Introduction
Dendrobium is a huge genus of orchids, and
Dendrobium candidum Wall. ex Lindl. (D. candidum) is
a synonym of Dendrobium moniliforme (L.) Sw. (1,2). It
is a traditional Chinese medicinal herb that is used raw or
processed for health care products in China (3). D. candidum contains
water-soluble polysaccharides, phenanthrenes and many amino acids.
It also has high contents of chrysotoxen and erianin, which may
have medical effects (4).
Constipation is defined medically as the passing of
fewer than three stools per week and is considered severe when
there is less than one stool per week. It may occur when the colon
absorbs too much water (5). In the
current study, activated carbon was orally administered to mice to
generate a model of constipation. Activated carbon attaches to the
mucosal surfaces of the gastrointestinal (GI) tract and reduces
drainage. This causes a reduction of GI fluids and slows down GI
movement, resulting in weakness of the spleen and stomach, and
ultimately to constipation.
The model of constipation induced by activated
carbon has been used to demonstrate the effects of drugs in the
treatment of constipation (6,7). A
megadose of activated carbon has been shown to result in digestive
tract obstruction (8). In the
present study, the functional effects of D. candidum in the
alimentary tract were investigated in a mouse model of activated
carbon-induced constipation. GI transit, time to the first
defecation of a black stool, and serum levels of motilin (MTL),
gastrin (Gas), endothelin (ET), somatostatin (SS),
acetylcholinesterase (AChE), substance P (SP) and vasoactive
intestinal peptide (VIP) were examined. Bisacodyl, a laxative drug
that stimulates intestinal peristalsis and acts directly on the
colon to produce a bowel movement, was used as a positive control.
Bisacodyl is typically prescribed for the relief of constipation
and for the management of neurogenic bowel dysfunction, as well as
for bowel preparation prior to medical examinations (9–11).
The current study was designed to investigate the
anti-constipation effects of D. candidum on activated
carbon-induced constipation and also to elucidate the association
of its protective effects with the chemical compounds it
contains.
Materials and methods
Preparation of D. candidum
A sample of D. candidum was purchased from
Shanghai Pharmacy Co., Ltd. (Shanghai, China). The D.
candidum was stored at −80°C and freeze-dried to produce a
powder. A twenty-fold volume of boiling water was added to the
powdered sample and the sample was extracted twice by stirring
overnight. The aqueous extract was evaporated and concentrated
using a rotary evaporator (N-1100; Eyala, Tokyo, Japan).
Animals
Seven-week-old female ICR mice (n=100) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The mice were maintained in a
temperature- and humidity-controlled (temperature 25±2°C, relative
humidity 50±5%) facility with a 12-h light/dark cycle and free
access to a standard rat chow diet and water. All experimental
procedures carried out in the present study were approved by the
Ethics Committee of Chongqing Medical University
Component analysis by nuclear magnetic
resonance (NMR)
Dried D. candidum was refluxed and extracted
3 times with a 10-fold quantity of ethyl acetate. An ethyl acetate
extract was obtained after 1 h for every reflux extraction and
concentrated by evaporation under reduced pressure. The combined
ethyl acetate extracts were extracted with anhydrous ethanol 3
times. The ethanol extract was suspended in water, and extracted by
petroleum ether, chloroform and butanol extraction, respectively.
The ethyl acetate extract was treated by gradient elution in a
silica gel (Shanghai Xinhuo Silica Gel Factory, Shanghai, China)
column with a petroleum ether-ethyl acetate solvent system. Then,
the chloroform extract was treated by gradient elution in a silica
gel column with a petroleum chloroform-methanol system. The butanol
extract was treated with water with ultrasonic irradiation, and the
extracting solution was isolated after filtering. The extract was
then eluted using an HP2MGL macroporous resin (Mitsubishi Chemical
Corporation, Tokyo, Japan) column with water, 10% ethanol, 30%
ethanol and 60% ethanol, respectively. After elution, the various
solvents contained different compounds, and their compositions were
determined by NMR (Varian Inova 400; Varian Inc., Palo Alto, CA,
USA). NMR was conducted using the following settings: 1H
frequency, 300 MHz; temperature, 25°C; pulse length, 8 μsec; spin
speed, 20 Hz; and scan number, 64 times. The 1H NMR
spectra were recorded using a standard high-resolution magic angle
spinning probe with magic-angle gradient.
Induction of constipation in mice
To investigate the preventive effects of D.
candidum against activated carbon-induced constipation, the
animals were divided into five groups with 20 mice in each. The
experimental design was as follows: the normal and control groups
were fed a normal diet for 9 days; the high and low concentration
D. candidum groups received 400 and 200 mg/kg body weight of
the aqueous extract orally in a volume of 2 ml; and the drug cure
group mice were treated with a 100 mg/kg dose of bisacodyl
dissolved in water for 9 days. The control and treatment groups
received an oral administration of activated carbon (0.2 ml of 10%
activated carbon, w/w; activated carbon dissolved in 10% arabic
gum) at 18:00 from the sixth to ninth day to induce constipation
(11). The body weight, stool
weight and stool moisture content were determined at 09:00 every
day.
Measurement of the defecation status of
the mice
This measurement was performed to determine whether
the prokinetic action of D. candidum was capable of
propagating a prokinetic signal along the length of the GI tract.
The excreted fecal pellets of individual mice were collected daily
at 09:00 for the duration of the experiment. The total number,
weight and water content of the pellets were determined. The water
content was calculated as the difference between the wet and dry
weight of the pellet. After 16 h, the mice in the control and
treatment groups received 10% activated carbon and the normal group
was administered 10% arabic gum by intragastric gavage. The animals
were then placed individually in small transparent cages and
allowed access to food and tap water ad libitum. The length
of time from carbon meal administration to the appearance of
darkened feces was recorded. Feces were collected, counted, weighed
and their water content was evaluated.
GI transit and defecation time
Mice were fasted for 16 h from the ninth day at
18:00; however, they were not deprived of water. After 16 h, the
mice in the control and treatment groups received an oral
administration of 10% activated carbon while the mice in the normal
group received an oral administration of 10% arabic gum. After 30
min, the mice were sacrificed by cervical dislocation under
anesthesia with diethyl ether. A total of 10 mice in each group
were dissected and the small intestine from the pylorus to the
cecum was carefully removed. The GI transit of each mouse was
calculated as the percentage of the distance traveled by the
activated carbon meal relative to the total length of the small
intestine. The following equation was used to calculate GI transit:
GI transit (%) = distance traveled by the activated carbon/total
length of the small intestine ×100. The remaining 10 mice of each
group were used to measure the time until the first defecation of
the black stool following the oral administration of 10% activated
carbon.
Levels of MTL, Gas, ET, SS, AChE, SP and
VIP in the serum
The levels of MTL, Gas, ET, SS, AChE, SP and VIP in
the serum were determined using radioimmunoassay kits (Beijing Puer
Weiye Biotechnology Co., Ltd., Beijing, China). The serum was
collected from the heart following surgery.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Differences between the mean values for individual groups
were assessed by one-way analysis of variance (ANOVA) with Duncan’s
multiple range test. P<0.05 was considered to indicate a
statistically significant difference. SAS version 9.1 (SAS
Institute Inc., Cary, NC, USA) was used to conduct the statistical
analyses.
Results
Constituents of D. candidum leaf
Eleven compounds were isolated and identified in
D. candidum leaf. Compound 1 was obtained as a clear
crystal; the 1H-NMR spectrum of this compound exhibited
peaks at δ 6.92 (2H, d), 6.62 (2H, d), 6.06 (2H, s), 6.03 (1H, s)
and 2.65 (4H, m), consistent with this compound being
dihydro-resveratrol. Compound 2 was obtained as a white powder; the
1H-NMR spectrum of this compound exhibited peaks at δ
6.98 (2H, d), 6.74 (2H, d), 6.62 (1H, s), 6.47 (1H, d), 4.83 (1H,
d), 4.63 (1H, d), 3.1–3.8 (12H), 3.73 (3H, s), 3.69 (3H, s) and
2.74 (4H, m), indicating that it was dendromoniliside E. Compound 3
was obtained as a black red needle; the 1H-NMR spectrum
of this compound exhibited peaks at δ 11.00 (1H, s), 8.15 (1H, d),
6.06 (2H, s), 8.07 (1H, d), 6.95 (1H, s), 6.83 (1H, s), 6.15 (1H,
s), 3.96 (3H, s) and 3.93 (3H, s), indicating that it was
denbinobin. Compound 4 was obtained as a colorless needle; the
1H-NMR spectrum of this compound exhibited peaks at δ
4.72 (2H, m), 3.85 (1H, d), 6.06 (2H, s), 2.53 (1H, d), 2.49 (1H,
t), 2.39 (1H, dd), 2.21 (1H, dd), 1.64 (1H, m), 1.35 (3H, s), 1.03
(3H, d) and 0.95 (3H, d), which suggests that this material was
aduncin. Compound 5 was obtained as a white needle; the
1H-NMR spectrum of this compound exhibited peaks at δ
8.25 (1H, s), 8.10 (1H, s), 5.90 (1H, d), 4.66 (1H, dd) and 3.5–4.2
(4H, m), and it was confirmed that material this was adenosine.
Compound 6 was obtained as a white powder; the 1H-NMR
spectrum of this compound exhibited peaks at δ 7.95 (1H, d), 5.85
(1H, d), 5.66 (1H, d) and 3.2–4.3 (5H, m), confirming that the
material was uridine. Compound 7 was obtained as a clear crystal;
the 1H-NMR spectrum of this compound exhibited peaks at
δ 10.60 (1H, s), 7.92 (1H, s), 6.45 (2H, s), 5.66 (1H, d), 3.4–4.4
(5H, m), indicating that the material was guanosine. Compound 8 was
obtained as a white powder; the 1H-NMR spectrum of this
compound exhibited peaks at δ 7.65 (1H, d), 7.41 (2H, d), 6.85 (2H,
d), 6.33 (1H, d), 4.17 (2H, t), 1.69 (2H, m), 1.25 (54H, m) and
0.85 (3H, t), indicating that this material was defuscin. Compound
9 was obtained as a white powder; the 1H-NMR spectrum of
this compound exhibited peaks at δ 7.45 (2H, d), 6.82 (2H, d), 6.81
(1H, d), 5.83 (1H, d), 4.16 (2H, t), 1.67 (2H, m), 1.23 (54H, m)
and 0.88 (3H, t), indicating that this material was
n-triacontyl cis-p-coumarate. Compound 10 was
obtained as a white powder; the 1H-NMR spectrum of this
compound exhibited peaks at δ 2.35 (2H, t), 1.62 (2H, m), 1.25
(24H, m) and 0.88 (3H, t), consistent with the material being
hexadecanoic acid. Compound 11 was obtained as a white powder; the
1H-NMR spectrum of this compound exhibited peaks at δ
3.85 (2H, t), 1.75 (2H, m), 1.45 (2H, m), 1.22 (54H, m) and 0.85
(3H, t), and was identified to be hentriacontane.
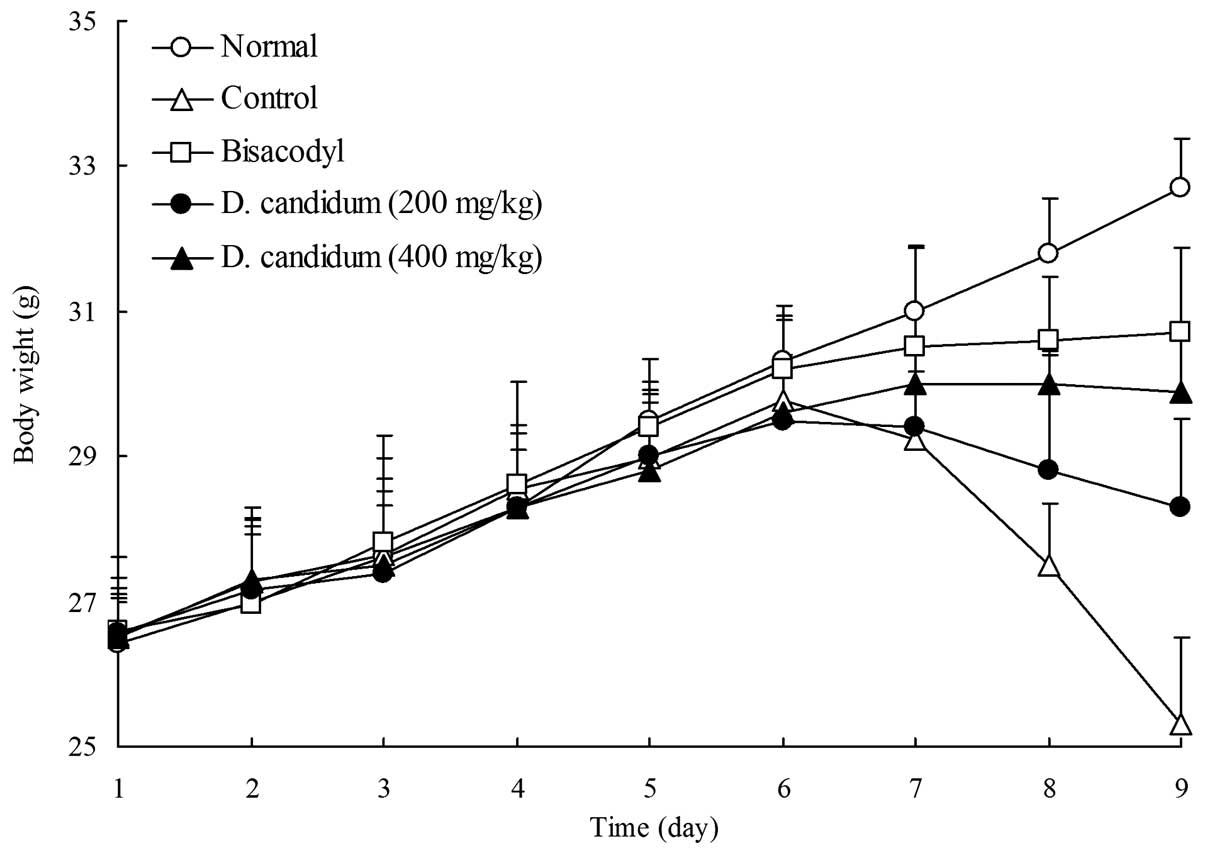
Body weight during the experiment
The body weights of the control mice with activated
carbon-induced constipation were significantly decreased after six
days. As shown in Fig. 1,
following the initiation of activated carbon-induced constipation,
the body weights of the mice in the D. candidum-treated
groups were significantly lower compared with those of the normal
mice and the bisacodyl-treated group, but higher than those of the
control mice with activated carbon-induced constipation. The high
dose of 400 mg/kg D. candidum alleviated the weight loss of
the mice to a greater extent than the lower 200 mg/kg dose.
Effect of D. candidum on defecation
status of mice
From the first to the sixth day, defecation weight,
particle counts of defecation and water content of defecation in
each group were not significantly different, although the
defecation weight and particle counts of defecation in the
bisacodyl group were slightly greater than those in the other
groups (Table I). When
constipation was induced, from the seventh day to the ninth day,
the defecation weight, particle counts of defecation and water
content of defecation were decreased to 0.31 g, 16 pieces and 18%,
respectively, in the control group. Particle counts were decreased
to 0.78 g (32 pieces), 0.58 g (22 pieces) and 0.67 g (27 pieces),
respectively, and the water content of defecation was decreased to
41, 26 and 35% in the bisacodyl and 200 and 400 mg/kg D.
candidum dose groups, respectively. These results demonstrated
that D. candidum was able to relieve constipation and had a
good effect in the treatment of constipation.
 | Table IDefecation status of the various
groups of mice during the experiment. |
Table I
Defecation status of the various
groups of mice during the experiment.
| | | | D. candidum
(mg/kg) |
|---|
| | | |
|
|---|
| Treatment | Normal | Control | Bisacodyl | 200 | 400 |
|---|
| Days 1–6a |
| Defecation weight
(g) | 0.92±0.07d | 0.93±0.08d | 1.04±0.08c | 0.94±0.03d | 0.96±0.06d |
| Particle counts of
defecation | 34±5d | 35±3d | 44±3c | 35±4d | 36±4d |
| Water content of
defecation (%) | 45±5d | 46±3d | 50±6c | 45±5d | 47±4c,d |
| Days 7–9b |
| Defecation weight
(g) | 0.93±0.06c | 0.31±0.03g | 0.78±0.11d | 0.58±0.03f | 0.67±0.05e |
| Particle counts of
defecation | 35±4c | 16±5g | 32±4d | 22±4f | 27±5e |
| Water content of
defecation (%) | 46±3c | 18±5g | 41±5d | 26±5f | 35±6e |
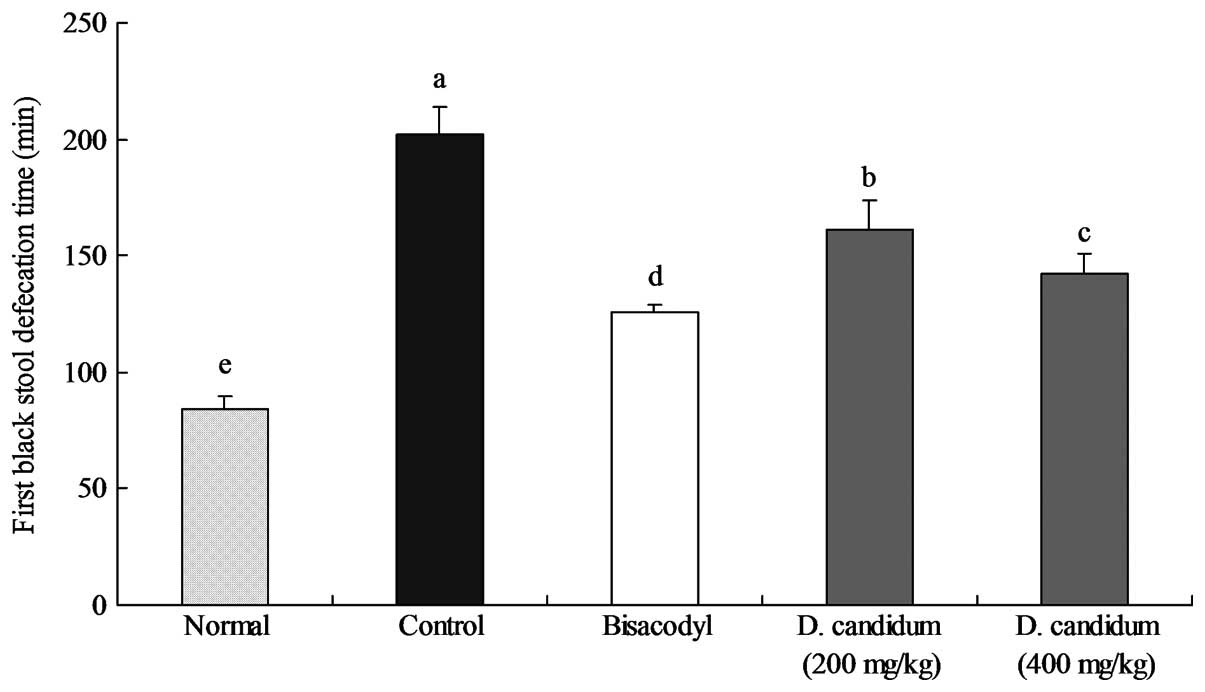
Time taken to the first defecation of a
black stool
The time taken for the first defecation of a black
stool in each group of mice following the administration of
activated carbon, which demonstrates the constipation-inhibiting
effect of the different treatments, is shown in Fig. 2. The defecation time was the
shortest (84±6 min) in the normal group and the longest (202±12
min) in the control group; the defecation time in the bisacodyl
group was 126±3 min, higher only than that of the normal group. The
times taken for the first defecation of a black stool for the mice
treated with 200 and 400 mg/kg D. candidum were 161±9 and
142±6 min, respectively. According to the defecation time, D.
candidum demonstrated a strong effect as an inhibitor of
constipation.
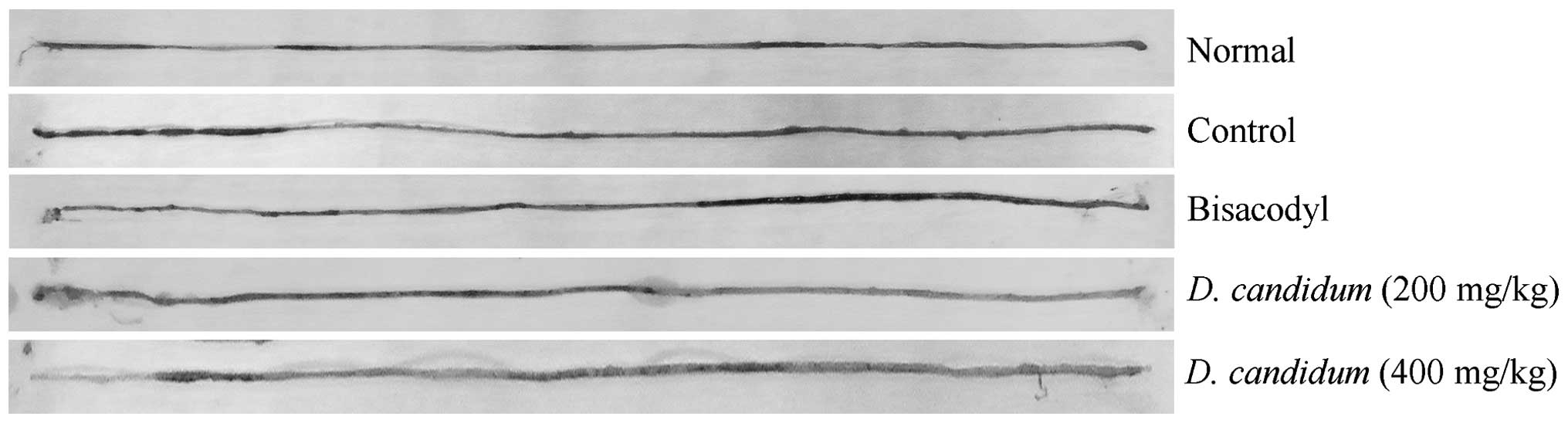
GI transit
The constipation-inhibiting effects of the
treatments were determined by GI transit in mice following the
administration of activated carbon (0.2 ml/mouse, 10% activated
carbon). In the bisacodyl-treated group, the mean GI transit was
90.2±4.8%, which was higher than that of the control group
(48.8±6.1%; Fig. 3). The GI
transits of the mice in the 200 and 400 mg/kg D. candidum
treatment groups were 57.7±4.6 and 74.6±2.8%, respectively. D.
candidum increased the GI transit compared with that of the
control, reduced constipation and had an increased functional
effect on GI transit.
MTL, Gas, ET, SS, AChE, SP and VIP levels
in the serum
Normal mice showed the highest MTL, Gas, ET, AChE,
SP and VIP levels; however, these levels in the control mice were
significantly decreased (P<0.05, Table II). The levels of these analytes
in the bisacodyl-treated mice were most similar to those of the
normal mice. In the D. candidum-treated mice, these analyte
levels were also comparable with those in the normal mice and
higher than those of the control mice. Additionally, the mice
treated with 400 mg/kg D. candidum exhibited higher levels
than did the mice treated with 200 mg/kg D. candidum. The
higher dose helped to promote these analyte levels to similar
values as were observed in the normal and bisacodyl-treated mice.
The SS levels in mice showed the opposite tendency.
 | Table IIEffect of D. candidum on serum
MTL (motilin), Gas (gastrin), ET (endothelin), SS (somatostatin),
AchE (acetylcholine enzyme), SP (substance P) and VIP (vasoactive
intestinal peptide) levels in activated carbon-induced constipation
model mice. |
Table II
Effect of D. candidum on serum
MTL (motilin), Gas (gastrin), ET (endothelin), SS (somatostatin),
AchE (acetylcholine enzyme), SP (substance P) and VIP (vasoactive
intestinal peptide) levels in activated carbon-induced constipation
model mice.
| | | | D. candidum
(mg/kg) |
|---|
| | | |
|
|---|
| Analyte
(pg/ml) | Normal | Control | Bisacodyl | 200 | 400 |
|---|
| MTL | 166.3±10.8a | 87.2.3±9.2e | 150.2±8.2b | 111.7±8.7d | 142.6±7.1c |
| Gas | 83.1±3.8a | 40.4±4.1e | 70.2±3.1b | 53.1±2.1d | 66.1±3.1c |
| ET | 14.6±0.6a | 6.4±0.5e | 12.6±0.5b | 7.4±0.4d | 11.7±0.2c |
| SS | 34.1±.1.2e | 69.2±4.3a | 38.9±2.1d | 50.8±2.2b | 41.1±0.3c |
| AchE | 33.8±1.4a | 11.6±0.3e | 28.2±1.1b | 17.7±0.8d | 24.2±0.3c |
| SP | 69.2±3.1a | 31.7±1.1e | 62.1±1.5b | 47.1±1.5d | 57.1±1.3c |
| VIP | 56.2±1.3a | 32.6±1.3e | 51.2±1.8b | 38.2±1.6d | 45.5±1.1c |
Discussion
The definition of constipation includes infrequent
bowel movements and difficulty during defecation (12,13).
Constipation most commonly occurs when the stool that forms after
food is digested moves too slowly as it passes through the
digestive tract. Dehydration, changes in diet and activity, and
certain drugs are frequently responsible for the slow transit of
stools. When stools move slowly, too much water is absorbed from
the stool and it becomes hard and dry (14). Defecation status, stool defecation
time and GI transit are important standards when investigating
constipation.
The serum levels of MTL, Gas, ET, AChE, SP and VIP
in patients with constipation are lower than those in healthy
individuals while the SS levels are higher (15–17).
The main function of MTL is to increase the migrating myoelectric
complex component of GI motility and stimulate the production of
pepsin. It is one of the intestinal hormones responsible for the
proper filling and emptying of the GI system in response to food
intake, as well as hunger stimuli and responses (18). Gas is a polypeptide hormone
secreted by certain cells of the pyloric glands, which strongly
stimulates the secretion of gastric acid and pepsin, and weakly
stimulates the secretion of pancreatic enzymes and gallbladder
contraction (15). Gas produces
effects throughout the GI tract, including promoting GI secretion,
increasing GI movement and promoting pyloric sphincter relaxation.
ET plays an important role in the stability of vascular tension and
maintains the basic cardiovascular system. Constipation, in
addition to causing intestinal obstruction, also induces or
aggravates cardiocerebrovascular diseases in the elderly (19). An SS analog, octreotide, has been
reported to stimulate intestinal motor complexes and this agent has
been used to treat sclerodermatous pseudo-obstruction (20). Stools are formed from the
non-digestible components of food after water is either absorbed or
secreted in the large intestine. Mucous is also produced in the
large intestine to provide viscosity. Thin segments of muscle line
the intestinal tract and contract and relax in concert to propel
the stool forward. Muscle contraction and mucous secretion are
regulated by acetylcholine (21).
Patients with slow-transit constipation have abnormalities of the
neurotransmitters in the muscular layer of their intestinal walls.
These abnormalities include a deficiency of a peptide known as SP,
which is thought to contribute to peristalsis (22). Disturbances in the normal neural
content of VIP in the bowel wall in idiopathic constipation and
diverticular disease may initiate or contribute to the functional
changes observed in these disorders (23).
D. candidum has been used as a traditional
Chinese medicine. It has been reported to have various therapeutic
effects on numerous pathologic conditions such as inflammation,
immunity, hyperglycemic and cancer (24). However, to the best of our
knowledge, there are no scientific studies concerning its
anti-constipation effects. In the present study, 11 compounds were
isolated from the leaf of D. candidum. These compounds might
possess anti-constipation activities and, with the high content of
functional compounds, may be the reason why D. candidum
demonstrated good functional effects in the prevention of
constipation. In addition, the synergistic activities of the
various bioactive components may increase the anti-constipation
effect of D. candidum. Resveratrol has been recommended for
the prevention of constipation (25), and adenosine A1 receptor
antagonists have been suggested to be of therapeutic potential in
constipation (26). Uridine,
guanosine, hexadecanoic acid and hentriacontane have also
demonstrated an association with decreased constipation (27–29).
Aduncin, a component only found in Dendrobium, may have
constipation-preventing effects; however, its functional effects
require investigation (30).
Defuscin, n-triacontyl cis-p-coumarate, hexadecanoic
acid and hentriacontane have also demonstrated showed numerous
functional activities in human health (31,32).
In summary, the present study found that D.
candidum has potent in vivo anti-constipation
activities, provided by various functional components. The
scientific data concerning serum content, defecation status, GI
transit and defecation time demonstrated the functional effects and
provide a scientific basis for the development of D.
candidum.
Acknowledgements
This study was supported by Program for Innovation
Team Building at Institutions of Higher Education in Chongqing (no.
KJTD201325).
References
|
1
|
Jones WE, Kuehnle AR and Arumuganathan K:
Nuclear DNA content of 26 orchids (Orchidaceae) genera with
emphasis on Dendrobium. Ann Bot. 82:189–194. 1998. View Article : Google Scholar
|
|
2
|
Wang Q, Sun P, Li G, Zhu K, Wang C and
Zhao X: Inhibitory effects of Dendrobium candidum Wall ex Lindl. on
azoxymethane- and dextran sulfate sodium-induced colon
carcinogenesis in C57BL/6 mice. Oncol Lett. 7:493–498.
2014.PubMed/NCBI
|
|
3
|
Xiao F and Zhang JZ: First report of
Fusarium oxysporum causing wilt of Dendrobium candidum in Zhejiang
Province, China. Plant Dis. 96:13772012. View Article : Google Scholar
|
|
4
|
Shao H, Zhang LQ, Li JM and Wei RC:
Advances in research of Dendrobium officinale. Zhong Cao Yao.
35:109–111. 2004.(In Chinese).
|
|
5
|
Ueki A and Otsuka M: Life style risks of
Parkinson’s disease: Association between decreased water intake and
constipation. J Neurol. 251:18–23. 2004. View Article : Google Scholar
|
|
6
|
Wexner SD, Beck DE, Baron TH, Fanelli RD,
Hyman N, Shen B, et al: American Society of Colon and Rectal
Surgeons; American Society for Gastrointestinal Endoscopy; Society
of American Gastrointestinal and Endoscopic Surgeons: A consensus
document on bowel preparation before colonoscopy: prepared by a
task force from the American Society of Colon and Rectal Surgeons
(ASCRS), the American Society for Gastrointestinal Endoscopy
(ASGE), and the Society of American Gastrointestinal and Endoscopic
Surgeons (SAGES). Gastrointest Endosc. 63:894–909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farrugia G, Miller SM, Rich A, Liu X,
Maines MD, Rae JL and Szurszewski JH: Distribution of heme
oxygenase and effects of exogenous carbon monoxide in canine
jejunum. Am J Physiol. 274:G350–G358. 1998.PubMed/NCBI
|
|
8
|
Farrugia G, Lei S, Lin X, Miller SM, Nath
KA, Ferris CD, et al: A major role for carbon monoxide as an
endogenous hyperpolarizing factor in the gastrointestinal tract.
Proc Natl Acad Sci USA. 100:8567–8570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller SM, Reed D, Sarr MG, Farrugia G and
Szurszewski JH: Haem oxygenase in enteric nervous system of human
stomach and jejunum and co-localization with nitric oxide synthase.
Neurogastroenterol Motil. 13:121–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue L, Farrugia G, Miller SM, Ferris CD,
Snyder SH and Szurszewski JH: Carbon monoxide and nitric oxide as
coneurotransmitters in the enteric nervous system: evidence from
genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA.
97:1851–1855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian Y, Zhao X and Kan J: Preventive
effect of resistant starch on activated carbon-induced constipation
in mice. Exp Ther Med. 6:228–232. 2013.PubMed/NCBI
|
|
12
|
Walia R, Mahajan L and Steffen R: Recent
advances in chronic constipation. Curr Opin Pediatr. 21:661–666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emmanuel AV, Tack J, Quigley EM and Talley
NJ: Pharmacological management of constipation. Neurogastroenterol
Motil. 21:41–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lubowski DZ, Chen FC, Kennedy Ml and King
DW: Results of colectomy for severe slow transit constipation. Dis
Colon Rectum. 39:23–29. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sjölund K, Ekman R, Akre F and Lindner P:
Motilin in chronic idiopathic constipation. Scand J Gastroenterol.
21:914–918. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
el-Salhy M and Norrgård O: Colonic
neuroendocrine peptide levels in patients with chronic idiopathic
slow transit constipation. Ups J Med Sci. 103:223–230. 1998.
View Article : Google Scholar
|
|
17
|
Silkoff P, Karmeli F, Goldin E, Ewenson A,
Gilon C, Chorev M, et al: Effect of substance P on rat
gastrointestinal transit. Dig Dis Sci. 33:74–77. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feighner SD, Tan CP, McKee KK, Palyha OC,
Hreniuk DL, Pong SS, et al: Receptor for motilin identified in the
human gastrointestinal system. Science. 284:2184–2188. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Preston DM, Adrian TE, Christofides ND,
Lennard-Jones JE and Bloom SR: Positive correlation between
symptoms and circulating motilin, pancreatic polypeptide and
gastrin concentrations in functional bowel disorders. Gut.
26:1059–1064. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soudah HC, Hasler WL and Owyang C: Effect
of octreotide on intestinal motility and bacterial overgrowth in
scleroderma. N Engl J Med. 325:1461–1467. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzavella K, Riepl RL, Klauser AG,
Voderholzer WA, Schindlbeck NE and Müller-Lissner SA: Decreased
substance P levels in rectal biopsies from patients with slow
transit constipation. Eur J Gastroenterol Hepatol. 8:1207–1211.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milner P, Crowe R, Kamm MA, Lennard-Jones
JE and Burnstock G: Vasoactive intestinal polypeptide levels in
sigmoid colon in idiopathic constipation and diverticular disease.
Gastroenterology. 99:666–675. 1990.PubMed/NCBI
|
|
24
|
Bao LJ, Wang JH and Luo JP: Inhibitory
effects of water extracts from four species of Dendrobiums on
HelaS3 cells and HepG2 cells. J Anhui Agri Sci. 36:15968–15970.
2008.(In Chinese).
|
|
25
|
Baile CA, Yang JY, Rayalam S, Hartzell DL,
Lai CY, Andersen C and Della-Fera MA: Effect of resveratrol on fat
mobilization. Ann NY Acad Sci. 1215:40–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christofi FL: Unlocking mysteries of gut
sensory transmission: is adenosine the key? News Physiol Sci.
16:201–207. 2001.PubMed/NCBI
|
|
27
|
Kunwar RM, Burlakoti C, Chowdhary CL and
Bussmann RW: Medicinal plants in farwest Nepal: Indigenous uses and
pharmacological validity. Med Aromat Plant Sci Biotechnol. 4:28–42.
2010.
|
|
28
|
Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL,
Rahman K and Qin LP: Antidepressant properties of bioactive
fractions from the extract of Crocus sativus L. J Nat Med.
64:24–30. 2010. View Article : Google Scholar
|
|
29
|
Gilani AH, Aziz N, Ali SM and Saeed M:
Pharmacological basis for the use of peach leaves in constipation.
J Ethnopharmacol. 73:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC,
Tong Y and Zhang KY: Review of research on Dendrobium, a prized
folk medicine. Appl Microbiol Biotechnol. 93:1795–1803. 2002.
View Article : Google Scholar
|
|
31
|
Benoit SC, Kemp CJ, Elias CF, Abplanalp W,
Herman JP, Migrenne S, et al: Palmitic acid mediates hypothalamic
insulin resistance by altering PKC-theta subcellular localization
in rodents. J Clin Invest. 119:2577–2589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang Q, Liang ZS, Wang JR and Xu WH:
Essential oil composition of Salvia miltiorrhiza flower. Food Chem.
113:592–594. 2009. View Article : Google Scholar
|