Introduction
Atrial fibrillation (AF) is the most common type of
cardiac arrhythmia; however, current therapies are not efficient in
the management of this condition (1). Although multiple treatment methods
exist, no single modality is effective in all patients. Pulmonary
vein isolation has emerged as an effective method for the treatment
of AF. However, recurrences are frequent and occur in 10–40% of
patients (2–4). An increasing number of clinical and
experimental studies have revealed that AF is associated with
atrial electrical and structural remodeling (5). Valsartan (recognized by the trade
name, Diovan) is an angiotensin II (Ang II) receptor antagonist,
also known as Ang II receptor blocker (ARB), which possesses a
particularly high affinity for the type I Ang II (AT1)
receptor (6). A previous study in
atrial rapid-pacing dogs has demonstrated that valsartan may
prevent the induction and promotion of AF through the attenuation
of calpain I upregulation and the suppression of atrial structural
remodeling (7). Furthermore, Ang
II has been demonstrated to be involved in the mediation of
inflammatory responses, which are also involved in the development
of AF (8). However, previous
studies on the comparative effects of the administration of
Ang-converting-enzyme inhibitor (ACEI) or ARB on the incidence of
AF have reported inconsistent findings (9–11).
Despite some studies demonstrating that ARB may not lower the
incidence of AF, further studies are required due to the multiple
biological effects of ARB. The aim of the present study was to
evaluate the efficacy of treatment with ARBs in patients undergoing
antral pulmonary vein isolation.
Materials and methods
Participants
In total, 120 randomly selected patients, referred
to the Arrhythmia Unit of the Beijing AnZhen Hospital (Beijing,
China) for AF ablation therapy between December 2008 and July 2012,
were enrolled into this study. Patients developing major
postablation complications or in which AF ablation was considered
to be unsuccessful (patients presenting permanent AF) were not
included in the analysis, due to the low possibility of late
conversion to sinus rhythm in these patients, and the effect of
renin-Ang-aldosterone system (RAAS) inhibitors in decreasing AF
could not be demonstrated. Further exclusion criteria included:
Secondary causes of hypertension; congestive heart failure with
symptoms classified as class II to IV, according to the New York
Heart Association functional classification (12); left ventricular ejection fraction
of <35%; previous coronary artery stenting or angioplasty;
previous AF ablation procedure; transverse left atrial diameter of
60 mm, detected by transthoracic echocardiography; administration
of amiodarone treatment; valvular diseases; and suffering from type
1 diabetes mellitus. This study was approved by the Medical Ethics
Committee of Beijing AnZhen Hospital. All the patients provided
informed written consent prior to participation in this study.
Study design
All the patients were randomly divided, by
computer-generated randomization, into the control (placebo), 80
mg/day valsartan or 160 mg/day valsartan (Novartis AG, Basel,
Switzerland) groups. The variables considered in this study
included: age, gender, body mass index, presence/absence of
coronary artery disease, type of AF (paroxysmal and persistent),
hypertension and diabetes mellitus. Baseline examinations were
performed prior to ablation. The patients administered ACEIs or
ARBs for the treatment of hypertension were also administered a
calcium antagonist at least four weeks before AF ablation. Three
weeks before ablation, all the patients were administered the
anticoagulant, warfarin (target international normalized ratio,
2.0–3.0; Shanghai Xinyi Pharmaceutical Co., Ltd., Shanghai, China),
unless contraindicated. Patients free from AF were further
adminsitered warfarin three months after ablation. Subsequently,
the patients were subjected to pulmonary vein isolation and were
administered valsartan. The valsartan dose of 160 mg/day was
reached by administration in two stages, lasting one week each; the
patients were admininstered 80 mg/day in the first week, and 160
mg/day in the next week. For all the patients, follow-up was
performed for at least one year after ablation. The initial three
months after AF ablation were considered to be the ‘blanking
period’, during which AF recurrence was not considered as an
indicator of ablation failure. The patients were examined each
month within the first 3 months, followed by examination at six and
twelve months after AF ablation, as well as on any other occasions
when the patients presented symptoms. A 24-h Holter recording was
performed at one, six and twelve months after ablation, in order to
monitor the electrocardiograms of the patients. Prior to AF
ablation, all the patients were subjected to delayed enhancement
cardiac magnetic resonance imaging to determine the extent of
atrial fibrosis (13). In order to
determine the extent of myocardial fibrosis, the patients received
intravenous gadolinium (0.1 mmol/kg body weight;
MultiHance®; Bracco Diagnostics Inc., Princeton, NJ,
USA).
Outcome measurements
The present study compared the administration of 80
mg/day valsartan, 160 mg/day valsartan and a placebo to assess the
efficacy of different doses of valsartan based on the cumulative
number of patients relapsing into AF following ablation to maintain
the sinus rhythm. The primary endpoint was failure of the AF
treatment during the follow-up. The beginning of the follow-up
period for this purpose was considered to be the day of the
ablation. A blanking period of three months was used after
ablation, and recurrences within the first three months were not
classified as failure of the ablation. Any patient suffering a
persistent atrial arrhythmia during this period was reverted to
sinus rhythm by electrical cardioversion.
Statistical analysis
Statistical analysis was performed using the SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). The results
are expressed as the mean ± standard deviation. Categorical
variables were compared by χ2 analysis, while continuous
variables were compared by Student’s t-test. Kaplan-Meier analysis
was performed to determine the probability of treatment success,
which was expressed as the percentage of patients remaining free
from AF. Differences in arrhythmia-free survival were assessed with
the log-rank test. The recurrence of AF was analyzed using the Cox
proportional-hazards regression, to regulate potentially
confounding factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
In total, 120 patients were included in the
analysis. A total of 40 patients were randomly selected for
treatment with a placebo, while 40 patients were treated with 80
mg/day valsartan and 40 patients were treated with 160 mg/day
valsartan. The baseline clinical characteristics (prior to
ablation) of each group are shown in Table I. No statistically significant
differences were observed in all the pre-treatment characteristics
among the three treatment groups. The median duration of AF prior
to randomization was 8.6±7.2, 9.7±8.2 and 9.1±7.8 months in the 80
mg/day valsartan, 160 mg/day valsartan and placebo groups,
respectively, with no statistically significant differences
observed among the three groups. No patients succumbed to the
disease or were not able to follow-up during the study.
 | Table IMain demographic, clinical and
echographic characteristics of patients in the three treatment
groups. |
Table I
Main demographic, clinical and
echographic characteristics of patients in the three treatment
groups.
| Variable | Control (n=40) | 80 mg/day valsartan
(n=40) | 160 mg/day valsartan
(n=40) |
|---|
| Age, years | 63.4±8.5 | 62.5±8.1 | 63.7±9.2 |
| Male gender, n
(%) | 18 (45.0%) | 17 (42.5) | 22 (55.0) |
| Weight, kg | 75.3±14.1 | 74.3±12.9 | 78.2±15.3 |
| SBP, mmHg | 135.6±19.3 | 136.6±20.1 | 135.8±18.7 |
| DBP, mmHg | 76.3±11.2 | 80.1±12.8 | 79.6±11.6 |
| TC, mg/dl | 187.3±13.2 | 179.5±11.8 | 189.5±14.2 |
| HDL-C, mg/dl | 34.6±5.2 | 38.7±5.3 | 33.5±4.8 |
| FPG, mg/dl | 106.2±10.0 | 110.7±11.1 | 109.7±10.8 |
| HR, beats/min | 76.2±12.1 | 72.7±10.1 | 77.9±13.1 |
| AF duration,
months | 8.6±7.2 | 9.7±8.2 | 9.1±7.8 |
| Episodes of AF,
n | 2.6±0.7 | 2.4±0.6 | 2.7±0.9 |
| Ejection fraction,
% | 61.0±8.2 | 60.8±8.2 | 60.1±7.9 |
| Pre-ablation atrial
fibrosis, n | 17.2±11.8 | 17.8±12.0 | 17.3±11.8 |
| BMI | 23.6±2.4 | 24.1±2.5 | 23.8±2.4 |
| Presence of CAD
(n) | 7 (17.5) | 8 (20) | 8 (20) |
| Type of AF, n
(%) |
| Paroxysmal | 33 (82.5) | 32 (80) | 30 (75) |
| Persistent | 7 (17.5) | 8 (20) | 10 (25) |
Following the completion of a mean follow-up period
of 12.1±9.6 months, 92 patients were found to be free from AF
(Table II). At the end of the
follow-up period, 21/40 patients treated with 80 mg/day valsartan,
38/40 patients treated with 160 mg/day valsartan and 18/40 patients
treated with a placebo were found to be free of AF. The systolic
blood pressure (SBP) and diastolic blood pressure (DBP) at the end
of the follow-up period were significantly different in patients
treated with 80 mg/day (SBP, 114±19 mmHg; DBP, 72±11 mmHg DBP) and
160 mg/day valsartan (SBP, 125±10 mmHg; DBP, 71±8 mmHg) valsartan,
when compared with the control patients (SBP, 130±15 mmHg; DBP,
78±13 mmHg). Univariate analysis identified that P=0.3 for SBP and
P=0.03 for DBP.
 | Table IIResults of the intention-to-treat
analysis. |
Table II
Results of the intention-to-treat
analysis.
| Variable | Control (n=40) | 80 mg/day valsartan
(n=40) | 160 mg/day valsartan
(n=40) |
|---|
| Sinus rhythm (three
months after ablation), n | 26 | 30 | 38 |
| Sinus rhythm (end of
follow-up), n | 18 | 21 | 36 |
| Systolic blood
pressure (end of follow-up), mmHg | 125±10 | 130±15 | 114±19 |
| Diastolic blood
pressure (end of follow-up), mmHg | 71±8 | 78±13 | 72±11 |
| Mortality rate (end
of follow-up), n | 0 | 0 | 0 |
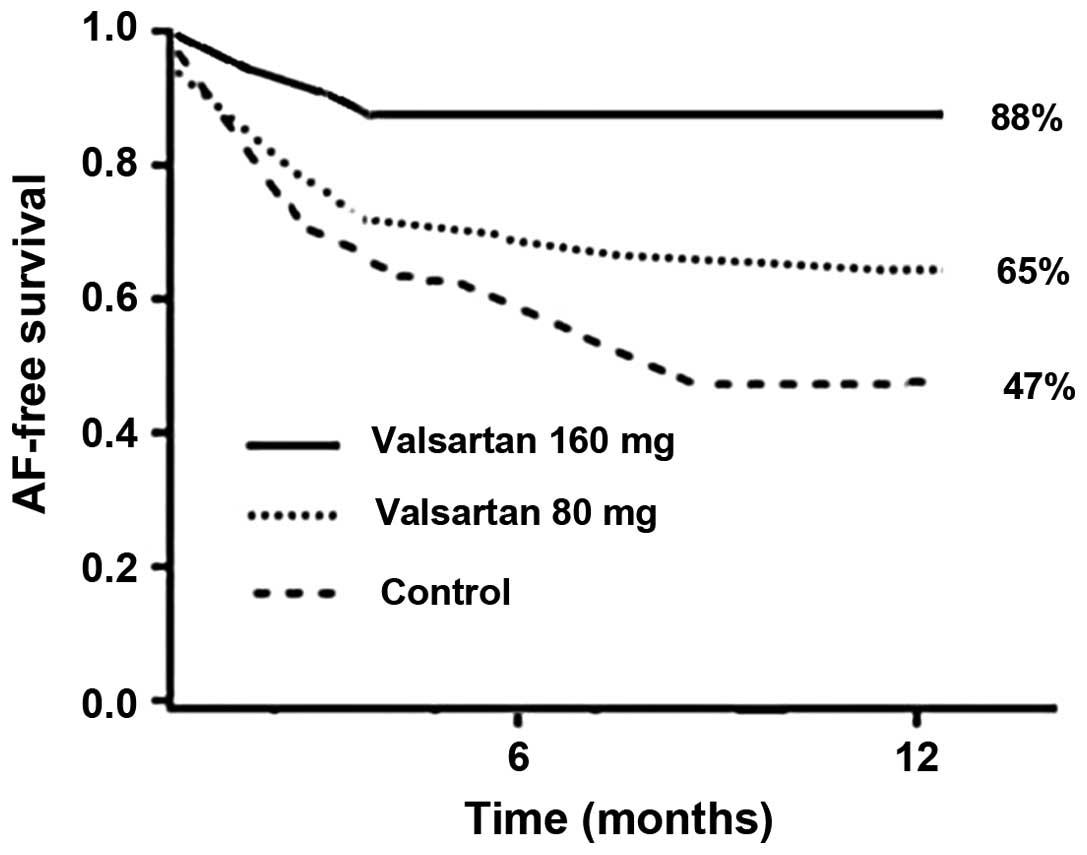
Kaplan-Meier analysis demonstrated that the 12-week
probability for maintaining sinus rhythm was 92% in patients
receiving 160 mg/day valsartan, whereas the probability was 76% in
patients receiving 80 mg/day valsartan and 67% in patients
receiving placebo treatment (P<0.05). The Kaplan-Meier analysis
of the time until the first recurrence during the follow-up period
revealed that patients treated with 160 mg/day valsartan presented
a greater probability of remaining free of AF (88% in the 160
mg/day valsartan group, vs. 65% in the 80 mg/day valsartan group
and 47% in the placebo group; Fig.
1). Treatment with 80 mg/day valsartan was not found to
decrease the recurrence rate of AF when compared with the control
group (P=0.07). No statistically significant difference was
observed between the 80 and 160 mg/day valsartan doses (P=0.06),
whereas the difference between the 160 mg/day valsartan and control
groups was statistically significant (P=0.01).
Multivariate analysis revealed that the use of 160
mg/day valsartan was the only significant variable associated with
the maintenance of sinus rhythm following ablation. The Cox
proportional model was used to correct for different variables
(including age, gender, AF duration, left atrium size, SBP and
DBP), which may influence the results. Treatment with 160 mg/day
valsartan was found to be associated with a lower risk of AF
recurrence (hazard ratio, 0.46; 95% confidence interval, 0.20–0.93;
P=0.01).
Discussion
A randomized trial was performed in the present
study, comparing the effect of two doses of valsartan (80 and 160
mg/day) on the recurrence rate of AF following ablation in 120
patients. The results indicated that the recurrence rate of AF was
affected in a dose-dependent manner and was lower in the group
treated with 160 mg/day valsartan.
Atrial fibrillation (AF) is the most common
sustained arrhythmia in clinical practice (14). Catheter ablation has emerged as an
effective therapy for AF that does not respond to other medical
treatments (15). However,
catheter ablation is limited by significant recurrence rates of
10–40%, depending on various factors, including left atrial size,
presence of persistent AF, scarring in the left atrium and AF
duration (15–17). These variables are associated with
the remodeling process, which may help trigger and maintain AF
(18). Electrical and structural
remodeling have been previously demonstrated to be associated with
inflammation and oxidative stress (19).
The progression of atrial alteration is a
fundamental component of AF pathophysiology. The RAAS is directly
and indirectly involved in the development of the AF substrate
(20,21). Previous animal studies have
demonstrated that inhibition of RAAS may prevent AF (22,23).
Furthermore, antagonists of the RAAS may have an antifibrotic
effect, due to their ability to decrease the synthesis of collagen
type I molecules, as well as increase the degradation of collagen
type I fibres (24). A previous
study has demonstrated that a blockade of the AT1
receptor reduces the development of atrial fibrosis and, thus,
chronic structural remodelling (9). Although the precise signaling
pathways resulting in atrial fibrosis remain unclear, evidence
indicates that activation of the RAAS may result in atrial
fibrosis. A number of studies have identified a positive
interaction between ARBs and potassium channel blockers on
transmembrane action potentials and currents (25,26).
In addition, ARBs have been demonstrated to modify the cardiac
delayed rectifiers, hKv1.5, HERG and Ks currents, indicating that
ARBs may possess antiarrhythmic properties.
Valsartan is a potential drug for the treatment of
hypertension, congestive heart failure or myocardial infarction
(27). A study by Li et al
demonstrated for the first time that valsartan may prevent the
induction and promotion of AF, through the attenuation of calpain I
upregulation and suppression of atrial structural remodeling in
atrial rapid-pacing dogs (7). In
addition, Harada et al identified that the transient
receptor potential canonical type-3 (TRPC3) channel plays a
critical role in AF-promoting fibroblast pathophysiology, which is
a novel potential therapeutic target (28). Furthermore, valsartan may affect
the fibrotic process by inhibiting TRPC3, which has been found to
be significantly upregulated in the atrium of canines suffering
from AF (29).
In the present study, treatment with 160 mg/day
valsartan resulted in a significantly reduced AF recurrence rate,
when compared with the control or 80 mg/day valsartan groups, which
is in agreement with the observations of previous studies (30,31).
For instance, Parving et al demonstrated the dose-dependent
effect of irbesartan in the prevention of diabetic nephropathy
(30). In addition, Madrid et
al reported that the treatment combination of irbesartan and
amiodarone decreased the rate of AF recurrences in lone AF
patients, in a dose-dependent manner (31).
In patients suffering from paroxysmal or persistent
AF, treatment with 160 mg/day valsartan following AF ablation was
found to be more effective in reducing the AF recurrence rate, when
compared with the administration of 80 mg/day valsartan. These
results indicated that the antiarrhythmic effect of valsartan may
be associated with atrial structural remodeling, as well as
specific and selective electric remodeling through the improvement
of atrial conduction disturbances. In conclusion, treatment with
160 mg/day valsartan markedly reduced the risk of AF recurrence in
a dose-dependent manner.
References
|
1
|
European Heart Rhythm Association;
European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof
P, Lip GY, et al: Guidelines for the management of atrial
fibrillation: the Task Force for the Management of Atrial
Fibrillation of the European Society of Cardiology (ESC). Eur Heart
J. 31:2369–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen MS, Marrouche NF, Khaykin Y, et al:
Pulmonary vein isolation for the treatment of atrial fibrillation
in patients with impaired systolic function. J Am Coll Cardiol.
43:1004–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan MN, Jaïs P, Cummings J, et al:
PABA-CHF Investigators: Pulmonary-vein isolation for atrial
fibrillation in patients with heart failure. N Engl J Med.
359:1778–1785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oral H, Scharf C, Chugh A, et al: Catheter
ablation for paroxysmal atrial fibrillation: segmental pulmonary
vein ostial ablation versus left atrial ablation. Circulation.
108:2355–2360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwasaki YK, Nishida K, Kato T and Nattel
S: Atrial fibrillation pathophysiology: implications for
management. Circulation. 124:2264–2274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Zhu X, Wang A, Fan H and Yuan H:
Effects of angiotensin-II receptor blockers on experimental
autoimmune myocarditis. Int J Cardiol. 137:282–288. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Li WM, Gong YT, et al: The effects
of cilazapril and valsartan on the mRNA and protein expressions of
atrial calpains and atrial structural remodeling in atrial
fibrillation dogs. Basic Res Cardiol. 102:245–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korantzopoulos P, Kolettis TM, Galaris D
and Goudevenos JA: The role of oxidative stress in the pathogenesis
and perpetuation of atrial fibrillation. Int J Cardiol.
115:135–143. 2007. View Article : Google Scholar
|
|
9
|
Kumagai K, Nakashima H, Urata H, et al:
Effects of angiotensin II type 1 receptor antagonist on electrical
and structural remodeling in atrial fibrillation. J Am Coll
Cardiol. 41:2197–2204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tayebjee MH, Creta A, Moder S, Hunter RJ,
et al: Impact of angiotensin-converting enzyme-inhibitors and
angiotensin receptor blockers on long-term outcome of catheter
ablation for atrial fibrillation. Europace. 12:1537–1542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
ONTARGET Investigators. Yusuf S, Teo KK,
Pogue J, et al: Telmisartan, ramipril, or both in patients at high
risk for vascular events. N Engl J Med. 358:1547–1559. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunt SA, Abraham WT, Chin MH, et al: 2009
focused update incorporated into the ACC/AHA 2005 Guidelines for
the Diagnosis and Management of Heart Failure in Adults: a report
of the American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines: developed in
collaboration with the International Society for Heart and Lung
Transplantation. Circulation. 119:e391–e479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oakes RS, Badger TJ, Kholmovski EG, et al:
Detection and quantification of left atrial structural remodeling
with delayed-enhancement magnetic resonance imaging in patients
with atrial fibrillation. Circulation. 119:1758–1767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benjamin EJ, Levy D, Vaziri SM, et al:
Independent risk factors for atrial fibrillation in a
population-based cohort. The Framingham Heart Study. JAMA.
271:840–844. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hocini M, Sanders P, Jaïs P, et al:
Techniques for curative treatment of atrial fibrillation. J
Cardiovasc Electrophysiol. 15:1467–1471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verma A, Wazni OM, Marrouche NF, et al:
Pre-existent left atrial scarring in patients undergoing pulmonary
vein antrum isolation: an independent predictor of procedural
failure. J Am Coll Cardiol. 45:285–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lipman EW, Madsen N, Lin AC, et al:
Incidence and determinants of left atrial scar in patients with
paroxysmal and persistent atrial fibrillation undergoing catheter
ablation. Heart Rhythm. 2(Suppl): S122005. View Article : Google Scholar
|
|
18
|
Everett TH 4th, Li H, Mangrum JM, et al:
Electrical, morphological, and ultrastructural remodeling and
reverse remodeling in a canine model of chronic atrial
fibrillation. Circulation. 102:1454–1460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dernellis J and Panaretou M: C-reactive
protein and paroxysmal atrial fibrillation: evidence of the
implication of an inflammatory process in paroxysmal atrial
fibrillation. Acta Cardiol. 56:375–380. 2001. View Article : Google Scholar
|
|
20
|
Cardin S, Li D, Thorin-Trescases N, et al:
Evolution of the atrial fibrillation substrate in experimental
congestive heart failure: angiotensin-dependent and -independent
pathways. Cardiovasc Res. 60:315–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehrlich JR, Hohnloser SH and Nattel S:
Role of angiotensin system and effects of its inhibition in atrial
fibrillation: clinical and experimental evidence. Eur Heart J.
27:512–518. 2006. View Article : Google Scholar
|
|
22
|
Zhao J, Li J, Li W, et al: Effects of
spironolactone on atrial structural remodelling in a canine model
of atrial fibrillation produced by prolonged atrial pacing. Br J
Pharmacol. 159:1584–1594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YJ, Chen YC, Tai CT, et al:
Angiotensin II and angiotensin II receptor blocker modulate the
arrhythmogenic activity of pulmonary veins. Br J Pharmacol.
147:12–22. 2006. View Article : Google Scholar
|
|
24
|
López B, Querejeta R, Varo N, et al:
Usefulness of serum carboxy-terminal propeptide of procollagen type
I in assessment of the cardioreparative ability of antihypertensive
treatment in hypertensive patients. Circulation. 104:286–291. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caballero R, Delpón E, Valenzuela C, et
al: Losartan and its metabolite E3174 modify cardiac delayed
rectifier K(+) currents. Circulation. 101:1199–1205. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreno I, Caballero R, González T, et al:
Effects of irbesartan on cloned potassium channels involved in
human cardiac repolarization. J Pharmacol Exp Ther. 304:862–873.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sestito A: Hypertension therapy and
cardiovascular protection. Effects of angiotensin II receptor block
with Valsartan. Eur Rev Med Pharmacol Sci. 15:1247–1255.
2011.PubMed/NCBI
|
|
28
|
Harada M, Luo X, Qi XY, et al: Transient
receptor potential canonical-3 channel-dependent fibroblast
regulation in atrial fibrillation. Circulation. 126:2051–2064.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Wang X, Hang P, et al: Valsartan
inhibits transient receptor potential canonical-3 channel in canine
atrial fibrillation. Int J Cardiol. 168:4417–4418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parving HH, Lehnert H, Bröchner-Mortensen
J, et al: Irbesartan in Patients with Type 2 Diabetes and
Microalbuminuria Study Group: The effect of irbesartan on the
development of diabetic nephropathy in patients with type 2
diabetes. N Engl J Med. 345:870–878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madrid AH, Marín IM, Cervantes CE, et al:
Prevention of recurrences in patients with lone atrial
fibrillation. The dose-dependent effect of angiotensin II receptor
blockers. J Renin Angiotensin Aldosterone Syst. 5:114–120. 2004.
View Article : Google Scholar : PubMed/NCBI
|