Shin JI, et al: Hempseed
oil induces reactive oxygen species- and C/EBP homologous
protein-mediated apoptosis in MH7A human rheumatoid arthritis
fibroblast-like synovial cells. J Ethnopharmacol. 154:745–752.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campos G, Schmidt-Heck W, Ghallab A, et
al: The transcription factor CHOP, a central component of the
transcriptional regulatory network induced upon CCl4
intoxication in mouse liver, is not a critical mediator of
hepatotoxicity. Arch Toxicol. 88:1267–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pavli M, Farmaki E, Merkourea S, et al:
Endoplasmic reticulum stress-associated chaperones, Bip/GRP78 and
calnexin are overexpressed in keratocystic odontogenic tumours. J
Oral Maxillofac Res. 5:e32014.PubMed/NCBI
|
|
6
|
Feng YX, Sokol ES, Del Vecchio CA, et al:
Epithelial-to-mesenchymal transition activates PERK-eIF2a and
sensitizes cells to endoplasmic reticulum stress. Cancer Discov.
4:702–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jerry Chiang WC and Lin JH: The effects of
IRE1, ATF6, and PERK signaling on adRP-linked rhodopsins. Adv Exp
Med Biol. 801:661–667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karali E, Bellou S, Stellas D, et al: VEGF
signals through ATF6 and PERK to promote endothelial cell survival
and angiogenesis in the absence of ER stress. Mol Cell. 54:559–572.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozpolat B, Akar U, Tekedereli I, Alpay SN,
et al: PKCδ regulates translation initiation through PKR and eIF2α
in response to retinoic acid in acute myeloid leukemia cells. Leuk
Res Treatment. 2012:4829052012.
|
|
10
|
Bruhat A, Chérasse Y, Maurin AC, et al:
ATF2 is required for amino acid-regulated transcription by
orchestrating specific histone acetylation. Nucleic Acids Res.
35:1312–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruhat A, Jousse C, Carraro V, Reimold AM,
Ferrara M and Fafournoux P: Amino acids control mammalian gene
transcription: activating transcription factor 2 is essential for
the amino acid responsiveness of the CHOP promoter. Mol Cell Biol.
20:7192–7204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakajima S, Hiramatsu N, Hayakawa K, Saito
Y, Kato H, Huang T, Yao J, Paton AW, Paton JC and Kitamura M:
Selective abrogation of BiP/GRP78 blunts activation of NF-κB
through the ATF6 branch of the UPR: involvement of C/EBPβ and
mTOR-dependent dephosphorylation of Akt. Mol Cell Biol.
31:1710–1718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayer P: Hematoxylin and eosin (H&E)
staining protocol. Mitt Zool Stn Neapel. 12:3031896.
|
|
15
|
Ramos-Vara JA: Technical aspects of
immunohistochemistry. Vet Pathol. 42:405–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mouw G, Zechel JL, Gamboa J, Lust WD,
Selman WR and Ratcheson RA: Activation of caspase-12, an
endoplasmic reticulum resident caspase, after permanent focal
ischemia in rat. Neuroreport. 14:183–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: a neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Braunwald E and Kloner RA: Myocardial
reperfusion: a double-edged sword? J Clin Invest. 76:1713–1719.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piper HM, García-Dorado D and Ovize M: A
fresh look at reperfusion injury. Cardiovasc Res. 38:291–300. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie LD, Chen DG, Zhang S, Wang HJ and Chen
HJ: Sympatholytic effect of captopril in regression of
cardiovascular remodeling in spontaneously hypertensive rats.
Zhongguo Yao Li Xue Bao. 15:123–128. 1994.PubMed/NCBI
|