Introduction
Embryo implantation is a key factor for a successful
pregnancy. In a previous study, Boomsma et al (1) demonstrated that the contribution of
implantation failure in the number of unsuccessful pregnancies was
higher in stimulated cycles (50%) compared with natural cycles
(30%). Therefore, the embryo quality and the status of the
endometrium are considered to be very important for achieving good
clinical outcomes in patients undergoing assisted reproductive
technology (ART) treatment (2).
ART involves a dynamic and complex process of interaction and
inter-acceptance between the embryo and matrix. A key step is the
synchronization of the receptive endometrium with the blastocyst at
a certain developmental stage, which has the ability to accept the
endometrium (3). According to a
number of animal experiments, the time period during which the
endometrium can accept a blastocyst implantation, known as the
implantation window, is limited and lasts only for a few hours in
rodents (4,5). Similarly, the human implantation
window appears to be between days 20 and 21 of the menstrual cycle,
which is consistent with the appearance of pinopodes on the surface
of endometrial epithelial cells (6,7).
Furthermore, a positive correlation between the number of pinopodes
and embryo implantation has been reported. Therefore, pinopodes are
recognized as an ultrastructural marker of endometrial receptivity
(3).
Zhuyun recipe (ZYR) is a traditional Chinese
medicine that has been widely used for infertility treatment and
in vitro fertilization (IVF) with embryo transfer in
clinical practice. Although the application results are desirable
and satisfactory, the therapeutic mechanism of ZYR remains unclear
(8). In a previous study, ZYR was
shown to significantly increase the pregnancy rate and number of
embryonic implantation sites, as well as the expression of
endometrial leukemia inhibitory factor (LIF) and integrin β3
subunit, which are markers of endometrial receptivity (9). In the present study, the effect of
ZYR treatment on pinopode expression was investigated in mice
subjected to ovulation stimulation (OS) and embryo implantation
dysfunction (EID). The aim of the study was to further explore the
effects of ZYR on endometrial receptivity.
Materials and methods
Plant material and extract
preparation
ZYR consists of Epimedium brevicornum Maxim,
Morinda officinalis How, Chinese Dodder and Eucommia
ulmoides, and was purchased from the Traditional Chinese
Medicine Department of Renmin Hospital of Wuhan University (Wuhan,
China). The ZYR formula was developed by our research group led by
Professor Yang, and the four aforementioned herbal materials were
mixed in a ratio of 15:12:20:15, respectively. An aqueous extract
of ZYR was produced using a previously described method (9). Briefly, the four medicinal materials
were mixed, macerated for 1 h in 8 volume/weight (v/w) distilled
water and decocted for 1 h. The filtrate was collected and the
residue was decocted for a further 1 h with 6 v/w distilled water.
Next, the filtrates were pooled and concentrated, and the extract
was sealed and stored at −20°C. The aseptic decoction contained 0.6
g/ml crude drug.
Animals
A total of 209 mature Kunming mice of
specific-pathogen-free grade (age, 6–8 weeks; weight, 25–28 g) were
provided by Wuhan University Laboratory Animal Center (Wuhan,
China). The female mice were virginal and the male mice had been
proven to be fertile. The mice were bred separately, with free
access to water and a standard diet. The animals were housed in the
laboratory on a 12 h light/dark regimen, at 18–22°C and 70–85%
relative humidity. The study protocol was conformed to the Guide
for the Care and Use of Laboratory Animals published by the
National Institutes of Health (NIH publication no. 85-23, revised
1996), and was approved by the Ethics Committee of Wuhan
University. The animals were handled according to the Wuhan
Directive for Animal Research.
Animal models and experimental
protocol
All 139 female mice were randomly divided into six
groups, including the control (22 mice), OS model (19 mice), OS +
ZYR (26 mice), EID model (20 mice), EID + ZYR (27 mice) and ZYR
only (25 mice) groups. OS was induced in the OS and OS + ZYR groups
with an intraperitoneal injection of 40 IU/100 g pregnant mare
serum gonadotropin (PMSG, Hangzhou Yunuo Chemical Co., Ltd,
Hangzhou, China), followed by 100 IU/100 g human chorionic
gonadotropin (HCG, Lizhu Pharmaceutical Trading Co., Ltd, Zhuhai,
China) after 48 h. After 3 h, the female mice were caged with the
male mice (ratio, 2:1) overnight and the mice were checked for the
presence of a vaginal plug the following morning. Presence of a
vaginal plug was considered as evidence of successful mating,
designating the day as day 1 postcoitum. To induce the EID model,
mice in the EID and EID + ZYR groups were injected subcutaneously
with 0.1 ml mifepristone dissolved in 0.08 mg/0.1 ml propanediol
(mifepristone, Hubei Gedian Renfu Pharmaceutical Co., Ltd, Ezhou,
China) on day 4 postcoitum (10).
In the three ZYR groups (OS + ZYR, EID + ZYR and ZYR
only), the female mice received a daily gastric perfusion of ZYR
decoction during the first four days of pregnancy, with an oral
administration volume of 1.5 ml/100 g per day. The OS and EID model
groups received the same amount of saline, while the control group
were not treated.
Two mice from each group were sacrificed by cervical
dislocation at 21:30–22:00 of day 4 postcoitum, regarded as time
point 1 (T1), while two further mice from each group were
sacrificed at 09:30–10:00 of day 5 postcoitum (T2). The uterine
horns were removed for scanning electron microscopic observation
(Type S_520; Hitachi Instruments, Inc., Tokyo, Japan). The
remaining female mice were sacrificed on day 8 postcoitum and the
uterine horns were excised to determine the number of implantation
sites. The number of pregnant mice and corresponding implanted
embryos in each uterine was recorded.
Ultrastructural observation
Murine uterine horns were cut open along the
longitudinal axis, rinsed with saline solution and fixed in sodium
cacodylate buffer (0.15 mol/l, pH 7.3), containing 2.5% w/v
glutaraldehyde. Next, the samples were refixed in 1% w/v osmium
tetroxide, dehydrated in a graded series of acetone, dried in a
critical-point dryer, mounted on a specimen holder and coated with
gold palladium. A scanning electron microscope (Type S-520; Hitachi
Instruments) was used to observe the samples.
All observations were performed by an appointed
observer. The pinopodes were divided according to their
developmental stage into developing, fully developed and regressing
pinopodes. In addition, the samples were divided into three grades,
based on the number of pinopodes present (11,12):
Few, when <20% pinopodes were observed in the whole area of the
endometrium; moderate, when 20–50% pinopodes were detected; and
abundant, when >50% pinopodes were observed.
Statistical analysis
Data were statistically analyzed and are expressed
as the mean ± standard error of mean. The mean values were analyzed
by analysis of variance, followed by the Newman-Keuls test, while
the number of pregnant mice was analyzed by the χ2 test.
Data analysis was performed using SPSS version 17.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the pregnancy rate and
number of implantation sites in the pregnant mice
The pregnancy rates in the OS (6.67%) and EID
(18.75%) model groups were significantly lower compared with the
control group (83.33%; P<0.001). However, the pregnancy rates of
the OS + ZYR (54.55%) and EID + ZYR (65.22%) groups were evidently
increased compared with the corresponding model groups (OS and EID,
respectively; P<0.01; Fig. 1).
The number of implanted embryos in the EID group (6.67±1.16) was
lower compared with the control group (13.80±1.42; P<0.01).
However, following ZYR treatment, the number of implanted embryos
in the EID + ZYR group (10.13±3.18) was higher compared with the
EID group (P<0.05). By contrast, no statistically significant
differences were observed in the pregnancy rate and number of
implanted embryos between the ZYR only and control groups.
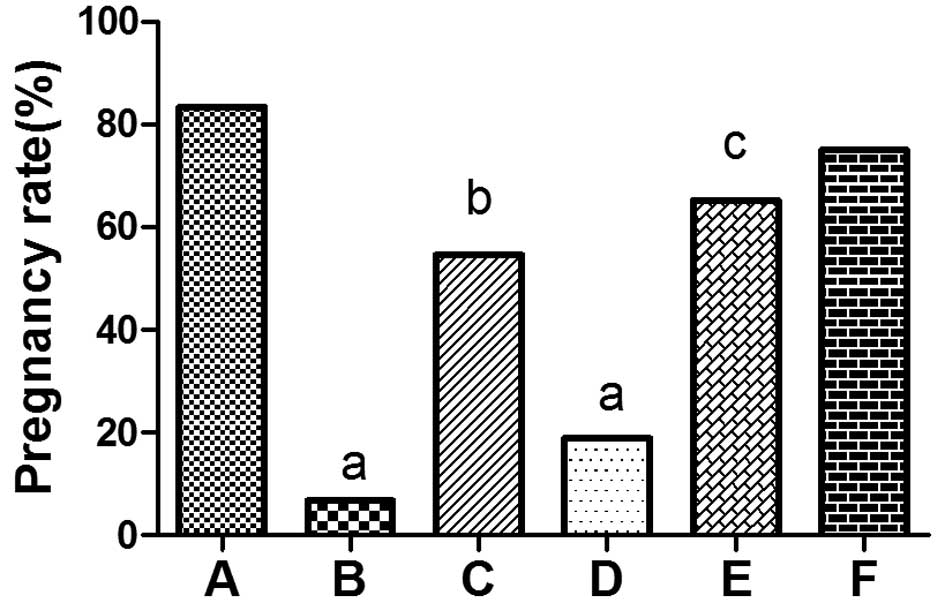 | Figure 1Pregnancy rate on day 8 postcoitum in
(A) control, (B) OS model, (C) OS + ZYR, (D) EID model, (E) EID +
ZYR and (F) ZYR only groups. aP<0.001, vs. control
group; bP<0.01, vs. OS group; cP<0.01,
vs. EID group. OS, ovulation stimulation; ZYR, Zhuyun recipe; EID,
embryo implantation dysfunction. |
Pinopode expression on the endometrial
surface during the implantation window
Murine embryo implantation normally initiates
between 10:00 and 22:00 on day 4 postcoitum, while the implantation
window is maintained between the afternoon of day 4 postcoitum and
the morning of day 5 postcoitum. In order to understand the
establishment of endometrial receptivity during the window of
implantation, two time-points were selected (T1, 21:30–22:00 of day
4 postcoitum; T2, 09:30–10:00 of day 5 postcoitum) for the
observation of pinopode expression.
In the control group, specimens collected at T1
revealed a large number of membranous projections, with
inconsistent shapes and sizes, unclear boundaries and rough
surfaces covered with short microvilli, which indicated the
presence of abundant developing pinopodes (Fig. 2A). At T2, the abundant pinopodes
were evenly distributed over the endometrial surface, more
protruded and consistent in shape and size, and had clearer
boundaries, while the microvilli merged together and disappeared
gradually, indicating abundant fully developed pinopodes (Fig. 2B).
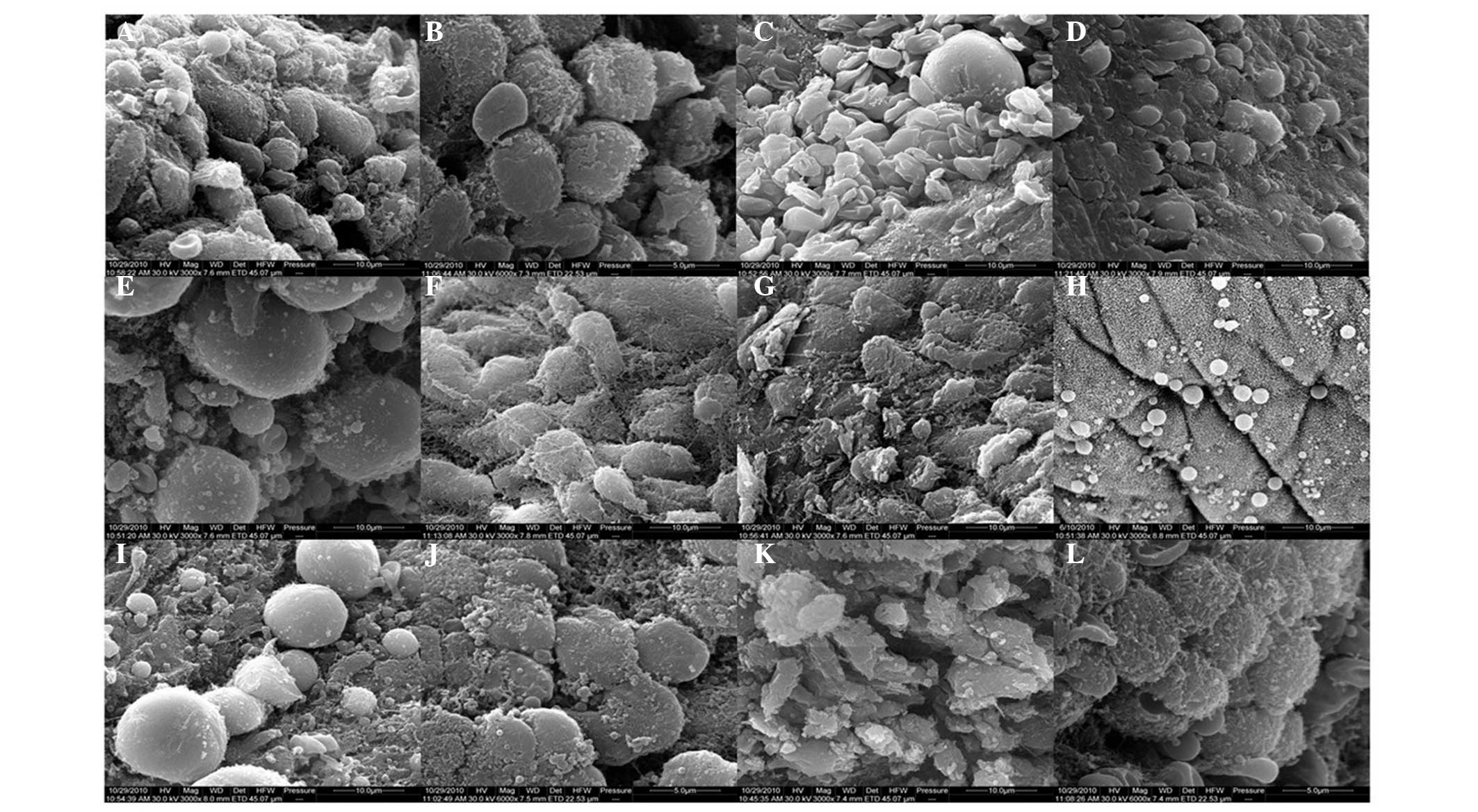 | Figure 2Scanning electron microscopy images
showing the expression of pinopodes on the endometrial surface
during the implantation window of mice. In the control group, (A)
developing pinopodes at T1 and (B) fully developed pinopodes at T2
were observed. In the OS group, (C) regressing pinopodes at T1 and
(D) fully regressing pinopodes at T2 were observed. In the OS + ZYR
group, (E) a number of fully developed pinopodes at T1 and (F)
developing pinopodes at T2 were observed. In the EID group, (G)
restrained expression of pinopodes at T1 and (H) fully restrained
expression of pinopodes at T2 were observed. In the EID + ZYR
group, (I) a number of fully developed pinopodes at T1 and (J)
fully developed pinopodes at T2 were observed. In the ZYR only
group, (K) irregularly developing pinopodes at T1 and (L) fully
developed pinopodes at T2 were observed. Magnification for a, c-i
and k, ×3,000; magnification for b, j and l, ×6,000. OS, ovulation
stimulation; EID, embryo implantation dysfunction; ZYR, Zhuyun
recipe. |
In the OS group, a moderate number of pinopodes
without microvilli were observed on the endometrial surface at T1,
which were mostly collapsed and flat, representing moderate
regressing pinopodes (Fig. 2C). At
T2, few, small and scattered pinopodes were distributed over the
endometrial surface (Fig. 2D),
which appeared earlier compared with the control group. By
contrast, in the OS + ZYR group, moderate pinopodes of different
sizes were observed on the endometrial surface at T1, a number of
which were well-developed with large projections (Fig. 2E). At T2, a greater number of cell
surfaces showed projections, which represented abundant developing
pinopodes (Fig. 2F). When compared
with the OS group, the appearance of pinopodes on the endometrial
surface was delayed following treatment with ZYR, which was
comparable to the control group.
In the EID group, few pinopodes with microvilli
appeared on the endometrial surface at T1 (Fig. 2G). However, at T2, fewer
well-developed pinopodes with small projections were observed
(Fig. 2H), indicating that
pinopode appearance was restrained by mifepristone. In the EID +
ZYR group, moderate pinopodes of different sizes were unevenly
distributed over the endometrial surface at T1, a number of which
were fully developed with large projections (Fig. 2I). At T2, abundant fully developed
pinopodes were observed (Fig. 2J).
Therefore, the restrained expression of pinopodes in the EID group
was improved following treatment with ZYR, and the expression was
similar to the control group.
In the ZYR only group, the majority of the
microvilli had disappeared at T1 and the pinopodes were transformed
into irregularly shaped projections, without distinguished cell
borders (Fig. 2K). At T2, the
pinopodes appeared to be well-developed, although a few had
collapsed (Fig. 2L), and were
similar to the pinopodes observed in the control group.
Discussion
In the present study, the effect of ZYR on the
expression of pinopodes on the endometrial surface of mice
subjected to ovarian stimulation (OS) or embryo implantation
dysfunction (EID) was demonstrated for the first time. A marked
decrease in the number of fully developed pinopodes on the
endometrial surface was observed in the OS and EID model mice,
which was in accordance with the decreased pregnancy rates and
embryonic implantation sites of the two model groups, when compared
with the control group. Following treatment with ZYR, an evident
increase was observed in the expression of fully developed
pinopodes in the EID + ZYR and OS + ZYR groups, while the pregnancy
rates and embryonic implantation sites of these groups also
increased. In a previous study (9), ZYR was found to significantly
increase the pregnancy rate and number of embryonic implantation
sites, and partly improve endometrial receptivity through
reinforcing the expression of endometrial LIF and integrin β3
subunit. In the present study, pinopode expression was investigated
to further explore the therapeutic mechanism of ZYR. The pinopodes
were found to be fully developed in the OS + ZYR and EID + ZYR
groups. Thus, the results of the present study reinforce the
hypothesis that the endometrial expression of pinopodes is
positively associated with endometrial receptivity and embryonic
implantation. In addition, ZYR was found to improve the endometrial
receptivity in OS and EID mice by significantly increasing the
spatial and temporal expression of pinopodes.
Pinopodes are widely known to be an important
morphological marker indicating the establishment of endometrial
receptivity and the opening of the implantation window. The
appearance and full development of pinopodes indicates that the
endometrium is ready for blastocyst adhesion and implantation
(7,13,14).
Pinopode deficiency in reproductive females undergoing in
vitro fertilization (IVF) and embryo transfer treatment results
in multiple implantation failures. Previous studies in humans have
revealed that the window of implantation, which begins upon the
formation of fully developed pinopodes, opens and closes earlier in
females undergoing ovarian hyperstimulation for IVF compared with
females with natural cycles (15–17).
In addition, a low dose of mifepristone, which is administered as a
progesterone (P4) receptor antagonist, restrains the development
and maturity of pinopodes (18).
In the current study, the appearance of pinopodes following OS was
earlier when compared with the control group, and the pinopode
expression was restrained by mifepristone, which is consistent with
the results of the aforementioned studies. Furthermore, previous
studies on mice and humans have revealed that changes in the
implantation window and abnormal expression of pinopodes may
severely influence the synchrony between embryo and endometrial
development, which is necessary for successful implantation
(19,20). Therefore, the lower expression of
fully developed pinopodes in the OS and EID model groups may be
associated with the lower rates of pregnancy and embryonic
implantation. The present study demonstrated that the spatial and
temporal expression of pinopodes changes following OS or the
administration of mifepristone, negatively impacting the synchrony
between the embryo and endometrial development.
ZYR is a traditional Chinese medicine composed of
Epimedium brevicornum Maxim, Morinda officinalis How,
Chinese Dodder and Eucommia ulmoides. Although ZYR has been
long used in infertility treatment, the underlying therapeutic
mechanism has only been studied recently. ZYR has been found to
attenuate the damage caused by superovulation and mifepristone by
increasing the expression of endometrial LIF and integrin β3
subunit (9). In the present study,
pinopodes were detected as a novel target of ZYR. The appearance of
pinopodes in OS mice was delayed following treatment with ZYR,
while the restrained expression of pinopodes in EID mice was
improved following ZYR treatment, which were similar to the control
mice. Previous studies have demonstrated that the duration of
pinopode formation in rodent and human endometrium is limited to a
short time period; in particular, pinopodes were found to persist
in humans for <48 h during the mid-luteal phase of the menstrual
cycle (15,16). In addition, pinopode appearance has
been shown to be P4-dependent (21), while administration of estradiol
(E2) results in the rapid loss of pinopodes (22). The P4 and E2 hormones
act together to regulate the development and regression of
pinopodes. Furthermore, oocyte donation studies have revealed that
females treated with E2 and P4 have a higher chance of
conception compared with individuals undergoing OS (15). In addition, the implantation window
may be regulated by hormone replacement therapy in humans (23). Notably, pharmacological studies
hypothesized that icarrin, Morinda officinalis How and
Chinese Dodder have estrogen-like effects (24–26),
while Eucommia ulmoides has progestin-like effects (27). Therefore, these observations led to
hypothesis that the effect of ZYR on pinopode expression may be due
to balancing the serum E2 and P4 levels in OS and EID
mice, which regulate the spatial and temporal expression of
pinopodes and synchronize the embryo and endometrial development at
the time of implantation.
Controlled ovarian hyperstimulation treatment has
been widely used as an important step in ART, aiming to increase
the number of oocytes retrieved for IVF and improve the overall
chance for a successful fertilization and pregnancy. However, due
to the negative influence of superstimulation on endometrial
receptivity, the pregnancy rate remains low, with the implantation
of a high number of transferred embryos unsuccessful. Despite the
considerable efforts of clinicians to improve endometrial
receptivity by cryoperservation of embryos or local injury to the
endometrium, the results remain unsatisfactory (28). In the present
study, the significantly higher expression of pinopodes, increased
pregnancy rate and increased number of embryonic implantation sites
following treatment with ZYR reveals an alteration in the
endometrial receptivity. Therefore, the results support the
aforementioned hypothesis, and may provide further insight into the
improved treatment of endometrial receptivity. As the effects of
ZYR on the safety of offspring, and other molecular and genetic
markers of endometrial receptivity in mice are currently unknown,
further studies are required to provide more definitive
answers.
In conclusion, the present study has revealed the
beneficial effects of ZYR on pinopode expression on the endometrial
surface of mice with EID and OS. The results demonstrated that
treatment of EID and OS mice with ZYR significantly synchronized
the expression of pinopodes and embryo development, and increased
the number of embryo implantation sites and pregnancy rate.
Therefore, ZYR may be used to improve endometrial receptivity and
embryonic implantation in humans. Furthermore, the current study
provides evidence on the mechanisms of traditional Chinese
medicine, which may be used to safely circumvent the negative
impact of OS on endometrial receptivity during the procedure of
IVF. Thus, ZYR may provide a new insight into improving the
treatment of endometrial receptivity. However, further studies are
required to provide more information on the use of ZYR and address
the safety of offspring.
Acknowledgements
The authors thank the Wuhan University Test Center
for providing the scanning electron microscope. This study was
supported by a grant from the Wuhan Science and Technology Plan
Projects (no. 200860423222).
References
|
1
|
Boomsma CM, Kavelaars A, Eijkemans MJ, et
al: Endometrial secretion analysis identifies a cytokine profile
predictive of pregnancy in IVF. Hum Reprod. 24:1427–1435. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santos MA, Kuijk EW and Macklon NS: The
impact of ovarian stimulation for IVF on the developing embryo.
Reproduction. 139:23–34. 2010. View Article : Google Scholar
|
|
3
|
Cavagna M and Mantese JC: Biomarkers of
endometrial receptivity - a review. Placenta 24 (Suppl B): S39–S47,
2003 Psychoyos A: Hormonal control of ovoimplantation. Vitam Horm.
31:201–256. 1973.
|
|
4
|
Psychoyos A: Hormonal control of uterine
receptivity for nidation. J Reprod Fertil Suppl. 17–28.
1976.PubMed/NCBI
|
|
5
|
Acosta AA, Elberger L, Borghi M, et al:
Endometrial dating and determination of the window of implantation
in healthy fertile women. Fertil Steril. 73:788–798. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikas G: Endometrial receptivity: changes
in cell-surface morphology. Semin Reprod Med. 18:229–235. 2000.
View Article : Google Scholar
|
|
7
|
Yu N, Yang J and Yin T: Extracts from a
traditional Chinese herbal remedy (Zhuyun recipe) improve
endometrial receptivity in mice with embryonic implantation
dysfunction and ovulation stimulation. J Ethnopharmacol.
137:389–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang M, Liu YF, Lv YY and Huang L: The
effect mechanism of Traditional Chinese medicine to improve the
receptivity of endometrium. Zhong Yi Za Zhi. 54:1064–1066. 2013.(In
Chinese).
|
|
9
|
Liu YJ, Huang GY, Lu F, et al:
Establishment of mice model with embryo implantation dysfunction.
Zhong Guo Yao Li Xue Tong Bao. 19:1315–1318. 2003.(In Chinese).
|
|
10
|
Nikas G: Pinopodes as markers of
endometrial receptivity in clinical practice. Hum Reprod. 14(Suppl
2): S99–S106. 1999. View Article : Google Scholar
|
|
11
|
Salehnia M: Different pattern of pinopodes
expression in stimulated mouse endometrium. Exp Anim. 54:349–352.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang DM, Huang GY and Lu FE: Effect of
Bushenyiqihexue recipe on the expression of endometrial pinopodes
in blastocyst implantation dysfunctional mice. Zhonghua Fu Chan Ke
Za Zhi. 39:230–233. 2004.(In Chinese). PubMed/NCBI
|
|
13
|
Pantos K, Nikas G, Makrakis E, et al:
Clinical value of endometrial pinopodes detection in artificial
donation cycles. Reprod Biomed Online. 9:86–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nikas G, Drakakis P, Loutradis D, et al:
Uterine pinopodes as markers of the ‘nidation window’ in cycling
women receiving exogenous oestradiol and progesterone. Hum Reprod.
10:1208–1213. 1995.PubMed/NCBI
|
|
15
|
Nikas G, Develioglu OH, Toner JP and Jones
HW Jr: Endometrial pinopodes indicate a shift in the window of
receptivity in IVF cycles. Hum Reprod. 14:787–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopata A, Bentin-Ley U and Enders A:
‘Pinopodes’ and implantation. Rev Endocr Metab Disord. 3:77–86.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DM, Nardo LG, Huang GY, et al:
Effect of a single dose of mifepristone on expression of pinopodes
in endometrial surface of mice. Acta Pharmacol Sin. 26:212–219.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Busso CE, Melo MA, Fernandez M, et al:
Implantation in IVF. Int Surg. 91(5 Suppl): S63–S76. 2006.
|
|
19
|
Diedrich K, Fauser BC, Devroey P and
Griesinger G; Evian Annual Reproduction (EVAR) Workshop Group. The
role of the endometrium and embryo in human implantation. Hum
Reprod Update. 13:365–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stavreus-Evers A, Nikas G, Sahlin L, et
al: Formation of pinopodes in human endometrium is associated with
the concentrations of progesterone and progesterone receptors.
Fertil Steril. 76:782–791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martel D, Monier MN, Roche D and Psychoyos
A: Hormonal dependence of pinopode formation at the uterine luminal
surface. Hum Reprod. 6:597–603. 1991.PubMed/NCBI
|
|
22
|
Kodaman PH and Taylor HS: Hormonal
regulation of implantation. Obstet Gynecol Clin North Am.
31:745–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Li E, Lu Z, et al: Effects of
icarrin on secretive function of cultured granulosa and adrenal
cortical cells of rats. Zhongguo Zhong Yao Za Zhi. 22:499–500.
1997.(In Chinese).
|
|
24
|
Qin D, Zuo B and Zuo Y: Effects of
flavonoids of Semen Cuscutae on reproductive function of animals.
Zhong Yao Xin Yao Yu Lin Chuang Yao Li. 11:349–351. 2000.(In
Chinese).
|
|
25
|
Chen XY, Cheng MJ and Ye ZQ: Study on
invigorating Yang effect of time-selecting administration of
Morinda officinalis How. Lin Chuang He Li Yong Yao. 2:31–32.
2009.(In Chinese).
|
|
26
|
Hunag WG, Zeng QZ, Pan ZX and Tang JK:
Study on the main pharmacodynamics and acute toxicity of eucommia
leaves electuary. Gui Zhou Yi Yao. 24:325–326. 2000.(In
Chinese).
|
|
27
|
Cakmak H and Taylor HS: Implantation
failure: molecular mechanisms and clinical treatment. Hum Reprod
Update. 17:242–253. 2011. View Article : Google Scholar :
|
















