Introduction
Hereditary spastic paraplegia (HSP) is a group of
genetic diseases of the nervous system with clinical and genetic
heterogeneity. Epidemiological studies have found that the
prevalence of HSP is an estimated 1.27–12.1 cases per 100,000
individuals in Europe (1,2). This disease is manifested as a slowly
progressive weakness of the lower extremities and spastic
paraplegia. HSP can be divided into two types: The pure form and
the complicated form. The pure form is only characterized by
spastic paraplegia, i.e. progressive muscular hypertonia and
weakness of the lower extremities (3,4). The
complicated form is accompanied with extramedullary damage, such as
mental retardation, extrapyramidal symptoms, ataxia, optic atrophy,
retinal pigment degeneration, deafness, muscle atrophy and
polyneuropathy (5). Hereditary
spinocerebellar ataxia (SCA) is another group of genetic diseases
involving the human nervous system. Spinocerebellar ataxia type 3
(SCA3)/Machado-Joseph disease (MJD), is the common subtype of SCA
in mainland China (6). In
addition, some cross symptoms are exhibited between HSP and
SCA3/MJD. In the present study, a family showing genetic
anticipation, spastic paraplegia and exophthalmos was
investigated.
Subjects and methods
Subjects
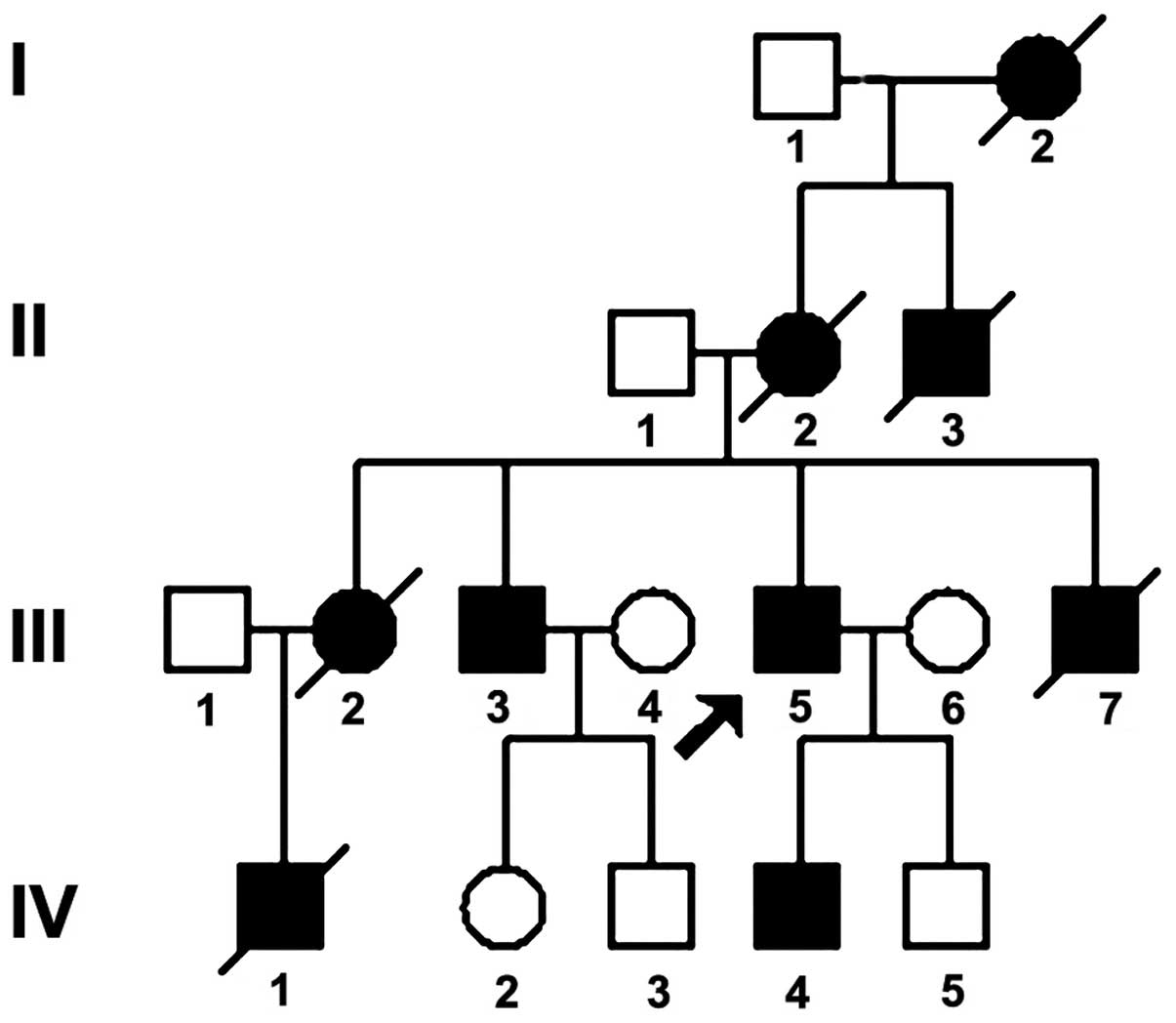
The pedigree of a Han family from Hunan, China, in
which four generations showed autosomal dominant inheritance, is
illustrated in Fig. 1. A detailed
inquiry was conducted into 17 individuals, six of whom have
succumbed. The nine patients included six males and three females.
The patients were diagnosed based on the Harding criteria (7): Progressive spasticity and weakness of
both lower extremities; pyramidal features in both lower
extremities; a positive family history; and exclusion of other
diseases (8). The study was
approved by the Ethics Committee of Xiangya Hospital, Central South
University (Changsha, China). The family members provided written
informed consent prior to undergoing a personal interview and a
complete neurological examination.
The proband (III5; male, 36 years old) was selected
as he was the first case brought to our attention, and had the
symptoms of spasticity, weakness and ataxia of both lower
extremities, which had persisted for over six years. The patient
began to have these symptoms, along with a choking cough following
drinking, >six years ago and the symptoms gradually became
aggravated. A physical examination revealed poor memory and
calculation ability, trouble with speaking clearly, bilateral
upper-eyelid contracture, horizontal nystagmus of both eyes, normal
muscle power in the upper extremities, inflexibility in alternating
movement tests, a lower extremity muscle force of grade 5 (MRC
scale) (9), muscle hypertonia,
tendon hyper-reflexia and bilateral positive pathological reflexes.
The patient was unable to finish the coordination movement
examination and had a scissor gait, but he had no sensory
abnormalities, muscular atrophy or arched feet. No Kayser-Fleischer
ring was noted in the eyes. Chromosome examination revealed a 46,
XY karyotype, and brain magnetic resonance imaging (MRI) showed
mild atrophy of the cerebellar hemispheres and upper cervical
spinal cord. Blood biochemistry tests (triiodothyronine, thyroxine
and thyroid stimulating hormone) showed no abnormality. The age of
onset in the remaining probands was as follows: I2, 50 years; II2,
42 years; III2, 30 years; III3, 30 years; III7, 27 years; and IV4,
15 years. The age of onset became younger and the symptoms more
aggravated in successive generations. The clinical features of
three family members are shown in Table I.
 | Table IClinical characteristics of three
patients in this pedigree. |
Table I
Clinical characteristics of three
patients in this pedigree.
| Clinical
characteristic | IV4 | III3 | III5 |
|---|
| Gender | Male | Male | Male |
| Age of onset
(years) | 15 | 30 | 29 |
| Course of disease
(years) | 1 | 12 | 7 |
| Bilateral upper
extremity weakness | − | − | − |
| Lower extremity
weakness | + | + | + |
| Bilateral upper-limb
tendon reflexes | Normal | Active | Active |
| Sensory
impairment | − | − | − |
| Bilateral upper-limb
muscle strength | Level 5 | Level 5 | Level 5 |
| Hypertonia of upper
limbs | + | ++ | ++ |
| Hoffmann’s sign | − | + | + |
| Bilateral lower-limb
tendon reflexes | Active | Active | Active |
| Bilateral lower-limb
muscle strength | Level 4 | Level 4 | Level 4 |
| Hypertonia of lower
limbs | ++ | + | + |
| Feeling of disorder
in lower limbs | − | − | − |
| Ankle clonus | + | + | + |
| Babinski sign | + | + | + |
| Gait | Scissor gait | Scissor gait | Scissor gait |
| Urination
obstacles | − | − | − |
| Dementia | − | − | − |
| Coordination movement
testing | Unable to
complete | Unable to
complete | Unable to
complete |
| Autonomic nerve
dysfunction | − | − | − |
| Foot deformities | − | − | − |
| Dysarthria | + | + | − |
| Exorbitism | − | + | + |
| Horizontal
nystagmus | − | + | + |
Extraction of genomic DNA (gDNA)
Peripheral venous blood samples (5 ml) were drawn
from the patients. Two healthy volunteers outside of the family (N1
and N2) were included as healthy controls. gDNA was extracted by
standard phenol-chloroform methods (10). III3 and IV4 did not consent to DNA
extraction.
Polymerase chain reaction (PCR) expansion
of Machado-Joseph disease 1 (MJD1) gene trinucleotide repeat
fragments
Since genetic anticipation was observed in this
family, and pseudo-exophthalmos caused by upper-eyelid retraction
is a specific manifestation of hereditary spinocerebellar ataxia
type 3 (SCA3)/MJD (7), the MJD1
gene trinucleotide sequences were amplified to detect any
mutations. MJD1 gene primers were designed according to the
literature (11,12) and were synthesized by Sagon Company
(Shanghai, China). The PCR system comprised 200 μmol/l
deoxynucleotide triphosphates (Roche Diagnostics GmbH, Nonnenwald,
Germany), 0.2 μmol/l of each primer, 50 ng gDNA, 1 unit Taq
polymerase (GE Healthcare Life Sciences, Chalfont, UK) and 1 μl 10X
PCR buffer (100 mM Tris-HCl, pH 8.5; 500 mM KCl, 1.5% Triton
X-100). Deionized water was added to a total volume of 10 μl. A
two-phase cycle PCR was used. Firstly, the sample was predenatured
at 95°C for 5 min. In the first-phase cycle the samples were
denatured at 95°C for 1 min. The initial annealing temperature was
62°C for 1 min, which was decreased by 1°C for each cycle, and
extension was performed at 72°C for 2 min, for a total of 10
cycles. In the second-phase cycle, the samples were denatured at
95°C for 1 min, annealed at 52°C for 1 min and extended at 72°C for
2 min, for a total of 25 cycles. The final step was extension at
72°C for 10 min.
PCR product detection
A 2-μl sample of PCR product was taken and mixed
with 2X denaturing buffer (95% formamide, 20 mM EDTA, 0.05%
bromophenol blue, 0.05% xylene cyanol). The PCR product was
denatured at 99°C for 10 min and cooled rapidly in an ice-bath, the
PCR product was then electrophoretically separated in an 8%
polyacrylamide gel containing 7 mol/l urea (the electrophoresis
buffer was 0.5X TBE; 44.5 Tris-HCl, 44.5 mM boric acid, 1 mM EDTA,
pH 8.0). Following 30 min of electrophoresis at <300 V,
electrophoresis at 300 V was applied for 4 h. Silver staining and
photographic analysis were then carried out. The PCR products of
abnormal bands were purified, cloned into the pGEM®-T
Easy vector (Promega Corp, Madison, WI, USA) and sequenced, and the
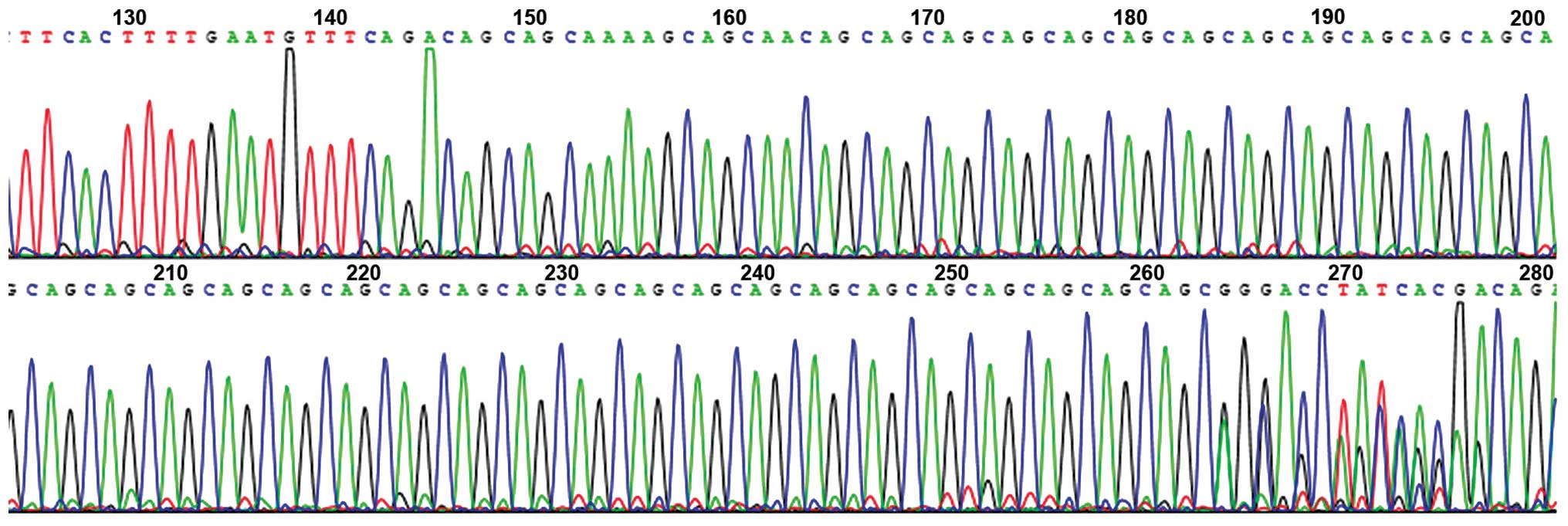
number of trinucleotide CAG repeats was directly read out from an
ABI Prism®377 DNA sequencer (Applied Biosystems, Foster
City, CA, USA).
Results
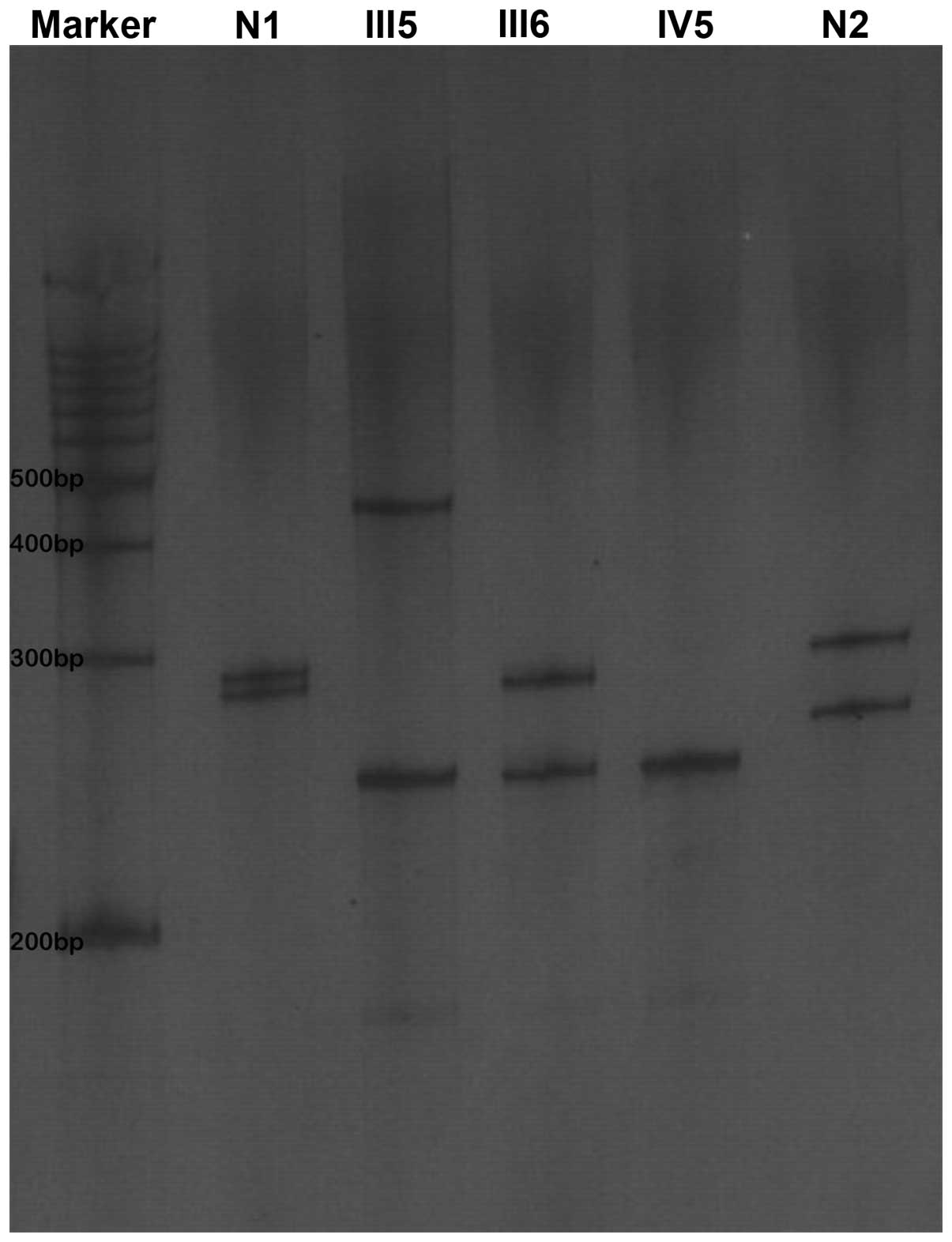
Proband III5 was found to be a heterozygote for an
MJD1 gene mutation, and the CAG repeat number of the mutant allele
was 80. The CAG repeat numbers of the wife of the proband (III6)
and the son (IV5) were 26 and 15, respectively. All of the patients
were heterozygotes. The CAG number of the normal controls was 29
(Figs. 2 and 3).
Discussion
SCA3/MJD and HSP are common genetic diseases in the
clinic and their disease spectrum shows mutual overlaps. The main
clinical manifestation of SCA3/MJD is cerebellar ataxia, while
other features include pyramidal signs, extrapyramidal symptoms,
muscle atrophy, peripheral neuropathy and dementia. It has been
reported that SCA3/MJD has several clinical variants. Clinical
manifestations include dystonia musculorum deformans, Parkinson’s
disease or spastic paraplegia without ataxia (13–16).
The clinical manifestation of the family studied in
the present investigation was spastic paraplegia but another
significant feature was genetic anticipation. Mild atrophy was
present in the cervical spinal cord, as observed using MRI, and the
patients exhibited eyelid contracture and horizontal nystagmus. The
most well-known disease clinically featuring dynamic mutation is
SCA3/MJD, so MJD1 mutation detection was performed. This gave a
genetic diagnosis of SCA3/MJD. It was not possible to obtain the
DNA from patients III3 and IV4; therefore the dynamic mutation of
the MJD1 gene of the family could not be verified at the DNA level.
Genetic diagnosis was performed, however, for patient IV5, who was
too young to have reached the age-segment of incidence, confirming
that the patient did not carry the pathogenic gene.
The cause of clinical variance in patients with SCA
is not clear. The larger the CAG repeat number, the earlier the age
of onset. Furthermore, the CAG repeat number is, to some extent,
associated with the severity of the disease. The more serious the
disease, the more impact it is likely to have on the family. The
molecular mechanism of genetic anticipation is intergenerational
unstable amplification of CAG repeat sequences. There is a trend
that the CAG repeat number increases with generation, and the
age-at-onset decreases in successive generations. The age of onset
is inversely correlated with the CAG repeat number. Maruyama et
al (17) reported that, in 89
to 100% of patients with SCA3/MJD, dynamic mutations of CAG
trinucleotide repeats existed, and the repeat number was 61 to 84.
The normal CAG repeat number was 14 to 37. In addition to the CAG
repeat number, the phenotype of the disease was affected by certain
regulating factors, such as regulating genes, the change in
polymorphism within and on either side of the gene loci, genetic
imprinting and environmental factors (17,18).
SCA has numerous subtypes with complex and
changeable phenotypes. Atypical SCA3/MJD can be manifested as
spastic paraplegia without evident ataxia. For those families with
HSP involving the nervous system and showing genetic anticipation
in the clinic, an MJD1 genetic diagnosis should be considered to
compensate for the insufficient diagnosis of HSP. This would
promote the prepotency health care and, therefore, gradually reduce
the incidence of the disease.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (no. 81201001).
References
|
1
|
McMonagle P, Webb S and Hutchinson M: The
prevalence of ‘pure’ autosomal dominant hereditary spastic
paraparesis in the island of Ireland. J Neurol Neurosurg
Psychiatry. 72:43–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silva MC, Coutinho P, Pinheiro CD, et al:
Hereditary ataxias and spastic paraplegias: methodological aspects
of a prevalence study in Portugal. J Clin Epidemiol. 50:1377–1384.
1997. View Article : Google Scholar
|
|
3
|
Fink JK: Hereditary spastic paraplegia.
Curr Neurol Neurosci Rep. 6:65–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coutinho P, Ruano L, Loureiro JL, et al:
Hereditary ataxia and spastic paraplegia in Portugal: a
population-based prevalence study. JAMA Neurol. 70:746–755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paulson H: Machado-Joseph
disease/spinocerebellar ataxia type 3. Handb Clin Neurol.
103:437–449. 2012. View Article : Google Scholar
|
|
6
|
Jiang H, Tang B, Xia K, et al:
Spinocerebellar ataxia type 6 in Mainland China: molecular and
clinical features in four families. J Neurol Sci. 236:25–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harding AE: Classification of the
hereditary ataxias and paraplegias. Lancet. 21:1151–1155. 1983.
View Article : Google Scholar
|
|
8
|
Li CM, Zhang C, Lu XL, et al: Clinical
features and zygosity diagnosis of hereditary spastic paraplegia in
identical twins. Zhoghua Shenjing Yixue Zazhi. 5:1122–1124.
2006.(In Chinese).
|
|
9
|
Medical Research Council. Aids to the
Examination of the Peripheral Nervous System. Memorandum no. 45
London: Her Majesty’s Stationery Office; 1976
|
|
10
|
Mo DH, Xu H, Zhou W, et al: Susceptibility
gene for stroke or cerebral infarction in the Han population in
Hunan Province of China. Neural Regen Res. 8:1519–1527.
2013.PubMed/NCBI
|
|
11
|
Han Y, Zheng H, Ding S, et al: The
clinical features and genomic diagnosis of hereditary
spinocerebellar ataxia type 1. Cuzhong Yu Shenjing Jibing.
5:520–522. 2006.(In Chinese).
|
|
12
|
Gallassi R, Morreale A, Montagna P, et al:
Fatal familial insomnia: behavioral and cognitive feaures.
Neurology. 46:935–939. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong Q, Surewicz WK, Petersen RB, et al:
Inherited prion diseases. Prion Biology and Diseases. Prusiner SB:
2nd edition. Cold Spring Harbor Laboratory Press; New York, NY: pp.
673–775. 2004
|
|
14
|
Sakai T and Kawakami H: Machado-Joseph
disease: A proposal of spastic paraplegic subtype. Neurology.
46:846–847. 1996.PubMed/NCBI
|
|
15
|
Yun JY, Lee WW, Kim HJ, et al: Relative
contribution of SCA2, SCA3 and SCA17 in Korean patients with
parkinsonism and ataxia. Parkinsonism Relat Disord. 17:338–342.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bettencourt C, Santos C, Coutinho P, et
al: Parkinsonian phenotype in Machado-Joseph disease (MJD/SCA3): a
two-case report. BMC Neurol. 11:1312011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maruyama H, Izumi Y, Morino H, et al:
Difference in disease-free survival curve and regional distribution
according to subtype of spinocerebellar ataxia: a study of 1,286
Japanese patients. Am J Med Genet. 114:578–583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maciel P, Gaspar C, DeStefano AL, et al:
Correlation between CAG repeat length and clinical features in
Machado-Joseph disease. Am J Hum Genet. 57:54–61. 1995.PubMed/NCBI
|