Introduction
Left-to-right shunt congenital heart disease (CHD)
causes pulmonary vascular wall endothelial cell damage by powerful
stretch stimulation and high shear stress due to the impact of a
large flow of blood through the pulmonary circulation (1). Endothelial injury destroys the
endothelial barrier function and the muscle-endothelial connection
between endothelial and smooth muscle cells. It also destroys the
balance between vascular endothelial cells and vasoactive
substances produced by the pulmonary circulation, as well as the
regulation of endothelial cell and smooth muscle cell formation,
thus contributing to the proliferation, hypertrophy and
disorganization of the pulmonary vascular smooth muscle cells and
causing pulmonary vascular structural remodeling. It has been
demonstrated that the nuclear factor-κB (NF-κB) activation pathway
exists in the vascular endothelium, smooth muscle cells and cardiac
myocytes (2,3). In the present study, the method of
establishing animal models of high pulmonary blood flow was used to
measure the NF-κB activity of the experimental and control groups,
as well as pulmonary artery pressure and NF-κB activity changes
subsequent to the administration of an NF-κB inhibitor [pyrollidine
dithiocarbamate (PDTC)]. The aim of the study was to determine the
correlation between NF-κB activation and pulmonary hypertension in
a high blood flow model.
Materials and methods
Animals
Fifty four-week-old male Wistar rats (purchased from
the Experimental Animal Center of Shandong University School of
Medicine, Jinan, China) with an average weight of 120 g were
randomly divided into four groups by computer: Surgical shunt group
(Tn, n=15); surgery + PDTC administration group (Ti, n=15); sham
surgery group (Co, n=10) and negative control group (Cn, n=10). The
present study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Shandong University.
High pulmonary blood flow modeling
The 30 rats from the Tn and Ti groups underwent
carotid artery-external jugular vein anastomosis to establish a
left-to-right shunt pulmonary hypertension model. Fifteen rats in
the Ti group were injected intraperitoneally with PDTC 1 h prior to
surgery with a dose of 120 mg/kg/day for two weeks of continuous
infusion. With the exception of the common carotid artery-jugular
venous anastomosis, the rats in the Co group underwent identical
surgical procedures to the experimental groups. The rats underwent
12 weeks of ad libitum under specific pathogen-free
conditions.
Measurement of pulmonary artery
pressure
Chloral hydrate (10%, 0.3 ml/100 g; Sigma-Aldrich,
St. Louis, MO, USA) was used to anesthetize the rats. A 3F right
heart catheterization was performed by inserting the catheter into
the right ventricle of the rats via the right external jugular vein
(in perspective). The right ventricular systolic pressure was
recorded through a pressure sensor pipeline (Omega, Stamford, CT,
USA), and was assumed to be theoretically equivalent to the
pulmonary artery systolic pressure (PASP).
Measurement of cardiac morphological
indicators
The heart was cut at the place where the right
ventricle and the ventricular septum meet. The weight of the right
and the left ventricles were obtained using an electronic balance
(Adventurer AR1530; Mettler Toledo, Shanghai, China), and the right
to left ventricle plus septum weight ratio was calculated.
Measurement of pulmonary vascular
index
Lung tissue sections were stained with hematoxylin
and eosin and the morphology of the pulmonary artery was observed
under a light microscope (Olympus BX41; Olympus Corporation, Tokyo,
Japan). The external diameter (ED) and media wall thickness of the
middle artery (50–200 μm in diameter) was measured and at least 10
middle pulmonary arteries were measured. The mean percentage of the
media wall thickness in the artery was calculated using the
following formula: (MT% = 2×MT/ED×100%), where MT is the medial
wall thickness.
Determination of NF-κB activity
Following thoracotomy, the pulmonary artery was
rinsed with phosphate-buffered saline (PBS), extracted and then
placed in 4°C PBS (ph=7.4) with 2 × 105 U/l penicillin
and 200 mg/l streptomycin, and the pulmonary artery endothelial
cells were isolated and cultured. The medium was aspirated and
discarded prior to the cells being washed with 5 ml ice-cold
PBS/phosphatase inhibitor. This rinsing fluid was subsequently
discarded and 3 ml ice-cold PBS/phosphatase inhibitor was added to
the cells. The cells were then carefully transferred from the
culture flask to a pre-cooled 15-ml cone-shaped bottle using a cell
scraper. The cell suspension was centrifuged at 4°C and 14,000 × g
for 5 min, the supernatant was discarded and the cell pellet was
placed on ice. A total of 500 μl 1× hypotonic buffer
(Sigma-Aldrich) was added to the cell pellet and the cells were
aspirated to form a suspension. The cell suspension was transferred
to a pre-cooled microfuge tube and incubated on ice for 15 min. A
total of 25 μl detergent was added and the suspension was
vortexed on the highest setting for 10 sec and centrifuged at 4°C
by microcentrifuge at 14,000 × g for 30 sec. The upper suspension
was then transferred to a pre-cooled microcentrifuge tube (−80°C)
and stored for use.
The precipitate was fully resuspended in 50
μl lysis buffer and vortexed for 10 sec. The suspension was
then placed on ice in a shaker (VWR International, Radnor, PA, USA)
at 4,200 × g to incubate for 30 min. Following high-speed vortexing
for 30 sec and centrifugation at 4°C and 14,000 × g for 10 min, the
supernatant was transferred to pre-cooled microcentrifuge tubes to
store at −80°C. The supernatant contained the desired
nucleoprotein. An NF-κB gene DNA probe was prepared with the
following sequences: Upper strand, 5′-AGT TGA GGG GAC TTT CCC AGG
C-3′; lower strand, 3′-TCA ACT CCC CTG AAA GGG TCC G-5′. The 5′ and
3′ ends were labeled with biotin digoxin. An electrophoretic
mobility shift assay (digoxin-labeled NF-κB kit; Roche Diagnostics,
Indianapolis, IN, USA) was used to measure NF-κB activity. The
procedures were performed according to the manufacturer’s
instructions and the results were obtained by computer image
analysis. Optical density represented the NF-κB activity.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL,
USA) was used for the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
General information
During the shunt surgery, one rat in the Tn group
died and one in the Ti group. During the observation period, one
rat in the Tn group died, one in the Ti group and one in the Co
group. When the animals were sacrificed following manometry, one
rat in the Ti group was excluded due to a shunt barrier. As a
result, the Tn group had 13 rats, the Ti group had 12 rats, the Cn
group had 10 rats and the Co group had nine rats. The mean ratio of
pulmonary to systolic flow of all shunt rats was 2.32±0.44,
indicating that the shunt was smooth and that pulmonary blood flow
was significantly increased.
PASP
The pulmonary artery contraction pressure of the Tn
group was significantly higher than that of the Cn group (41.4±2.7
vs. 16.1±3.6 mmHg, P<0.01) while the pulmonary artery
contraction pressure of the Ti and Cn groups showed no significant
difference (22.5±5.9 vs. 16.1±3.6 mmHg, P>0.05). No significant
difference was observed between the Co and Cn groups (15.7±3.1 vs.
16.1±3.6 mmHg, P>0.05) (Table
I).
 | Table IHemodynamics and vascular
morphological parameter changes in the four model groups. |
Table I
Hemodynamics and vascular
morphological parameter changes in the four model groups.
| Groups | N | PASP (mmHg) | RV/LV+SP | MT (%) |
|---|
| Tn | 13 | 41.4±2.7b | 0.57±0.07b | 55.6±3.3b |
| Ti | 12 | 22.5±5.9a | 0.37±0.02a | 15.8±4.1a |
| Cn | 10 | 16.1±3.6 | 0.32±0.03 | 13.5±2.1 |
| Co | 9 | 15.7±3.1a | 0.32±0.01a | 14.2±1.6a |
Cardiac morphological indicators
The ratio of the mass of the right ventricle to that
of the left ventricle plus septum was significantly higher in the
Tn group than that in the Cn group (0.57±0.07 vs. 0.32±0.03,
P<0.01). No significant difference was observed between the Ti
and Cn groups (0.37±0.02 vs. 0.32±0.03, P>0.05) or the Co and Cn
groups (0.32±0.01 vs. 0.32±0.03, P>0.05) (Table I).
Pulmonary vascular index
The pulmonary vascular MT% of the Tn group was
increased significantly compared with that of the Cn group
(55.6±3.3 vs. 13.5±2.1, P<0.01). No significant difference was
observed between the Ti and Cn groups (15.8±4.1 vs. 13.5±2.1,
P>0.05) or the Co and Cn groups (14.2±1.6 vs. 13.5±2.1,
P>0.05) (Table I).
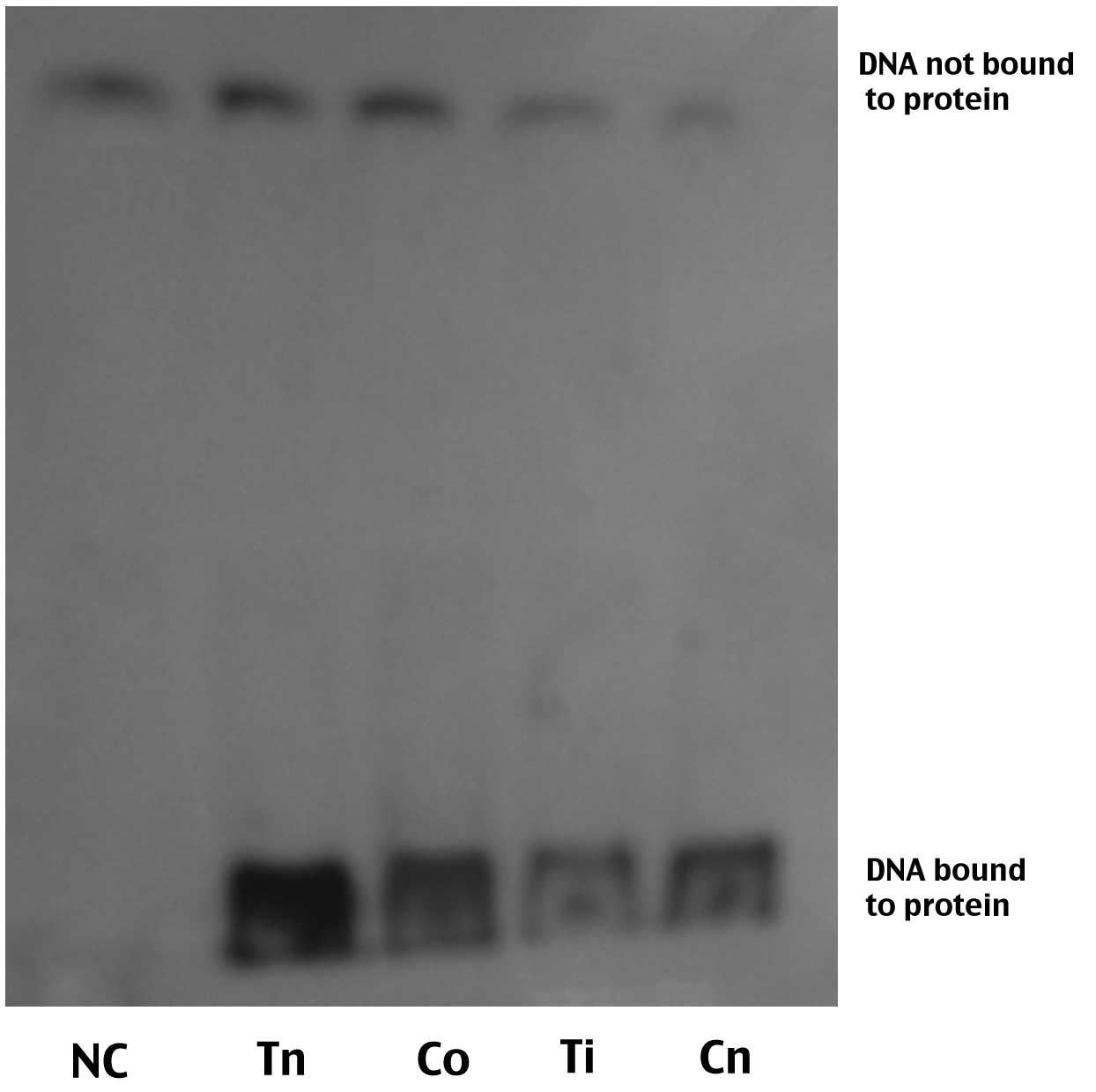
NF-κB activity
NF-κB activity in the Tn group was significantly
higher than that in the Cn group (529±15.1 vs. 298±18.7,
P<0.01). The NF-κB activity of the rats in the Ti group was
significantly lower than that in the Cn group (228±19.6 vs.
298±18.7, P<0.01). A significant difference was also observed
between the Ti and Tn groups (529±15.1 vs. 228±19.6, P<0.01),
but no significant difference was found between the Cn and Co
groups (314±20.5 vs. 298±18.7, P>0.05) (Table II and Fig 1).
 | Table IIComparisons of NF-κB activity in the
four groups. |
Table II
Comparisons of NF-κB activity in the
four groups.
| Groups | N | NF-κB OD value |
|---|
| Cn | 10 | 298±18.7 |
| Co | 9 | 314±20.5a |
| Tn | 13 | 529±15.1b,c |
| Ti | 12 | 228±19.6b |
Discussion
The pathogenesis and treatment of pulmonary
hypertension caused by left-to-right shunt CHD is a worldwide
clinical concern. Left-to-right shunt CHD results in pulmonary
endothelial structural and functional changes due to the pulmonary
blood flow, causing significant damage to the vascular endothelium.
The endothelial cell damage destroys endothelial barrier functions
and muscle-endothelial connections between endothelial cells and
smooth muscle cells, as well as undermines the regulatory
mechanisms of endothelial cell and smooth muscle cell formation so
that vascular smooth muscle cells undergo uncontrollable
proliferation (4). In the present
study, carotid artery-external jugular vein anastomosis was used to
establish the left-to-right shunt animal model, and cardiac
catheterization techniques were used to measure the pulmonary
artery pressure of the right ventricle. The results showed that the
pulmonary artery contraction pressure of the Tn group was
significantly higher than that of the Cn group, and this was
accompanied by increased right ventricular weight and significant
pulmonary vascular structural changes, indicating that the
established pulmonary hypertension model was in line with
experimental requirements.
Although the pathogenesis of pulmonary hypertension
is unknown, evidence suggests that numerous inflammatory cytokines
and chemokines play an important role in the course of the disease
(5,6). The upregulation of pro-inflammatory
genes in response to environmental stimuli has been shown to be
coordinated by transcription factors, among which NF-κB has a
pivotal role (7). An
immunoglobulin κ light-chain gene-enhancer κB sequence (GGG ACT
TTC)-specific binding nuclear protein factor was first detected in
the nucleus and extracted from B lymphocytes in 1986 and was named
NF-κB (8). NF-κB is a nuclear
transcription factor with multiple actions. Previous studies showed
that, in resting cells, NF-κB combined with the inhibitor I-κB to
produce NF-κB in an inactive form in the cytoplasm. When the cells
were stimulated by various factors, I-κB rapidly underwent
ubiquitination and proteolysis, and NF-κB was activated and rapidly
translocated to the nucleus to combine with the κB motif in the
promoter regions of target genes, promoting the gene transcription
and protein synthesis (9,10). Since then, NF-κB has been found to
exist in several cell types, including endothelial, smooth muscle
and cardiac pulmonary vascular cells. It plays an important role in
the immune response, inflammation and cell-growth control, and is
associated with the occurrence and development of cardiovascular
diseases (11–14).
The present study confirmed that, in the high-flow
model of pulmonary hypertension, NF-κB activity of the shunt group
was significantly higher than that of the negative control group;
therefore, NF-κB activation was assumed to exist. It can be
speculated that high-flow shear stress acted on the vascular
endothelium to activate the NF-κB signaling pathway. Subsequent to
administering the NF-κB inhibitor PDTC, pulmonary artery pressure
was not significantly increased, and NF-κB activity was decreased,
which further indicated that NF-κB mediated the development of
pulmonary high blood pressure. The studies of Bartman and Hove
(15) and Mironov et al
(16) also found that mechanical
interference could regulate the activity and translocation of
NF-κB, thus affecting the regulation of gene expression. The study
of the cell-stretch injury model additionally found that
over-stretch of the cells allowed NF-κB activation, the initiation
of inflammatory gene transcription and major cytokine release
(17,18). Studies have demonstrated that shear
stress increase caused by high pulmonary blood flow can induce the
release of endothelial cells, thus promoting the synthesis and
secretion of vasoactive substances increasing smooth muscle cell
proliferation, such as endothelin, angiotensin, vascular
endothelial growth factor, platelet-derived growth factor and
vasoactive peptide U-II (19–22),
and thereby inhibiting the synthesis and secretion of the cytokines
causing smooth muscle cell proliferation, such as prostaglandins,
atrial natriuretic peptide, adrenomedullin, nitric oxide and carbon
monoxide (23–26). Pulmonary vascular endothelial cells
regulate blood quality and the quantity of vasoactive substances
via transformation and uptake, pulmonary vascular endothelial cells
regulate blood quality and the quantity of vasoactive substances.
Under normal physiological conditions, endothelial cells mainly
produce various growth inhibitory factors in order to maintain the
normal structure of blood vessels; however, in pathological
conditions a variety of stimuli can induce the synthesis and
secretion of a number of endothelial cell growth factors to promote
pulmonary vascular structural remodeling (3,27).
According to the results of the present study, it
can be speculated that the high blood flow shear stress acted on
the pulmonary artery endothelial cells, activating NF-κB signaling
pathways and initiating gene transcription to produce vasoactive
mediators, pulmonary vasoconstriction and pulmonary vascular
remodeling, and thus leading to pulmonary hypertension. In the
present study, the morphology of the heart, pulmonary vascular
index and NF-κB activity were obtained after the rats had been
sacrificed. Therefore the abovementioned indexes in rats were not
tested in vivo. However such tests remain to be investigated
in future studies using neuroimaging or by obtaining living
specimens.
References
|
1
|
Rabinovitch M, Haworth SG, Castaneda AR,
Nadas AS and Reid LM: Lung biopsy in congenital heart disease: a
morphometric approach to pulmonary vascular disease. Circulation.
58:1107–1122. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loscalzo J: Endothlial dysfuntion in
pulmonary hypertension. N Engl J Med. 327:117–119. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuder RM, Marecki JC, Richter A,
Fijalkowska I and Flores S: Pathology of pulmonary hypertension.
Clin Chest Med. 28:23–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humbert M, Morrell NW, Archer SL and
Stenmark KR: Cellular and molecular pathobiology of pulmonary
arterial hypertension. J Am Coll Cardiol. 43:13S–24S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Price LC, Wort SJ, Perros F, et al:
Inflammation in pulmonary arterial hypertension. Chest.
141:210–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quarck R, Nawrot T, Meyns B and Delcroix
M: C-reactive protein: a new predictor of adverse outcome in
pulmonary arterial hypertension. J Am Coll Cardiol. 53:1211–1218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Tan Y, Stenmark KR and Tan W: High
pulsatility flow induces acute endothelial inflammation through
overpolarizing cells to activate NF-κB. Cardiovasc Eng Technol.
4:26–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen F, Castranova V, Shi X and Demers LM:
New insights into the role nuclear factor kappaB, a ubiquitous
transcription factor in the initiation of diseases. Clin Chem.
45:7–17. 1999.PubMed/NCBI
|
|
10
|
Adib-Conquy M, Adrie C, Moine P, et al: NF
kappaB expression in mononuclear cells of patients with sepsis
resembles that observed in lipopolysaccharide tolerance. Am J
Respir Crit Care Med. 162:1877–1883. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan Q, Mercurius KO and Davies PF:
Stimulation of transcription factors NF kappa B and AP1 in
endothelial cells subjected to shear stress. Biochem Biophys Res
Commun. 201:950–956. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagel T, Resnick N, Dewey CF Jr and
Gimbrone MA Jr: Vascular endothelial cells respond to spatial
gradients in fluid shear stress by enhanced activation of
transcription factors. Arterioscler Thromb Vasc Biol. 19:1825–1834.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davies RJ, Holmes AM, Deighton J, et al:
BMP type II receptor deficiency confers resistance to growth
inhibition by TGF-β in pulmonary artery smooth muscle cells: role
of proinflammatory cytokines. Am J Physiol Lung Cell Mol Physiol.
302:L604–L615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Price LC, Caramori G, Perros F, et al:
Nuclear factor κB is activated in the pulmonary vessels of patients
with end-stage idiopathic pulmonary arterial hypertension. PLoS
One. 8:e754152013. View Article : Google Scholar
|
|
15
|
Bartman T and Hove J: Mechanics and
function in heart morphogenesis. Dev Dyn. 233:373–381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mironov V, Visconti RP and Markwald RR: On
the role of shear stress in cardiogenesis (Review). Endothelium.
12:259–261. 2005. View Article : Google Scholar
|
|
17
|
Yamamoto H, Teramoto H, Uetani K, Igawa K
and Shimizu E: Cyclic stretch upregulates interleukin-8 and
transforming growth factor-betal production through a protein
kinase C-dependent pathway in alveolar epithelial cells.
Respirology. 7:103–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vlahakis NE, Schroeder MA, Limper AH and
Hubmayr RD: Stretch induces cytokine release by alveolar epithelial
cells in vitro. Am J Physiol. 277:L167–L173. 1999.PubMed/NCBI
|
|
19
|
Zheng JP, Zhang Y, Edvinsson L, Hjalt T
and Xu CB: NF-kappaB signaling mediates vascular smooth muscle
endothelin type B receptor expression in resistance arteries. Eur J
Pharmacol. 637:148–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schermuly RT, Dony E, Ghofrani HA, et al:
Reversal of experimental pulmonary hypertension by PDGF inhibition.
J Clin Invest. 115:2811–2821. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Merklinger SL, Jones PL, Martinez EC and
Rabinovitch M: Epidermal growth factor receptor blockade mediates
smooth muscle cell apoptosis and improves survival in rats with
pulmonary hypertension. Circulation. 112:423–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakao S, Taraseviciene-Stewart L, Cool CD,
et al: VEGF-R blockade causes endothelial cell apoptosis, expansion
of surviving CD34+ precursor cells and
transdifferentiation to smooth muscle-like and neuronal-like cells.
FASEB J. 21:3640–3652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tuder RM, Cool CD, Geraci MW, et al:
Prostacyclin synthase expression is decreased in lungs from
patients with severe pulmonary hypertension. Am J Respir Crit Care
Med. 159:1925–1932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsui H, Shimosawa T, Itakura K, Guanqun
X, Ando K and Fujita T: Admnomedullin can protect against pulmonary
vascular remodeling induced by hypoxia. Circulation. 109:2246–2251.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Couture R, Blaes N and Girolami JP: Kinin
receptors in vascular biology and pathology. Curr Vasc Pharmacol.
12:223–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeh PY, Li CY, Hsieh CW, Yang YC, Yang PM
and Wung BS: CO-releasing molecules and increased heme oxygenase-1
induce protein S-glutathionylation to modulate NF-κB activity in
endothelial cells. Free Radic Biol Med. 70:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YH, Xiang RL, Hu XT, et al: Changes
of serum leptin and vascular endothelial growth factor in children
with congenital heart disease. Zhongguo Dang Dai Er Ke Za Zhi.
11:802–804. 2009.(In Chinese). PubMed/NCBI
|