Introduction
Spinal cord injury (SCI) is a serious threat to the
health and quality of life of those affected and its treatment has
become a global issue. Brain-derived neurotrophic factor (BDNF) is
a nerve growth factor that been reported to play an important role
in the growth, development, differentiation, maintenance and damage
repair of several types of neurons in the central nervous system.
It also induces axonal regeneration and promotes neural pathways
(1–3). As a class of pluripotent stem cells,
mesenchymal stem cells (MSCs) have been widely used in cell
transplantation for the treatment of SCI. However, studies have
revealed that the lack of secretion of neurotrophic factors at the
site of injury in the spinal cord and inadequately inducing
conditions in the microenvironment influence the survival rate of
transplanted MSCs and their differentiation into neurons, resulting
in unsatisfactory neurological recovery (4–6).
Therefore, developing a procedure to maintain the long-term
survival of MSCs and raise their differentiation rate into neurons
has become an important issue for the treatment of SCI by MSC
transplantation.
With the advance of genetic modification technology,
targeted genes have been used to transfect transplanted stem cells
and stably express the gene products, thereby enhancing the effect
of cell and gene therapies by incorporating the features of the
gene. In a previous study, BDNF gene-modified MSCs were shown to
promote functional recovery and reduce infarct size in a middle
cerebral artery occlusion model of SCI (7). In the present study, the effect of
the BDNF gene on the survival rate of MSCs and the rate of their
differentiation into neuron-like cells was observed in BDNF
gene-transfected MSCs.
Materials and methods
Isolation, culture and identification of
bone marrow stromal cells (BMSCs)
Eight-week-old Sprague-Dawley (SD) rats (male or
female) were sacrificed by cervical dislocation. The femur and
tibia marrow cavities of the rats were exposed under sterile
conditions and flushed with D-Hank’s solution containing heparin
(100 U/ml). The fluid was collected and the BMSCs were isolated by
density gradient centrifugation and an adherent method. Briefly,
1×106 nucleated cells were loaded onto 25 ml of 1.073
g/ml Percoll solution and centrifuged at 1,100 × g for 30 min at
20°C. Cells were collected from the upper layer and interface,
diluted with 2 volumes of Dulbecco’s phosphate-buffered saline
(PBS) and collected by centrifugation at 900 × g. The cells were
cultured in 25-ml culture flasks at 37°C with 5% CO2 in
low glucose Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; Hyclone, Logan, UT, USA), which was changed for the
first time after 48 h and subsequently changed once every three
days. The cells were digested with 0.25% trypsin (when cell fusion
reached 80–90%), with subculture at a ratio of 1:3 (0.25%
trypsin:cells) in different tubes. BMSCs growing on glass
coverslips were washed three times with PBS and fixed with 0.3
mol/l NaCl and 75% ethanol for 30 min. Rabbit anti-mouse cluster of
differentiation (CD)34, CD44, CD45 and CD90 antibodies (BD
Biosciences, Franklin Lakes, USA) were added at a ratio of 1:100 to
the BMSCs, which were incubated at 37°C in an incubator for 1 h.
After washing twice with PBS, FITC-labeled goat anti-rabbit IgG
secondary antibody (Pierce, Rockford, IL, USA) was added and the
BMSCs were incubated at room temperature for 1 h. An inverted
fluorescence phase contrast microscope (DFM-60; Shanghai Caikon
Optical Instrument Ltd., Shanghai, China) was used to analyze the
images.
Fourth generation cells with good growing conditions
were selected to prepare single cell suspensions with
~3×105 cells/sample. Following centrifugation at 500 × g
for 5 min, the cells were resuspended in 500 μl PBS. The
resuspended cell suspension was transferred to a test tube for flow
cytometric analysis and 5 μl CD34, CD44, CD45 and CD90 antibodies
were added, respectively. The cells were incubated for 30 min at
4°C. A total of 500 μl PBS was added to the cells, which were
centrifuged at 500 × g for 5 min and the supernatant discarded.
Following the addition of 300 μl PBS and mixing, the cells were
analyzed using a flow cytometer (BD FACSCanto™ II; BD
Biosciences). The data was analyzed using CellQuest software (BD
Biosciences). The present study was approved by the Institutional
Animal Care and Use Commitee of Zhengzhou Railway Vocational and
Technical College (Zhegzhou, China).
Grouping and gene transfection
A lentiviral gene expression vector carrying hBDNF
and enhanced green fluorescent protein (eGFP) genes was produced by
Cyagen Biosciences Inc. (Guanghzou, China) and amplified from
HT1080 cells (acquired from Shanghai Meixuan Biotechnology Co.,
Ltd, Shanghai, China).
The cells were divided into three groups: hBDNF and
eGFP gene transfection group (group A), empty lentiviral vector
(LV-EGFP-0101; Cyagen Biosciences Inc.) transfection group (group
B) and untransfected group (group C). Third-generation cells were
used to perform viral transfection with a multiplicity of infection
(MOI) of 25. The solution was changed after 8 h and cultured at
37°C in a saturated humidity incubator with a CO2 volume
fraction of 0.05. The infection efficiency and cell growth were
observed under a fluorescence microscope (DFM-60; Shanghai Caikon
Optical Instrument Ltd.). G418 was added to the screen when the
transfected cells had been cultured for 48 h.
Methylthiazolyldiphenyl-tetrazolium
bromide (MTT) assay
Cells from each group were seeded into 96-well
plates with 1×104/ml cells per well with eight wells for
each group. A total of 200 μl cell culturing medium was added to
each well and 20 μl MTT was added after 24 h at room temperture.
The cells were incubated at 37°C for 4 h and the culture
supernatant was removed. A total of 150 μl dimethyl sulfoxide
(DMSO) was added to each well, the plate was oscillated for 10 min
and the absorbance (A) value of each well at a wavelength of 490 nm
was measured using a microplate reader (2550 EIA reader; Bio-Rad,
Hercules, CA, USA).
Western blot analysis
The medium of the hBDNF and eGFP gene transfection
(group A), empty lentiviral vector transfection (group B) and
non-transfected (group C) cells was aspirated and a lysis buffer
was added. The cells were lysed using ultrasound (JYD-900; Shanghai
Credibility Instrument Co., Ltd, Shanghai, China). A BCA Protein
assay kit (Pierce) was used to detect the protein concentrations. A
total of 20 μg of the protein was processed by 12% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis and electrically
transferred to a polyvinylidene difluoride (PVDF) membrane. The
membrane was sealed with 5% skimmed milk for 1 h, the mouse
anti-human GFP primary antibody (Pierce) was added and the membrane
was incubated at 4°C overnight. Following washing of the membrane
with Tris-buffered saline and Tween 20 (TBST), goat anti-mouse
IgG-HRP secondary antibody (Pierce) was added and the membrane was
incubated at room temperature for 60 min. Following washing with
TBST, the membrane was analyzed by enhanced chemiluminescence [ECL;
Santa Cruz Biotechnology (Shanghai) Co., Ltd., Shanghai, China].
The murine monoclonal anti-β-actin antibody [Santa Cruz
Biotechnology (Shanghai) Co., Ltd.]. acted as the internal
reference.
Cell differentiation
The sixth generation cells of groups A, B and C were
used for subculture. The induction of differentiation into neurons
was performed when the adherent cell fusion rate reached 80%
(8–11). The culture medium was aspirated,
and 100% FBS and a medium containing 10 ng/ml basic fibroblast
growth factor (bFGF) and DMEM/F12 were added for pre-induction for
24 h. The culture medium was aspirated and the cells were washed
three times with PBS. DMEM/F12 serum-free inducer containing 20
ml/l DMSO, l0 ng/ml bFGF, 100 μmol/l butylated hydroxyanisole
(BHA), 10 μmol/l forskolin, 25 mmol/l KCl, 2 mmol/l malonic acid
and 5 μg/ml insulin was added. Half of the inducing agent was
replaced every 24 h for three days.
Immunofluorescence
Following removal of the medium, the cells were
washed with PBS and fixed for 20 min with 4% polyformaldehyde at
room temperature. The cells were subsequently washed twice with
PBS, subjected to permeabilization with 0.1% Triton-X 100/PBS at
room temperature for 1 h, then washed with PBS three times, for 5
min each time. Fresh blocking solution was prepared, and the cells
were blocked with 5 mg/ml FBS/PBS for 30 min at room temperature.
Polyclonal rabbit anti-mouse antibody [anti-TUJ-1 antibody; 1:50;
Santa Cruz Biotechnology (Shanghai) Co., Ltd.] in blocking buffer
was added to the cells, which were incubated at room temperature
for 2 h. The cells were subsequently washed with PBS three times,
for 10 min each time. PBS was used to prepare Cy3
fluorescence-labeled goat anti-rabbit IgG secondary antibody
[1:400; Santa Cruz Biotechnology (Shanghai) Co., Ltd.], which was
added to the cells and incubated overnight at 4°C. After washing
three times with PBS, for 10 min each time, the cover slips were
incubated with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear
staining and mounted with anti-fade mounting medium. The slides
were observed under a fluorescence microscope (DFM-60; Shanghai
Caikon Optical Instrument Ltd.) and images were captured. A total
of 300 cells from each group were randomly selected following
differentiation for 24 h and TUJ-1-positive cells were counted.
Statistical analysis
Results are expressed as mean ± standard deviation.
SPSS statistical software, version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used to carry out the statistical analyses and the
factorial analysis of variance (ANOVA) was calculated. ANOVA was
used to analyze the comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Microscopic observation
The adherent growth of primary cultured cells was
visible following culture for 24 h, and a small colony appeared
after 72 h. The cells grew in a tubular shape after 7–9 days and
were basically fused after ~2 weeks. Immunofluorescence revealed
that CD44 expression was positive and expression of the other
surface antigens was negative. Following transfection by the hBDNF
and eGFP genes, the appearance of the cells did not change
significantly compared with the normal passage of cells, with long
spindle or polygonal adherent growth, although the proliferation
rate accelerated rapidly. Under a fluorescence microscope, green
fluorescence was visible after 12 h with a low extent and weak
intensity. Cells emitted strong fluorescence after 48 h,
demonstrating the distribution of the whole cells.
Determination of MSC surface markers
The results of flow cytometry detection and Cell
Quest software analysis revealed that the positive rate of
MSC-specific surface markers was 99.67% for CD90 (+), 99.81% for
CD44 (+), 0.48% for CD34 (−) and 0.03% for CD45 (−).
MTT assay
As shown in Table
I, the A values of the cells in each group were detected at 490
nm following an MTT assay. The A value in the gene transfection
group was significantly different from that in the empty
vector-transfected and non-transfection groups (P<0.01). No
statistically significant difference was identified between the
empty vector and the non-transfected groups (P>0.05).
 | Table IEffect of gene transfection on the
proliferative activity of BMSCs (n=8 per group). |
Table I
Effect of gene transfection on the
proliferative activity of BMSCs (n=8 per group).
| Group | A value |
|---|
| BMSCs transfected
with hBDNF and eGFP | 0.84±0.05 |
| BMSCs transfected
with empty vectors | 0.42±0.04a |
| Non-transfected
BMSCs | 0.50±0.04a |
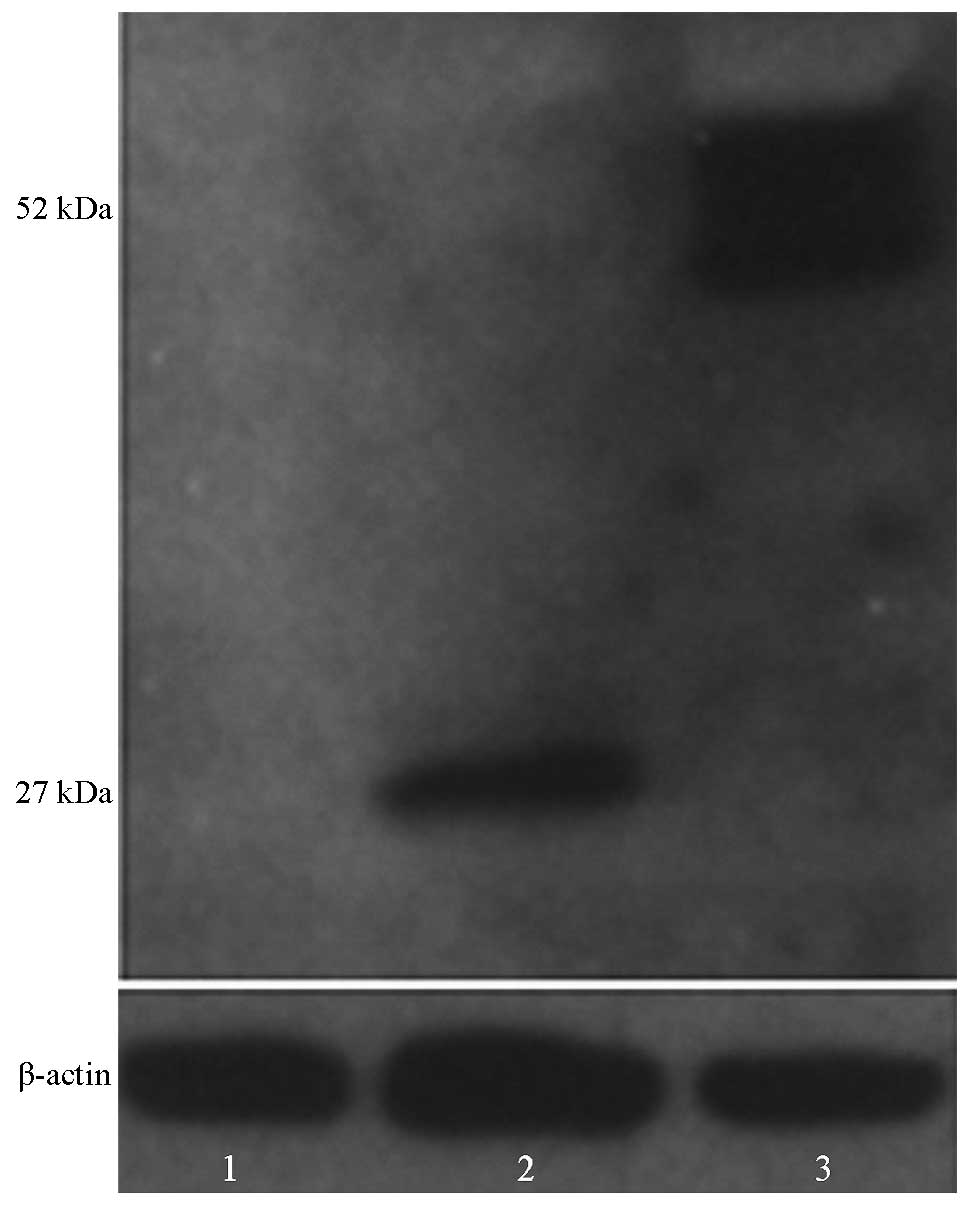
Western blot analysis
Stably expressing MSCs were obtained following BDNF
and eGFP transfection. The expression of BDNF and GFP in the cells
of the three groups was detected by western blotting using a GFP
antibody. The results revealed that BDNF-GFP bands (~52 kDa) could
be detected from the BDNF and eGFP-transfected cells via the GFP
antibody. However, only GFP (~27 kDa) was detected in the empty
vector-transfected cells (Fig.
1).
Neuronal differentiation of MSC-like
cells in vitro
Partial retraction of the cytoplasm to the nucleus
occurred in the three MSC groups following the addition of the
inducer. In addition, the cell bodies were reduced in size,
irregular or rounded in shape, with three-dimensional, surrounding
strong refraction. Frequently, several short projections and a
longer projection were observed. Neuron-like cells exhibited a
typical bipolar, multi-polar and tapered shape with a strong
refraction. The neuron-specific marker TUJ-1 was used to stain the
induced MSCs. TUJ-1 positive cells were detected in the three
groups following the addition of the inducing agent. By comparing
the TUJ-1-positive rate of the BDNF-transfected group with those of
the empty plasmid and non-transfected negative control groups, it
was demonstrated that the TUJ-1 positive rate in the experimental
BDNF-transfected group was higher compared with that in the empty
plasmid-transfected group (71.11±4.72 vs. 56.67±6.89%; P<0.05).
The difference between the empty vector-transfected and negative
control groups was not statistically significant (56.67±6.89 vs.
57.36±2.41%; P>0.05).
Discussion
SCI is a serious central nervous system trauma.
Although conventional treatment, including surgical intervention,
drug treatment and postoperative rehabilitation training, following
SCI is effective to an extent, limitations remain in the functional
recovery of the spinal cord. In recent years, with the development
of stem cell technology, the prospects for SCI treatment have
improved through alternative neuronal cell transplantation
technologies. Stem cells have a pluripotent ability and may
differentiate into neurons under certain conditions, which have a
significant recovery effect on neurological dysfunction caused by
SCI. A number of sources of stem cells are available for the repair
of SCI, including embryonic stem cells, neural stem cells, MSCs and
cord blood stem cells. Due to certain characteristics of stem
cells, including their ready availability, no transplant rejection
occurs and directed differentiation of neuronal cells occurs. MSCs
may be used as seed cells for wide application in the study of cell
transplantation in the treatment of SCI (12).
However, due to local tissue hemorrhage, edema and
cell necrosis following SCI, secondary ischemic changes and tissue
cavity formation occur gradually at the site of injury. The damaged
area lacks differentiation-inducing conditions, including
neurotrophic factors, that are essential for the seed cells to
differentiate into neurons. These factors result in low survival
rates following MSC transplantation and lack of differentiation
into neurons, which affects nerve function recovery in the
treatment of SCI by cell transplantation (7,13).
Previous studies have demonstrated that MSCs differentiate into
neuron-like cells, which is associated with expression of the
tyrosine kinase (Trk)B receptor (14–16).
TrkB is a BDNF receptor for the cellular transmembrane protein. The
binding of BDNF to TrkB receptors on the MSC cell membrane
activates a series of cell bioreactors to promote the development
of undifferentiated MSCs into mature neurons. BDNF is a member of
the neurotrophic factor family that consists of α, β and γ subunits
involved in the regulation of growth and differentiation of neurons
in the central nervous system (8).
Accordingly, gene modification technology was used in the current
study to transfect exogenous BDNF gene into the MSCs. The results
revealed that the expression of cell surface markers CD90 (+), CD44
(+), CD34 (−) and CD45 (−) by the cells was in line with that
expected for MSCs, confirming that the cultured cells were MSCs
from bone marrow and not hematopoietic cells from bone marrow.
At present, viral vectors including adenoviral,
adeno-associated viral, retroviral and lentiviral vectors, are used
for the transfer of targeted genes into seed cells, and each has
both advantages and disadvantages. However, the application of
non-viral vectors is relatively rare as their application in tissue
engineering is challenging due to their low transfection efficiency
(17,18).
In the current study, a BDNF-carrying lentiviral
vector was used to infect rat MSCs, resulting in stable expression
of the BDNF fusion protein by the MSCs. The successful transfection
of BDNF into the MSCs and secretory expression of BDNF was
confirmed by western blot analysis. The MSCs of the
BDNF-transfected, empty vector-transfected and non-transfected
negative control groups were induced to differentiate into neurons
under similar conditions. All cells of the three groups expressed
the neuronal specific antibody TUJ-1 following differentiation. The
results revealed that the differentiation rate of the BDNF
gene-modified MSCs into neural-like cells was significantly higher
compared with that of the MSCs transfected with an empty vector and
the negative control group (P<0.05), which was consistent with
the results of Jouhilahti et al (11). This suggests that BDNF played an
important role in neuron-like cell differentiation of the MSCs,
although the exact mechanism remains unclear. In conclusion, the
hBDNF gene carried by lentiviral vectors may promote the
differentiation of MSCs into neuron-like cells in vitro.
Further study is required to determine the function of the hBDNF
gene in vivo.
References
|
1
|
Kovalchuk Y, Hanse E, Kafitz KW and
Konnerth A: Postsynaptic induction of BDNF-mediated long-term
potentiation. Science. 295:1729–1734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang EJ and Reichardt LF: Neurotrophins:
roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coles LS, Diamond P, Lambrusco L, Hunter
J, Burrows J, Vadas MA and Goodall GJ: A novel mechanism of
repression of the vascular endothelial growth factor promoter, by
single strand DNA binding cold shock domain (Y-box) proteins in
normoxic fibroblasts. Nucleic Acids Res. 30:4845–4854. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuszynski MH, Peterson DA, Ray J, Baird A,
Nakahara Y and Gage FH: Fibroblasts genetically modified to produce
nerve growth factor induce robust neuritic ingrowth after grafting
to the spinal cord. Exp Neurol. 126:1–14. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Senut MC, Tuszynski MH, Raymon HK, et al:
Regional differences in responsiveness of adult CNS axons to grafts
of cells expressing human neurotrophin 3. Exp Neurol. 135:36–55.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofstetter CP, Schwarz EJ, Hess D,
Widenfalk J, El Manira A, Prockop DJ and Olson L: Marrow stromal
cells form guiding strands in the injured spinal cord and promote
recovery. Proc Natl Acad Sci USA. 99:2199–2204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
et al: Adult bone marrow stromal cells differentiate into neural
cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eriksson NP, Aldskogius H, Grant G,
Lindsay RM and Rivero-Melian C: Effects of nerve growth factor,
brain-derived neurotrophic factor and neurotrophin-3 on the laminar
distribution of transganglionically transported choleragenoid in
the spinal cord dorsal horn following transection of the sciatic
nerve in the adult rat. Neuroscience. 78:863–872. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kesser BW and Lalwani AK: Genetherapy and
stem cell transplantation: strategies for hearing restoration. Adv
Otorhinolaryngol. 66:64–86. 2009.
|
|
11
|
Jouhilahti EM, Peltonen S and Peltonen J:
Class III beta-tubulin is a component of the mitotic spindle in
multiple cell types. J Histochem Cytochem. 56:1113–1119. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siminiak T and Kurpisz M: Myocardial
replacement therapy. Circulation. 108:1167–1171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pisati F, Bossolasco P, Meregalli M, et
al: Induction of neurotrophin expression via human adult
mesenchymal stem cells: implication for cell therapy in
neurodegenerative diseases. Cell Transplant. 16:41–55. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang SK, Jun ES, Bae YC and Jung JS:
Interactions between human adipose stromal cells and mouse neural
stem cells in vitro. Brain Res Dev Brain Res. 145:141–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satomura K, Derubeis AR, Fedarko NS, et
al: Receptor tyrosine kinase expression in human bone marrow
stromal cells. J Cell Physiol. 177:426–438. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murer MG, Yan Q and Raisman-Vozari R:
Brain-derived neurotrophic factor in the control human brain, and
in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol.
63:71–124. 2001. View Article : Google Scholar
|
|
17
|
Mason MR, Tannemaat MR, Malessy MJ and
Verhaagen J: Gene therapy for the peripheral nervous system: a
strategy to repair the injured nerve? Curr Gene Ther. 11:75–89.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng N, Yin L, Song Z, et al: Maximizing
gene delivery efficiencies of cationic helical polypeptides via
balanced membrane penetration and cellular targeting. Biomaterials.
35:1302–1314. 2014. View Article : Google Scholar
|