Introduction
Bell’s palsy, also known as idiopathic facial nerve
paralysis (IFNP), is an acute, idiopathic and unilateral paralysis
of the face. This common condition affects 20–45 cases per 100,000
people (1). At present, although
the aetiology remains unknown, autoimmune disorders and
inflammations due to viral infections are considered to play two
key roles in the development of the disease (2–4). The
prognosis of the disease is favourable, and 70–80% of all patients
with IFNP are likely to have complete or near complete recovery,
regardless of whether treatment is given (5). However, poor recovery of facial
muscle control, facial disfigurement, psychological trauma and
facial pain are likely to affect <30% of patients.
Improving the recovery of facial functions and
preventing associated complications are the priorities in
treatment. During the acute stage of the disease, corticosteroid
and antiviral medications have been proved to be effective
treatments (6,7). During the sequelae stage, botulinum
toxin A and surgical reconstruction are the treatment options for
facial spasms and synkinesis. Physical therapy and acupuncture are
also used to treat Bell’s palsy (8).
Nerve growth factor (NGF) was the first member of
the neurotrophin family to be identified (9). Neurotrophins are critical to the
development and maintenance of the central and peripheral nervous
systems. They prevent or reverse neuronal degeneration, promote
neurite regeneration and enhance synaptic plasticity (10,11).
Given its potential roles, NGF has been widely used in neurological
disorders, particularly in China. Three brands of NGF drug are
available in China. A number of doctors use the drug as an adjuvant
treatment for peripheral neuropathy, including Bell’s palsy
(12). To date, however, no
systematic review has provided evidence on the efficacy of NGF for
Bell’s palsy. Clearly, the efficacy and safety of NGF should be
strictly assessed prior to being recommended for routine use in
patients with Bell’s palsy. Therefore, we conducted a systematic
review to critically evaluate the effect of NGF treatment on Bell’s
palsy.
Materials and methods
Search sources and strategy
The following electronic databases were searched,
starting from their inception to December 2013: PubMed, the
Cochrane Central Register of Controlled Trials, Embase, the China
National Knowledge Infrastructure, China Biology Medicine disc, VIP
Database for Chinese Technical Periodicals and Wan Fang Data. The
search strategy combined the following specific Medical Subject
Heading and free-text words using the following terms: Bell’s
palsy, facial paralysis, idiopathic facial palsy, herpetic facial
paralysis and nerve growth factor or NGF. In addition, manual
searches were performed in specialised journals. No restriction was
applied on publication date or language.
Study selection and inclusion/exclusion
criteria
Identified trials were evaluated independently by
two of the co-authors of the present study, Dr Yipeng Su (SYM) and
Dr Xiaomeng Dong (DXM), following a defined protocol. The inclusion
criteria were as follows: i) The study design had to be a
randomised controlled trial (RCT); ii) the study had to include
patients with unilateral facial nerve weakness with no identifiable
cause observed within seven days of the onset of weakness; iii)
treatment had to start within seven days of paralysis onset; and
iv) the follow-up had to last at least four weeks. By contrast,
studies that satisfied the following criteria were excluded: i) Any
study that included patients with suppurative otitis media,
multiple sclerosis, traumatic facial paralysis, encephalitis and
Lyme disease; ii) any study that included children or pregnant or
breastfeeding females; iii) any study where the study design
involved animals, case reports, case series, retrospective studies
or cohort studies; and iv) redundant studies or duplicate
publications. If a study did not provide the necessary information
to assess potential eligibility, then the authors were contacted
and asked to provide the missing data.
Data extraction and quality
assessment
The following variables were extracted from all
studies: General characteristics (type of study, year of
publication, number of patients included and their baseline
characteristics); procedural data (type of randomisation, inclusion
criteria, NGF dose, follow-up protocol and number of patients who
dropped out of the study) and outcome data (definition for facial
recovery and facial muscle recovery outcome, and adverse effects of
each therapy strategy). Any disagreement was resolved by
discussion.
Two independent investigators (SYP and DXM)
evaluated study quality. The modified Jadad score (13) was used to assess study quality.
This score considers randomisation technique, allocation
concealment, blinding, intention to treat and patient attrition.
Studies with a score of 4–7 were considered to be of high quality,
whereas those with a score of 0–3 were considered of poor quality.
Any disagreement was resolved by discussion.
Primary outcome
The primary outcome of the meta-analysis was the
proportion of patients who attended a follow-up visit at least four
weeks after treatment initiation, with at least partial facial
muscle recovery. Complete recovery was defined as a score of ≤2 on
the House-Brackmann Facial Recovery (H-B) scale (14–16).
Partial recovery was defined as a score of 3–6 according to H-B
scale. The secondary outcome was the range of recovery of the
compound muscle action potential (CMAP) of the facial nerve
measured by electroneurography (ENoG).
Statistical analysis
To summarise the effects of NGF on disease response
rate, the risk estimates [odds ratio (OR) and mean difference (MD)]
and 95% confidence interval (CI) from each study were calculated
using the Cochrane Collaboration’s Review Manager 5.2 software (The
Nordic Cochrane Centre, Copenhagen, Denmark). The heterogeneity of
ORs was assessed using Cochrane’s Q test and I2. If
heterogeneity was present, the ORs were pooled using the
random-effects model (the DerSimonian and Laird method). Otherwise,
the fixed-effects model (the Mantel-Haenszel method) was used. The
statistical significance of the pooled ORs and MDs was analysed
using the Z test. A funnel plot was used to detect publication
bias. For studies with insufficient information, the authors of the
present study contacted the primary authors of the studies
undergoing analysis to acquire and verify data whenever
possible.
Results
Study description and quality
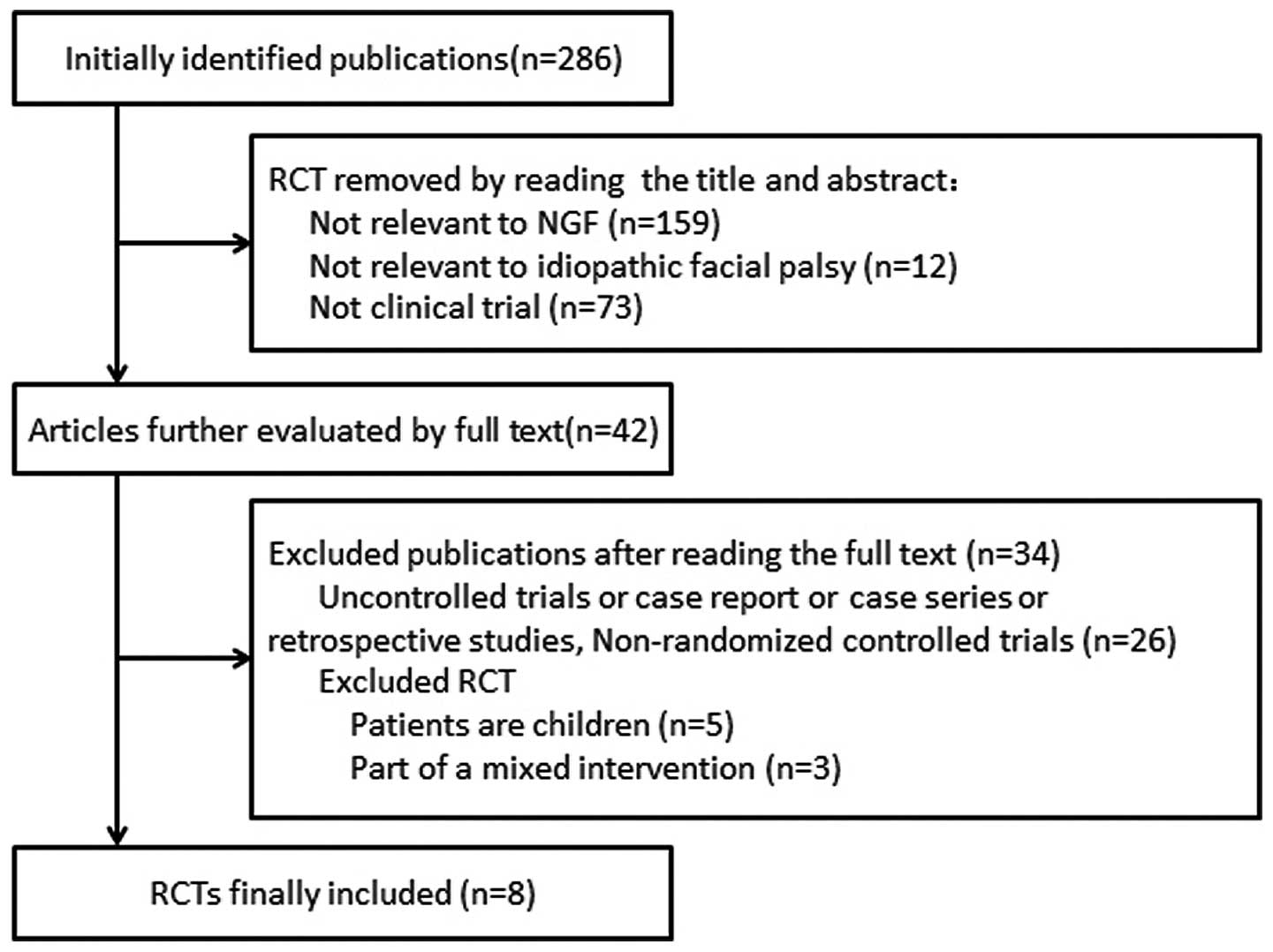
The literature search from the various databases
identified 286 relevant studies. From this pool of relevant
studies, eight (17–24) met our inclusion criteria (Fig. 1). All trials originated in China.
The characteristics of the eight studies are summarised in Table I. All the participants in these
studies suffered from Bell’s palsy. The total number of
participants was 642 (NGF group, n=317 and Control group, n=325),
all of whom were adults, excluding pregnant and breastfeeding
females. All trials employed a two-arm parallel group design.
 | Table ISummary of clinical studies of NGF for
Bell’s palsy. |
Table I
Summary of clinical studies of NGF for
Bell’s palsy.
| First author, year
(reference) | Age in years | Gender (M/F) | Intervention group
NGF regimea | Control group drug
therapy regime | Main outcome/days
from onset | Jadad score |
|---|
| Zhang, 2006 (14) | A:
28.5±10.6
B: 28.7±8.9 | 32/13
35/17 | 4 μg i.m. q.d. 28
days (n=45) | p.o.: Prednisone 1
mg/kg for 3 days tapered to 5 mg/day, 15 days; vitamin B6 +
fursultiamine + mecobalamin for 4 weeks (n=52) | H-B score/28 | 2 |
| Tang, 2008 (15) | 38±8b | 52/42 | 4 μg i.m. q.d. 21
days (n=48) | p.o.: Prednisone 1
mg/kg for 3 days tapered to 5 mg/day, 21 days; mecobalamin for 3
weeks (n=46) | H-B score/21 | 2 |
| Zhang, 2010 (16) | A:
25.5±10.6
B: 28.7±8.9 | 19/11
21/9 | 4 μg i.m. q.d. 28
days (n=30) | p.o.: Prednisone 1
mg/kg for 3 days tapered to 5 mg/day, 15 days; vitamin B6 +
fursultiamine + mecobalamin for 4 weeks (n=30) | H-B score/28 | 2 |
| Mo, 2011 (17) | A: 45.3±3.4
B: 43.4±4.2 | 18/12
20/10 | 4 μg i.m. q.d. 7 days
(n=30) | i.v. drip:
Dexamethasone 10 mg for 7 days + Danhong injection 20 ml for 7
days; p.o.: Mecobalamin for 7 days; physical therapy for 20 days
(n=30) | H-B score/30 | 2 |
| Liu, 2012 (18) | A: 45.3±3.4
B: 43.4±4.2 | 18/10
20/10 | 30 μg i.m. q.d. 7
days (n=28) | i.v. drip:
Dexamethasone 10 mg for 7 days + PNS 300 mg for 7 days; p.o.:
Vitamin B1 + mecobalamin for 7 days; physical therapy for 21 days
(n=30) | H-B score/30 | 3 |
| Wang, 2012 (19) | A:
43.7±14.9
B: 42.5±15.2 | 29/28
31/27 | 30 μg i.m. q.d. 7
days (n=57) | p.o.: Prednisone 1
mg/kg for 3 days tapered to 5 mg/day, 30 days; i.m.: Vitamin B1 +
mecobalamin q.d. for 30 days (n=58) | H-B
score/28
ENoG/28 | 3 |
| Li, 2013 (20) | A:
48.6±13.7
B: 48.9±14.5 | 25/24
26/23 | 30 μg i.m. q.d. 7
days (n=30) | i.v. drip: Acyclovir
500 mg b.i.d. for 7 days; p.o.: Prednisone 1 mg/kg for 3 days
tapered to 5 mg/day, 15 days; i.m.: Vitamin B1+ vitamin B12 for 7
days (n=30) | H-B score/28 | 2 |
| Tian, 2013 (21) | A:
29.6±12.3
B: 34.7±11.2 | c
c | 30 μg i.m. q.d. 7
days (n=49) | i.v.drip:
Dexamethasone 10 mg for 7 days; i.m.: Vitamin B1+ vitamin B12 for 7
days (n=49) | H-B
score/28
ENoG/28 | 2 |
The specific drug and dosage used for all trials are
provided in Table I. All trials
employed the recovery of facial nerve motor function as the main
outcome measure based on the H-B scale. In two studies, ENoG was
used to evaluate the effect of NGF on facial nerve recovery; the
CMAP of the facial nerve was also measured. Four studies (17–19,24)
reported adverse events during the trial. However, adverse events
such as gastrointestinal disorders were likely caused by
corticosteroids rather than NGF.
The majority of the RCTs included in this review had
a high risk of bias. Only two RCTs employed an appropriate
sequence-generation method (21,22).
None of the studies reported allocation concealment or blinding.
Table I provides the results of
the assessment conducted on the quality of the included trials.
Meta-analysis
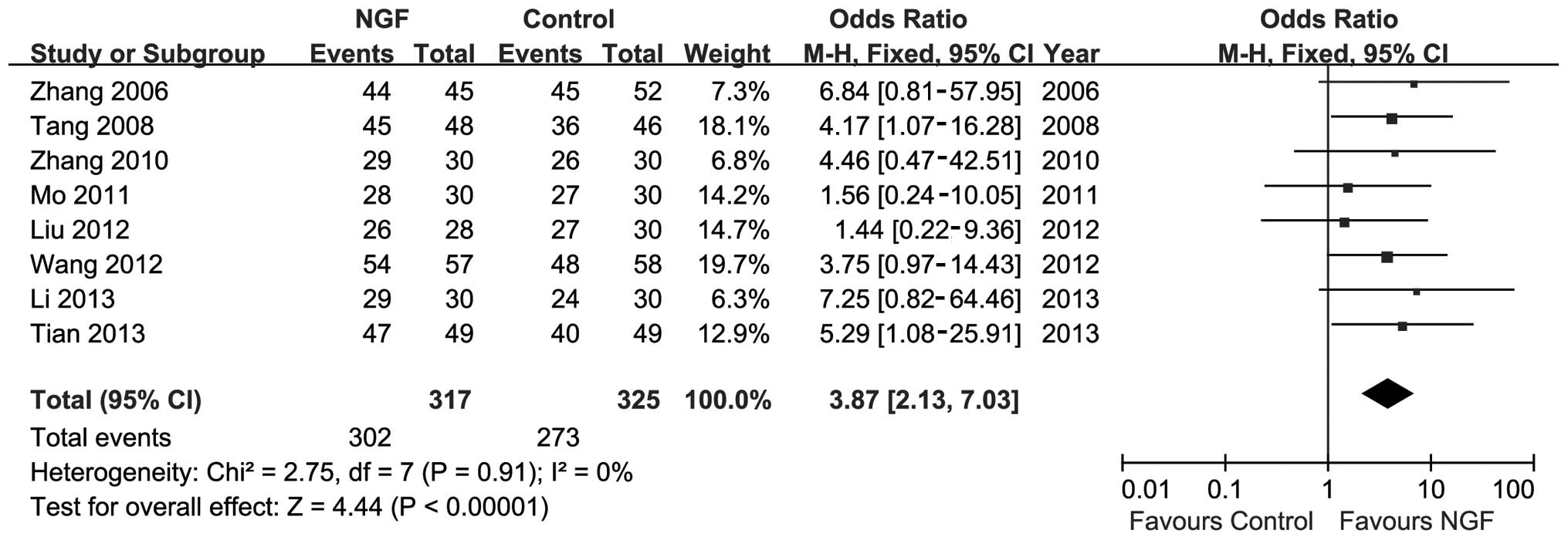
All the RCTs indicated the number of participants
who responded to the therapy. As shown in Fig. 2, moderate heterogeneity was
observed among trials (P=0.91; I2=0%). A meta-analysis
was conducted using the fixed-effect model. The result of the
meta-analysis showed favourable effects of NGF on response rate
(n=642; OR, 3.87; 95% CI, 2.13–7.03; P<0.01; Fig. 2).
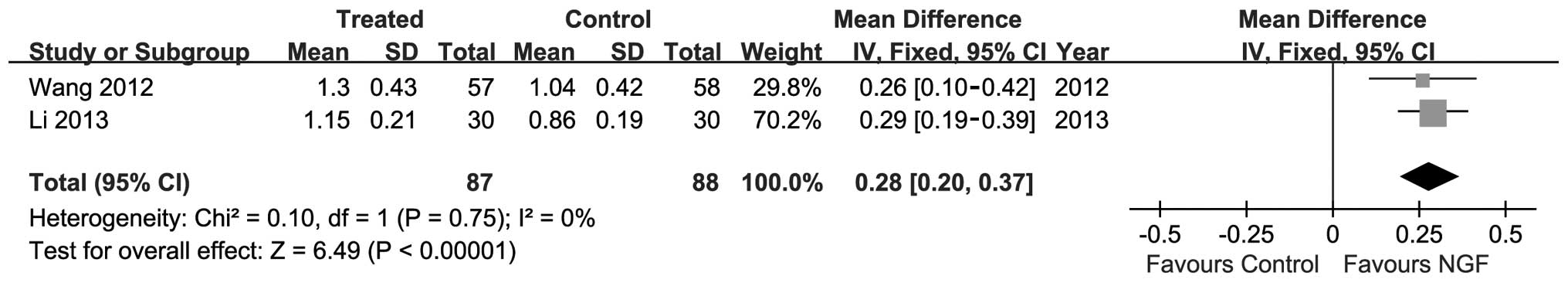
For the secondary outcome, i.e., facial nerve CMAP,
the meta-analysis also showed the favourable effects of NGF on
response rate. Heterogeneity was also observed between two trials
(P=0.75; I2=0%), and the fixed-effect model was used to
perform the meta-analysis (n=175; MD, 0.28; 95% CI, 0.2–0.37;
P<0.01; Fig. 3).
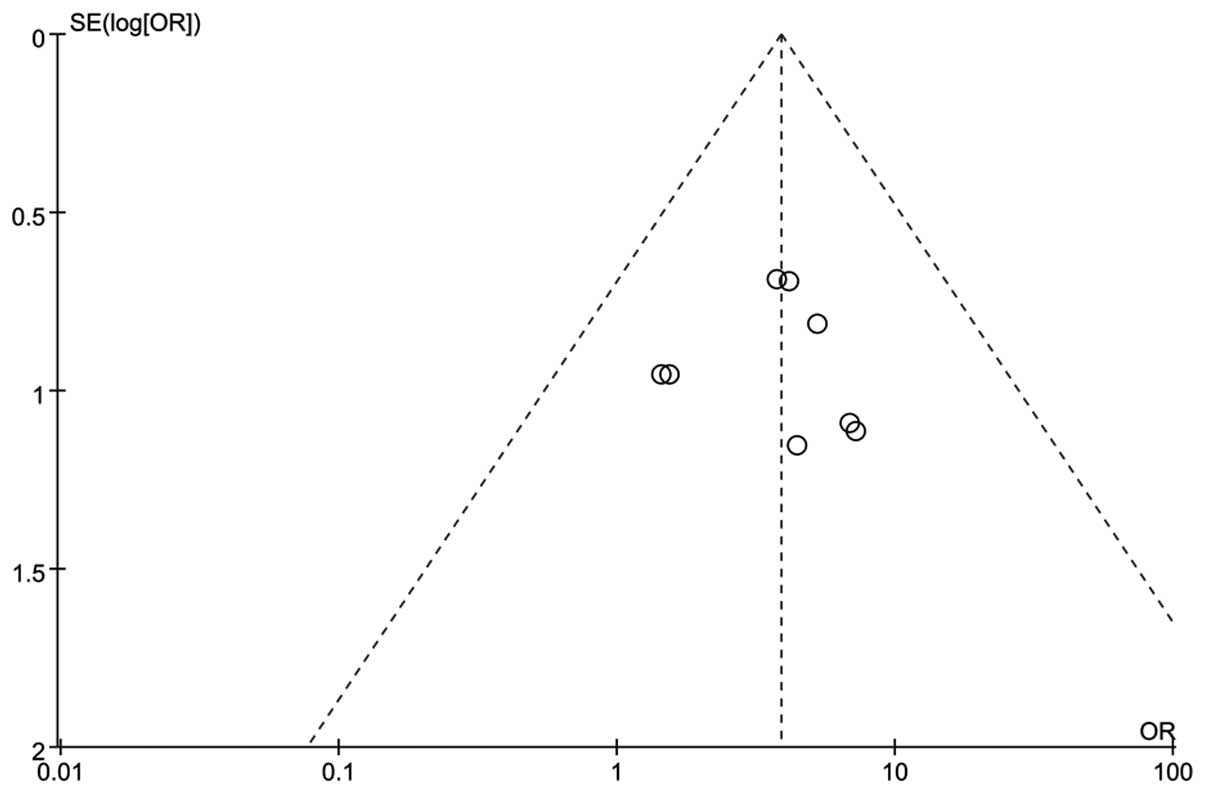
A funnel plot was constructed based on the
comparison of the total efficacy rate of two groups (NGF versus
Control), and the presence of publication bias was visually
assessed. The resultant funnel shape was almost symmetrical
(Fig. 4), thus indicating that no
potential publication bias occurred.
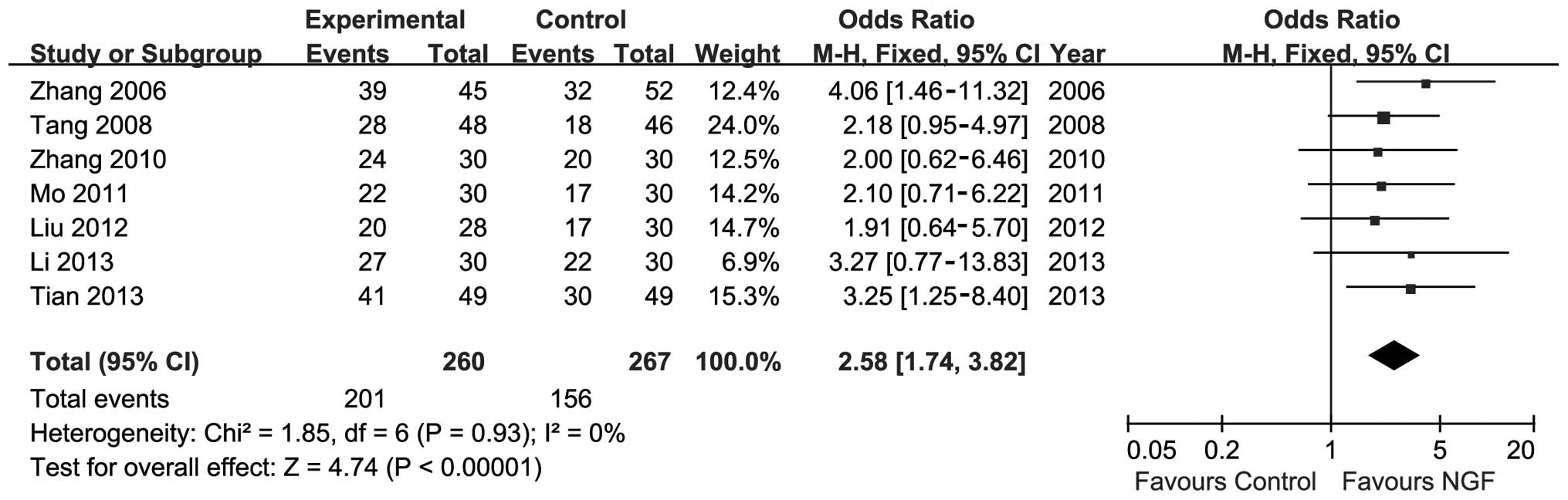
Sensitivity analysis was conducted by substituting
complete recovery for partial recovery as the primary outcome. The
meta-analysis showed significant improvements in the NGF group
(n=527; OR, 2.58; 95% CI, 1.74–3.82; P<0.01; I2=0%;
Fig. 5). Risk ratio was also used
instead of OR, and the data did not change relative to the
outcomes.
Discussion
Bell’s palsy is an idiopathic peripheral nerve palsy
that involves the facial nerve and is a common peripheral
neuropathy worldwide. Although prognosis is favorable, <30% of
patients are likely to experience sequelae and the recovery period
can last for more than two months. At present, corticosteroid and
anti-viral therapy are the most effective treatments (6,7).
Reports on the use of NGF to treat Bell’s palsy are lacking, and
few trials have examined the effects of NGF treatment on Bell’s
palsy. The results from the limited number of studies showed a more
favourable effect when NGF was used as an adjunct to conventional
drug treatment compared with conventional drug treatment alone
(17–24). However, the number of trials is
small and the risk of bias is too high to draw any solid
conclusions. The findings of the present study provide limited
evidence that NGF is beneficial to the symptomatic treatment of
Bell’s palsy.
A strong attempt was made in the present study to
search the available literature comprehensively. However, there
cannot be absolute certainty that all the relevant articles were
identified. Furthermore, selective publishing is another major
cause of bias. NGF drug has not been used in the USA or Europe;
thus, the majority of the clinical studies on NGF were conducted in
China. As such, existing potential regional bias is an issue. All
the available trails exhibited the positive effects of NGF in
treating Bell’s palsy. Considering that no other systematic study
of NGF as a facial palsy treatment is available, it was not
possible to compare the results of the present study with other
meta-analyses. The paucity and sub-optimal methodological quality
of the primary data create further shortcomings in the available
evidence, thereby markedly limiting the conclusiveness of this
systematic review (25).
Of the eight RCTs included in the present
meta-analysis, all the trials compared NGF with conventional drug
therapy versus conventional drugs only. This type of pragmatic
design may generate favourable effects on at least one outcome
measure. Considering this design, the aforementioned trials are
unable to demonstrate specific therapeutic effects (26).
Since 1986, when Levi-Montalcini and Cohen won the
Nobel Prize in Physiology or Medicine for their work on NGF
(27,28), numerous pharmaceutical companies
and research institutions have begun to identify the mechanisms of
action and protective effects of NGF. Assuming that NGF is
beneficial in the treatment of Bell’s palsy, possible mechanisms of
action are of interest. NGF has an important role in the
neurorepair process following the occurrence of damage (29). One previous study (30) arrived at the same conclusion
subsequent to using NGF to treat traumatic facial paralysis.
In conclusion, the present available trials provide
limited evidence on the effectiveness of NGF in the treatment of
Bell’s palsy. The number and quality of trials are too low to draw
firm conclusions. Further rigorous RCTs that can overcome the
numerous limitations of the current evidence are required.
References
|
1
|
Peitersen E: Bell’s palsy: the spontaneous
course of 2,500 peripheral facial nerve palsies of different
etiologies. Acta Otolaryngol Suppl. 4–30. 2002. View Article : Google Scholar
|
|
2
|
Greco A, Gallo A, Fusconi M, Marinelli C,
Macri GF and de Vincentiis M: Bell’s palsy and autoimmunity.
Autoimmun Rev. 12:323–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murakami S, Mizobuchi M, Nakashiro Y, Doi
T, Hato N and Yanagihara N: Bell palsy and herpes simplex virus:
identification of viral DNA in endoneurial fluid and muscle. Ann
Intern Med. 124:27–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorber B: Are all diseases infectious? Ann
Intern Med. 125:844–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peitersen E: Natural history of Bell’s
palsy. Acta Otolaryngol Suppl. 492:122–124. 1992. View Article : Google Scholar
|
|
6
|
Salinas RA, Alvarez G, Daly F and Ferreira
J: Corticosteroids for Bell’s palsy (idiopathic facial paralysis).
Cochrane Database Syst Rev. CD0019422010.
|
|
7
|
Lockhart P, Daly F, Pitkethly M, Comerford
N and Sullivan F: Antiviral treatment for Bell’s palsy (idiopathic
facial paralysis). Cochrane Database Syst Rev. CD0018692009.
|
|
8
|
Baugh RF, Basura GJ, Ishii LE, et al:
Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck
Surg. 149(3 Suppl): S1–S27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ebendal T: Function and evolution in the
NGF family and its receptors. J Neurosci Res. 32:461–470. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chao MV, Rajagopal R and Lee FS:
Neurotrophin signalling in health and disease. Clin Sci (Lond).
110:167–173. 2006. View Article : Google Scholar
|
|
11
|
Deppmann CD, Mihalas S, Sharma N, Lonze
BE, Niebur E and Ginty DD: A model for neuronal competition during
development. Science. 320:369–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Apfel SC: Neurotrophic factor therapy -
prospects and problems. Clin Chem Lab Med. 39:351–355. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egger M, Smith GD and Altman DG:
Systematic Reviews in Health Care: Meta-Analysis in Context. Second
edition. John Wiley & Sons, Inc; Hoboken, NJ: 2008
|
|
14
|
Sullivan FM, Swan IR, Donnan PT, et al:
Early treatment with prednisolone or acyclovir in Bell’s palsy. N
Engl J Med. 357:1598–1607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeo SG, Lee YC, Park DC and Cha CI:
Acyclovir plus steroid vs steroid alone in the treatment of Bell’s
palsy. Am J Otolaryngol. 29:163–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Diego JI, Prim MP, De Sarriá MJ, Madero
R and Gavilán J: Idiopathic facial paralysis: a randomized,
prospective, and controlled study using single-dose prednisone
versus acyclovir three times daily. Laryngoscope. 108:573–575.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suai Z, Suqin L and Xuhua C: The clinical
efficacy of the treatment of Bell’s palsy by nerve growth factor in
45 patients. Lin Chuang Shen Jing Bing Za Zhi. 19:3922006.(In
Chinese).
|
|
18
|
Xiaomei T and Ye W: Outcome of treatment
with nerve growth fator in patients with Bell’s palsy. Lin Chuang
Yi Xue. 28:23–24. 2008.(In Chinese).
|
|
19
|
Guofen Z: Outcome of treatment with nerve
growth factor in 30 patients with Bell’s palsy. Lin Chuang He Li
Yong Yao. 3:422010.(In Chinese).
|
|
20
|
Mo J, Huang Y and Zhou W: The efficacy of
mouse nerve growth factor for injection in idiopathic facial
paralysis. Zhong Guo Yi Yao Zhi Nan. 20:32–33. 2011.(In
Chinese).
|
|
21
|
Liu XD: The efficacy of mouse nerve growth
factor for idiopathic facial paralysis in 58 patients. Zhong Guo
Zhong Xi Yi Jie He Er Bi Yan Hou Ke Za Zhi. 20:462–463. 2012.(In
Chinese).
|
|
22
|
Wang Z, Ma MJ and Han Q: Analysis the
efficacy of nerve growth factor in the treatment of facial
paralysis. Shen Jing Sun Shang Yu Gong Neng Chong Jian. 7:286–288.
2012.(In Chinese).
|
|
23
|
Li GS, Wu SG, Liu XF, Li P and Long ZZ:
Clinical efficacy analysis of the nerve growth factor in the
treatment of facial neuritis. Hei Long Jiang Yi Xue. 37:927–928.
2013.(In Chinese).
|
|
24
|
Jianwei T and Chuan D: The observation of
nerve growth factor for idiopathic facial paralysis. Jian Kang Da
Shi Ye :Yi Xue Ban. 21:46–47. 2013.(In Chinese).
|
|
25
|
Schulz KF, Chalmers I, Hayes RJ and Altman
DG: Empirical evidence of bias. Dimensions of methodological
quality associated with estimates of treatment effects in
controlled trials. JAMA. 273:408–412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ernst E and Lee MS: A trial design that
generates only ‘positive’ results. J Postgrad Med. 54:214–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levi-Montalcini R: The nerve growth factor
35 years later. Science. 237:1154–1162. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen S, Levi-Montalcini R and Hamburger
V: A nerve growth-stimulating factor isolated from sarcomas 37 and
180. Proc Natl Acad Sci USA. 40:1014–1018. 1954. View Article : Google Scholar
|
|
29
|
Shao H, Shu H, Wang C, Yuan W and Li Y:
Expression of nerve growth factor and its receptor in distracted
tibial nerve after limb lengthening. Anat Rec (Hoboken).
296:333–339. 2013. View
Article : Google Scholar
|
|
30
|
Yildiz M, Karlidag T, Yalcin S, et al:
Efficacy of glial growth factor and nerve growth factor on the
recovery of traumatic facial paralysis. Eur Arch Otorhinolaryngol.
268:1127–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|