Introduction
At present, coronary heart disease (CHD) is a
serious concern in China. Diabetes is considered as harmful to
health as CHD, and glycosylated hemoglobin A1c (HbA1c) is an
important indicator for monitoring blood glucose levels (1–3).
HbA1c can indicate the average blood glucose concentration and is
highly reproducible and responsive to minor degrees of abnomality
in glucose tolerance. Studies have shown that high HbA1c levels can
increase the incidence of CHD and adversely affected its prognosis
(4,5). Ueda et al found that the
levels of HbA1c were significantly higher in patients with major
adverse cardiac events compared to those without (4). Fatima et al revealed that
HbA1c is a reliable predictor of coronary artery disease and that
the magnitude of perfusion defects, left ventricular dysfunction
and the incidence rate of nonfatal myocardial infarction were
higher at an HbA1c level >7.3% (6). A study by Rocco et al
demonstrated that HbA1c was an important predictor of symptomatic
hemorrhage following thrombolysis for acute stroke. The results
also suggested that hemorrhage following thrombolysis was a
consequence of long-term vascular injury rather than of acute
hyperglycemia, and that HbA1c may be a more effective predictor
than acute blood glucose or a history of diabetes (7). However, the correlation of low HbA1c
levels with coronary artery stenosis and prognosis is not yet
clear. The present study investigated the correlation between
different levels of HbA1c and coronary artery stenosis and
evaluated whether HbA1c is associated with the 1-year risk of major
adverse cardiac events (MACE) following successful drug-eluting
stent (DES) implantation.
Materials and methods
Study subjects
A total of 2,825 consecutive patients with CHD who
received the first call for drug-eluting coronary stent
implantation at the Department of Cardiology, Zhongda Hospital of
Southeast University (Nanjing, China) from June 2008 to June 2012
were included in the study. According to HbA1c levels the patients
were divided into three groups. These were the low HbA1c (group A,
HbA1c ≤5.9% or 41 mmol/mol; n=1,035), medium HbA1c (group B,
5.9%< HbA1c <6.8% or 41< HbAlc <51 mmol/mol; n=1,025)
and high HbA1c groups (group C, HbA1c ≥6.8% or 51 mmol/mol; n=765).
Exclusion criteria: i) Had received prior percutaneous coronary
intervention or coronary artery bypass surgery; ii) simultaneously
implanted bare-metal stents and drug-eluting stents; iii) acute
coronary syndrome (ACS); iv) severe anemia and other blood system
disease, severe infection, trauma, malignancy, severe liver and
kidney dysfunction. Ethical approval was obtained from the Ethics
Committee of the Faculty of Medicine, Southeast University
(Nanjing, China); all patients provided written informed consent
prior to participation in the current study. The study complied
with the Declaration of Helsinki.
Procedure
Criteria for coronary stenting and coronary artery
stenosis were assessed. All patients underwent coronary
angiography, and the angiography results were judged by one or two
experienced specialist physicians. Any main branch with coronary
artery stenosis ≥70% underwent coronary stent implantation. The
Gensini scoring system was used to quantify the degree of coronary
artery stenosis (8): 1 point,
≤25%; 2 points, 26–50%; 4 points, 51–75%; 8 points, 76–90%; 16
points; 91–99%; 32 points, 100%. This stenosis score multiplied by
the appropriate factor for the coronary segment provided the branch
score. Each patient’s Gensini score was the sum of the scores for
every branch.
Clinical follow up
Follow-up via phone, outpatient and/or readmission,
and the 1-year MACE rate were recorded during hospitalization and
discharge. MACE includes all-cause mortality, non-fatal myocardial
infarction (MI) and target lesion revascularization (TLR, involving
thrombolysis, stents or bypass).
Statistical analysis
Values are expressed as means ± standard deviation.
Categorical variables were compared using the Chi square test.
Differences in the mean values between two groups were compared
using an unpaired t-test or a Wilcoxon rank-sum test. The
Kaplan-Meier technique was used to plot cumulative event-free
estimates. The Cox proportional hazards model was used to analyze
the cumulative survival rate among the groups; differences between
groups were assessed with the log-rank test. The Backwald method
was used to determine the independent risk factors that will
influence clinical outcomes. P<0.05 was considered to indicate a
statistically significant result. Statistical analyses were
performed using the SPSS software package, version 19.0 (IBM,
Armonk, NY, USA).
Results
Baseline characteristics
The baseline characteristics are shown in Table I for all 2,825 patients with stent
implantation according to the baseline HbA1c level (group A: ≤5.9%,
group B: 5.9%< HbA1c <6.8%; group C: ≥6.8%) and by MACE
follow up (MACE or no-MACE). The mean duration of follow-up was
327±86.2 days. Family history of CHD, arrhythmia history, chronic
kidney disease, cerebrovascular disease, obesity and dual
anti-platelet time were similar for the three HbA1c groups. Age,
male gender, smoking, hypertension, pulse rate, hemoglobin level,
HbA1c level, hyperlipidemia, diabetes and history of heart failure
differed among the three HbA1c groups. Hyperlipidemia and diabetes
were different between the two MACE groups, whereas the other
baseline characteristics were similar between the two MACE
groups.
 | Table IBaseline clinical characteristics in
all 2,825 patients, grouped by baseline HbA1c and by MACE. |
Table I
Baseline clinical characteristics in
all 2,825 patients, grouped by baseline HbA1c and by MACE.
| Groups by baseline
HbA1c, % | | MACE | |
|---|
|
| |
| |
|---|
| Variable | ≤5.9 | 5.9–6.8 | ≥6.8 | P-value | MACE | No-MACE | P-value |
|---|
| Age (years) | 59.21±11.17 | 59.07±11.12 | 61.02±10.43 | <0.001 | 60.56±10.59 | 59.53±11.04 | 0.108 |
| Male (%) | 731 | 456 | 317 | 0.003 | 1689 | 221 | 0.266 |
| Smoking (%) | 493 | 456 | 317 | 0.038 | 1106 | 160 | 0.138 |
| Hypertension (%) | 596 | 630 | 526 | <0.001 | 1535 | 217 | 0.129 |
| Pulse (bpm) | 70.3 | 70.9 | 73.19 | <0.001 | 71.52 | 71.30 | 0.752 |
| Hemoglobin (g/l) | 137.45 | 137.27 | 135.56 | 0.029 | 136.50 | 136.93 | 0.652 |
| Hemoglobin A1c,
% | 4.49 | 5.39 | 7.69 | <0.001 | 5.79 | 5.78 | 0.966 |
| Family history of CHD
(%) | 11 | 20 | 16 | 0.174 | 44 | 3 | 0.156 |
| Arrhythmia (%) | 178 | 156 | 114 | 0.335 | 401 | 47 | 0.176 |
| Hyperlipidemia
(%) | 380 | 427 | 342 | 0.001 | 995 | 154 | 0.025 |
| Diabetes (%) | 77 | 120 | 384 | <0.001 | 496 | 85 | 0.013 |
| Chronic kidney
disease (%) | 617 | 595 | 453 | 0.809 | 1472 | 193 | 0.241 |
| Cerebrovascular
disease (%) | 93 | 72 | 64 | 0.234 | 201 | 28 | 0.453 |
| Body mass index
(kg/m2) | 20.0 | 23.4 | 24.5 | 0.376 | 24.3 | 23.2 | 0.144 |
| History of heart
failure (%) | 8 | 24 | 41 | <0.001 | 65 | 8 | 0.487 |
| Dual antiplatelet
time (days) | 197.1 | 196.8 | 197.8 | 0.920 | 199.01 | 196.94 | 0.488 |
The baseline angiographic characteristics of the
study group are summarized in Table
II. Gensini score, number of lesions and incidence of
multi-vessel disease differed among the three HbA1c groups. The
other baseline angiographic characteristics were similar in the
three HbA1c groups. Gensini score, number of lesions, number of
stents, number of target vessels, incidence of multi-vessel
disease, multi-stent use, overlapping stents and ostial lesions
were different between the two MACE groups, whereas the other
baseline angiographic characteristics were similar in the two MACE
groups.
 | Table IIBaseline angiographic characteristics
according to baseline HbA1c group and MACE after follow-up. |
Table II
Baseline angiographic characteristics
according to baseline HbA1c group and MACE after follow-up.
| Groups by baseline
HbA1c, % | | MACE | |
|---|
|
| |
| |
|---|
| Variable | ≤5.9 | 5.9–6.8 | ≥6.8 | P-value | MACE | No-MACE | P-value |
|---|
| Gensini score | 29.09±34.48 | 28.70±33.69 | 37.77±38.95 | <0.001 | 40.87±41.49 | 30.01±34.66 | <0.001 |
| Number of
lesions | 1.40 | 1.45 | 1.70 | <0.001 | 1.77 | 1.46 | <0.001 |
| Number of
stents | 1.94 | 1.25 | 1.87 | 0.394 | 1.73 | 1.94 | 0.020 |
| Number of target
vessels | 1.56 | 1.54 | 1.53 | 0.776 | 1.44 | 1.56 | 0.005 |
| Multi-vessel
disease | 240 | 228 | 238 | <0.001 | 583 | 123 | <0.001 |
| Multi-stent | 581 | 573 | 414 | 0.661 | 1401 | 167 | 0.016 |
| Overlapping
stents | 298 | 312 | 226 | 0.715 | 752 | 84 | 0.030 |
| Small vessels | 67 | 79 | 53 | 0.544 | 178 | 21 | 0.324 |
| Long lesions | 511 | 485 | 365 | 0.618 | 1213 | 148 | 0.066 |
| Calcification | 93 | 113 | 68 | 0.199 | 248 | 26 | 0.118 |
| CTO | 41 | 47 | 21 | 0.132 | 96 | 13 | 0.537 |
| Bifurcation | 348 | 342 | 251 | 0.936 | 834 | 107 | 0.308 |
| Ostial lesions | 189 | 188 | 160 | 0.290 | 459 | 78 | 0.022 |
| Type C lesions | 401 | 374 | 277 | 0.450 | 2032 | 270 | 0.351 |
Survival analysis
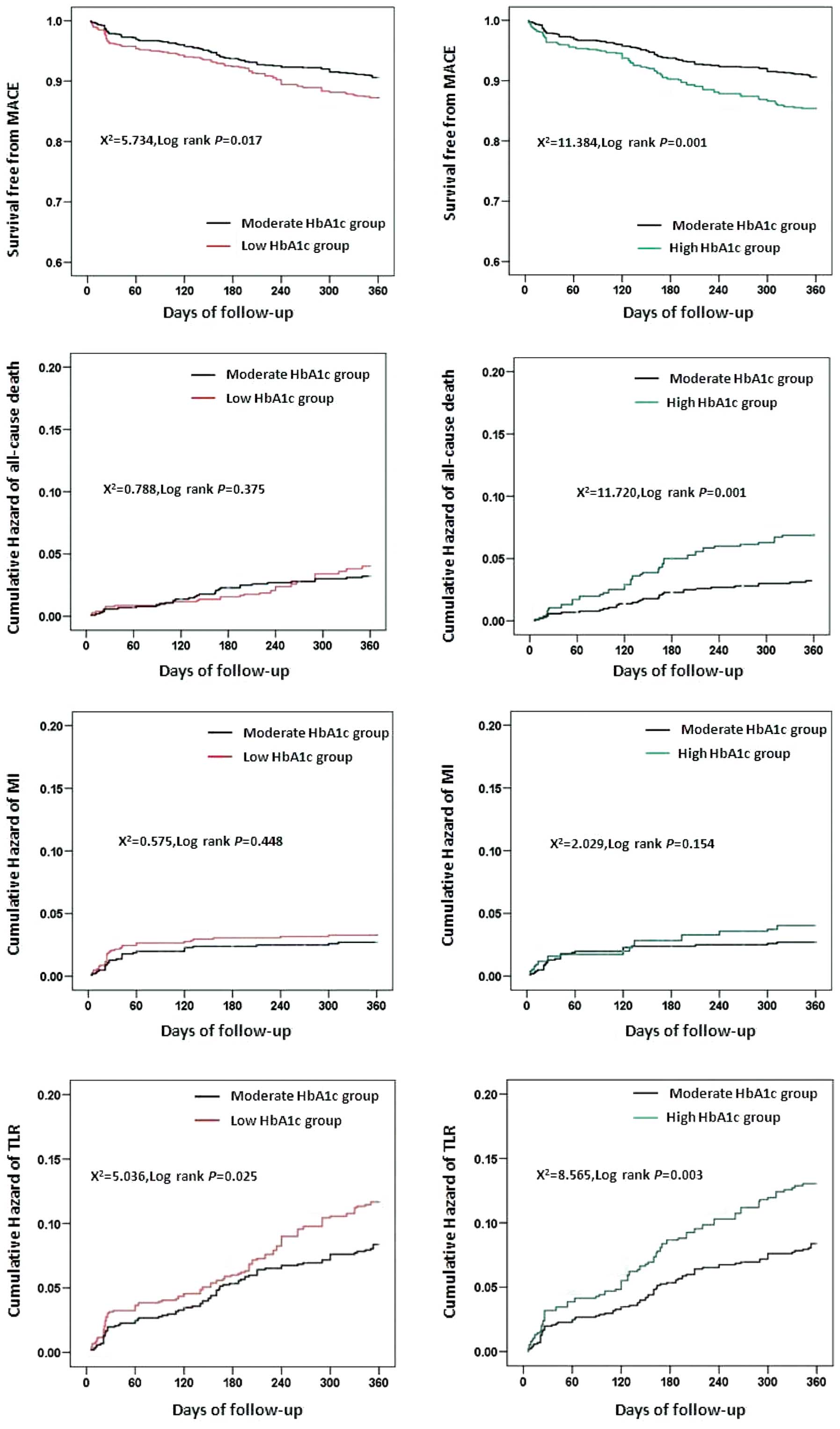
As shown in Fig. 1,
Kaplan-Meier analysis demonstrated a significantly higher survival
rate free from MACE in patients with moderate HbA1c levels
(P<0.01, log-rank test). The risk of all-cause mortality of
group C was significantly higher than that of group B. The TLR
risks of group A and group C were significantly higher than that of
group B, while group A and group B, and group B and group C
exhibited no significant differences in the risk of MI.
Correlation analysis
Table III shows
that a high or low HbA1c level significantly predicted MACE and TLR
after adjusting for age, gender, smoking, hypertension, heart rate,
hemoglobin, hyperlipidemia, diabetes, heart failure, Gensini score,
number of lesions, number of stents, number of target vessels,
multi-vessel disease, multi-stent, overlapping stents and ostial
lesions (high HbA1c level, hazard ratio 1.626 and 1.522,
respectively; low HbA1c level, hazard ratio 1.505 and 1.478,
respectively). A high HbA1c level significantly predicted all cause
mortality after adjusting for age, gender, smoking, hypertension,
heart rate, hemoglobin, hyperlipidemia, diabetes, heart failure,
Gensini score, number of lesions, number of stents, number of
target vessels, multi-vessel disease, multi-stent, overlapping
stents and ostial lesions (hazard ratio 2.008). Both low and high
HbA1c levels are indicated to be risk factors of all-cause
mortality, MI and TLR; however, the results are not statistically
significant. Analysis of HbA1c as a continuous variable showed that
each 1% increase of HbA1c was significantly associated with a
decreased risk of MACE and TLR by 53.5 and 54.2%, respectively, in
those with a low HbA1c level, and with an increased risk of MACE
and TLR by 10 and 9.2%, respectively, in those with a moderate or
high HbAlc level, suggesting a U-shaped association between HbA1c
and the risk of MACE and TLR, even after adjusting for baseline
potential confounders.
 | Table IIIAdjusted hazard ratios of MACE,
all-cause mortality, MI and TLR in 2,825 patients with CHD
following PCI by continuous and grouped HbA1c levels (mean
follow-up, 1 year). |
Table III
Adjusted hazard ratios of MACE,
all-cause mortality, MI and TLR in 2,825 patients with CHD
following PCI by continuous and grouped HbA1c levels (mean
follow-up, 1 year).
| HbA1c
continuousa,% | HbA1c groups,% |
|---|
|
|
|
|---|
| Variable | ≤5.9b | >5.9c | ≤5.9 | 5.9–6.8 | ≥6.8 |
|---|
| MACE |
| Crude hazard
ratio | 0.435
(<0.001)d | 1.100
(0.004)e | 1.379
(0.017)f | Ref | 1.101 (0.084) |
| Model 1 | 0.474 (0.098) | 1.004 (0.956) | 1.483
(0.005)f | Ref | 1.712
(<0.001)c |
| Model 2 | 0.465
(0.002)e | 1.095
(0.01)e | 1.505
(0.004)f | Ref | 1.626
(0.001)b |
| Mortality |
| Crude hazard
ratio | 0.324 (0.115) | 1.172
(0.034)f | 1.234 (0.376) | Ref | 1.172
(0.034)a |
| Model 1 | 0.780 (0.104) | 0.985 (0.892) | 1.246 (0.380) | Ref | 2.092
(0.002)b |
| Model 2 | 0.535 (0.158) | 1.049 (0.466) | 1.264 (0.354) | Ref | 2.008
(0.004)b |
| MI |
| Crude hazard
ratio | 0.128
(0.005)e | 1.002 (0.989) | 1.217 (0.449) | Ref | 1.002 (0.989) |
| Model 1 | 0.663
(0.033)f | 1.003 (0.985) | 1.359 (0.262) | Ref | 1.712 (0.056) |
| Model 2 | 0.6 (0.295) | 1.116 (0.068) | 1.418 (0.202) | Ref | 1.582 (0.106) |
| TLR |
| Crude hazard
ratio | 0.228
(<0.001)d | 1.127
(0.042)f | 1.385
(0.026)f | Ref | 1.127
(0.042)a |
| Model 1 | 0.577 (0.110) | 1.194 (0.864) | 1.460
(0.013)f | Ref | 1.595
(0.004)b |
| Model 2 | 0.458
(0.003)e | 1.092
(0.021)f | 1.478
(0.01)e | Ref | 1.522
(0.009)b |
Discussion
In this study of 2,825 older patients with CHD in a
hospital cohort followed up for an average of 1 years, an
approximately U-shaped association between HbA1c and MACE and TLR
was found. Patients with low (≤41 mmol/mol or 5.9%) or high (≥51
mmol/mol or 6.8%) HbA1c levels had a risk of MACE or TLR that was
higher than that of participants with moderate (41–51 mmol/mol or
5.9–6.8%) HbA1c levels after adjusting for multiple potential
confounders (hazard ratios for low HbAlc, 1.505 and 1.478,
respectively, and for high HbAlc, 1.626, 1.522, respectively).
Analysis of HbA1c as a continuous variable showed that each 1%
increase of HbA1c was significantly associated with decreased risks
of MACE and TLR of 53.5 and 54.2%, respectively, in patients with a
low level of HbAlc and with increased risks of MACE and TLR of 9.5
and 9.2%, respectively, in those with a moderate or high HbA1c
level, suggesting a U-shaped association between HbA1c and the risk
of MACE and TLR.
A number of studies have focused on the use of HbA1c
levels for the prognosis of patients with diabetes (6,7,9). A
meta-analysis of four randomized clinical trials (10), Action to Control Cardiovascular
Risk in Diabetes (ACCORD) (11),
Action in Diabetes and Vascular Disease: Preterax and Diamicron
Modified Release Controlled Evaluation (ADVANCE) (12), the UK Prospective Diabetes Study
(13) and Veterans Affairs
Diabetes Trial (14), found that
all-cause and cardiovascular mortality did not increase in the
intensive glycemic control group (target glycated hemoglobin ≤6.5%,
fasting blood glucose <6.0 mmol/l or absolute HbA1c reduction
1.5%) compared with a non-intensive control group, with a median
follow-up period from 3.4–5.6 years. The External Peer Review
Program (EPRP) study of a cohort of 5,815 heart failure patients
without diabetes found a U-shaped association between HbA1c and
all-cause mortality (15). Xu
et al found that higher and lower HbA1c levels increased the
risk of mortality compared with that for moderate glycemic control
(7.1–7.8%), even after adjustment for potential baseline
confounders. In comparison with patients with HbA1c levels of
7.5–8.4%, those with lower (<48 mmol/mol, 6.5%) or higher
(>69 mmol/mol, 8.5%) HbA1c levels had increased stroke mortality
risks of 112% and 143%, respectively (16).
However, the relationship between HbA1c and CHD
prognosis remains controversial. Ciciek et al (17) found that HbA1c is an independent
predictor of the in-hospital mortality of ST elevation MI (STEMI)
patients treated with PCI. Corpus et al (18) demonstrated that HbA1c is a
significant predictor of MACE, target vessel revascularization
(TVR) and cardiac death 1 year following PCI in non-diabetic
patients. Hadjadj et al (19) found that HbA1c was not associated
with STEMI prognosis in a small population. Lemesle et al
(20). demonstrated that HbA1c is
not a predictor for cardiac events in diabetic patients with
coronary artery disease. The data in the present study show that,
compared with patients with moderate HbAlc levels (range,
5.9–6.8%), those with higher HbA1c levels had higher risks of MACE
and TLR of 62.6 and 52.2%, respectively. The conflicting
relationship between HbA1c levels and prognosis may have several
interpretations. One reason may be due to different definitions and
follow-up times of the end point. Another reason may be differences
in the study. Corpus, et al (18) defined the primary endpoint as TVR,
secondary endpoint as cardiac death, MI, recurrent angina, stroke,
congestive heart failure, renal failure and cardiac
rehospitalization, and the study was of non-diabetic patients with
CHD. Lemesle et al (20)
defined the primary endpoint as mortality, MI and TVR, used a
1-year follow-up, ~70% of patients received stents and studied CHD
patients with DM. In the present study, the endpoint is defined as
mortality, non-fatal MI and TLR through PCI, and the subjects were
CHD patients with or without DM; all patients received stent
implantation. HbA1c and a composite endpoint (cardiovascular death,
nonfatal MI or TLR) to assess whether the definition of the end
result affected the correlation between HbA1c and the endpoint.
Cardiovascular death and TLR were found to be significantly
correlated with HbA1c.
There have been relatively few studies concerning
the correlation between low HbA1c level and the prognosis of CHD
patients. The present study suggests that compared with patients
with moderate HbAlc levels (range, 5.9–6.8%), those with lower
HbA1c levels had increased risks of MACE and TLR of 50.5 and 47.8%,
respectively. The results are consistent with a U-shaped
correlation between HbA1c and the prognosis of diabetic patients.
Intensive glucose control, resulting in low levels of HbA1c and
severe hypoglycemia, may lead to subsequent higher mortality, which
may explain the results of this study.
The present study has several limitations. First,
antidiabetic drugs may have affected the results. This study was
not designed to test the results of the treatment of diabetes; due
to lack of data, it was not possible to study the therapeutic
effect of such treatment. Secondly, the HbA1c level measurements
were not repeated, and in subsequent years, HbA1c levels may have
changed. Thirdly, the observed increases in MACE and TLR were
independent of sex, gender, hypertension, hyperlipidemia and
history of heart failure. Nonetheless, it is not possible to
exclude residual confounding by other known and unknown risk
factors, such as fasting blood glucose or inflammatory markers,
which were not covered in the analysis. Fourthly, due to telephone
interviews and the cause of mortality being reported by a family
member or loved one, misjudgment of the cause of mortality cannot
be completely ruled out. Finally, the severity of cardiovascular
disease at baseline cannot be assessed based on the self-reported
history. Therefore, misjudgment of cardiovascular disease cannot be
eliminated.
In summary, this study found that HbA1c levels,
either as a continuous variable or a categorical variable, have a
U-shaped correlation with MACE and TLR in CHD patients with stent
implantation, even following adjustment for multiple confounders.
Based on the aforementioned limitations of this study, more
rigorous and comprehensive studies are required to confirm these
findings.
References
|
1
|
Baber U, Gutierrez OM, Levitan EB, Warnock
DG, Farkouh ME, et al: Risk for recurrent coronary heart disease
and all-cause mortality among individuals with chronic kidney
disease compared with diabetes mellitus, metabolic syndrome, and
cigarette smokers. Am Heart J. 166:373–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim J, Chae YK and Chernoff A: The risk
for coronary heart disease according to insulin resistance with and
without type 2 diabetes. Endocr Res. 38:195–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saely CH and Drexel H: Is type 2 diabetes
really a coronary heart disease risk equivalent? Vascul Pharmacol.
59:11–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueda H, Mitsusada N, Harimoto K, Miyawaki
M, Yasuga Y and Hiraoka H: Glycosylated hemoglobin is a predictor
of major adverse cardiac events after drug-eluting stent
implantation in patients with diabetes mellitus. Cardiology.
116:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kataoka Y, Shao M, et al: Multiple risk
factor intervention and progression of coronary atherosclerosis in
patients with type 2 diabetes mellitus. Eur J Prev Cardiol.
20:209–217. 2013. View Article : Google Scholar
|
|
6
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fatima N, u Zaman M, Ishaq M, Baloch DJ,
Bano M, et al: Impact of glycosylated hemoglobin (HBA1C) on the
extent of perfusion abnormalities and left ventricular dysfunction
using gated myocardial perfusion imaging and clinical outcomes in
diabetic patients. Nucl Med Commun. 34:489–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocco A, Heuschmann PU, Schellinger PD,
Köhrmann M, Diedler J, et al: Glycosylated hemoglobin A1 predicts
risk for symptomatic hemorrhage after thrombolysis for acute
stroke. Stroke. 44:2134–2138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su G, Mi SH, Tao H, Li Z, Yang HX, et al:
Impact of admission glycemic variability, glucose, and glycosylated
hemoglobin on major adverse cardiac events after acute myocardial
infarction. Diabetes Care. 36:1026–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Control Group. Turnbull FM, Abraira C,
Anderson RJ, Byington RP, Chalmers JP, et al: Intensive glucose
control and macrovascular outcomes in type 2 diabetes.
Diabetologia. 52:2288–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Action to Control Cardiovascular Risk in
Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC
Jr, Bigger JT, et al: Effects of intensive glucose lowering in type
2 diabetes. N Engl J Med. 358:2545–2559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoungas S, Chalmers J, Ninomiya T, Li Q,
Cooper ME, et al: ADVANCE Collaborative Group: Association of HbA1c
levels with vascular complications and death in patients with type
2 diabetes: evidence of glycaemic thresholds. Diabetologia.
55:636–643. 2012. View Article : Google Scholar
|
|
13
|
Holman RR, Paul SK, Bethel MA, Matthews DR
and Neil HA: 10-year follow-up of intensive glucose control in type
2 diabetes. N Engl J Med. 359:1577–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agrawal L, Emanuele NV, Abraira C,
Henderson WG, Levin SR, et al: Ethnic differences in the glycemic
response to exogenous insulin treatment in the Veterans Affairs
Cooperative Study in Type 2 Diabetes Mellitus (VA CSDM). Diabetes
Care. 21:510–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguilar D, Bozkurt B, Ramasubbu K and
Deswal A: Relationship of hemoglobin A1C and mortality in heart
failure patients with diabetes. J Am Coll Cardiol. 54:422–428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Chan WM, Hui YF and Lam TH:
Association between HbA1c and cardiovascular disease mortality in
older Hong Kong Chinese with diabetes. Diabet Med. 29:393–398.
2012. View Article : Google Scholar
|
|
17
|
Cicek G, Uyarel H, Ergelen M, Ayhan E,
Abanonu GB, et al: Hemoglobin A1c as a prognostic marker in
patients undergoing primary angioplasty for acute myocardial
infarction. Coronary Artery Disease. 22:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corpus RA, O’Neill WW, Dixon SR, Timmis GC
and Devlin WH: Relation of hemoglobin A1c to rate of major adverse
cardiac events in nondiabetic patients undergoing percutaneous
coronary revascularization. Am J Cardiol. 92:1282–1286. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hadjadj S, Coisne D, Mauco G, Ragot S,
Duengler F, et al: Prognostic value of admission plasma glucose and
HbA in acute myocardial infarction. Diabet Med. 21:305–310. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lemesle G, Bonello L, de Labriolle A,
Maluenda G, Syed AI, et al: Prognostic value of hemoglobin A1C
levels in patients with diabetes mellitus undergoing percutaneous
coronary intervention with stent implantation. Am J Cardiol.
104:41–45. 2009. View Article : Google Scholar : PubMed/NCBI
|