Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy, with poor prognosis in East Asian
populations, particularly in China. The annual number of
mortalities worldwide is estimated as 250,000. Multiple treatment
modalities have been applied to HCC; however, the condition remains
one of the most difficult tumors to treat when multiple foci of the
tumor or distant metastases are present (1,2).
Thus, novel treatment modalities are urgently required (3).
Since gene therapy clinical trials began in 1990,
gene therapy modalities for malignancies have been investigated
extensively (4). One approach is
based on the insertion of a suicide gene. The analgesic-antitumor
peptide (AGAP) gene is found in the venom of the scorpion,
Buthus martensii Karsch. AGAP has been shown to exert
analgesic and antitumor activities, indicating that the gene has
potential in clinical situations as an antitumor drug (5). The α-fetoprotein (AFP) gene is
normally expressed in fetal livers and is transcriptionally silent
in adult livers; however, the gene is known to be overexpressed in
human HCC patients (6). Thus, the
AFP promoter is generally used in HCC-specific gene therapy
strategies (7). However, this
approach is limited by the weak activity of the AFP promoter.
Linking the AFP enhancer and promoter has been shown to generate a
stronger and HCC-selective promoter (8). These observations indicate that
utilization of AFP promoter and enhancer driven expression in a
plasmid vector can confer the selective expression of a
heterologous suicide gene in HCC cells. Therefore, it may be
feasible to produce a high local concentration of AGAP at the tumor
site by targeting the drug in a tumor cell-specific manner,
subsequently resulting in the targeted killing of cancer cells
(9,10).
Materials and methods
Plasmid construction
Total RNA of the Chinese Buthus martensii
Karsch scorpion was used to amplify the AGAP DNA fragment by
reverse transcription polymerase chain reaction (RT-PCR). For
amplification, a 100-mg sample of fresh scorpion tail was prepared,
and the total RNA was isolated using TRIzol reagent, according to
the manufacturer’s instructions (Takara Bio, Inc., Tokyo, Japan).
The present study was approved by the Ethics Committee of the First
Affiliated Hospital of Wenzhou Medical College (Wenzhou, China).
The RNA was detected with a UV spectrophotometer (UV1900; Shanghai
Shangtian Precision Instrument Co., Ltd., Shanghai, China). Using
the full sequence of the AGAP gene obtained from GenBank (accession
no. AF464898), the following primers were designed and synthesized:
AGAP forward, 5′-CATGCCATGGCCGTACGCGATGGTTATATTGCCG-3′ (containing
an NcoI site), and reverse, 5′-TGCTCTAGAGAC
CGCCATTGCATTTTCCTG-3′ (containing an XbaI site).
The conditions for PCR (Takara Bio, Inc.) were as
follows: Initial denaturation at 94°C for 5 min (one cycle),
followed by 28 cycles of 94°C for 30 sec, 54°C for 30 sec and 72°C
for 60 sec, and a final elongation at 72°C for 5 min. The final
product was visualized by 10% agarose gel electrophoresis (Biowest,
Nuaillé, France).
The pAFP plasmid was provided by Dr Cheng and Dr
Cristian Smerdou, and contained the minimal essential 1,002-bp DNA
fragment with the AFP promoter and enhancer (11). The AGAP DNA fragment was
subsequently inserted into the NcoI-XbaI site of the
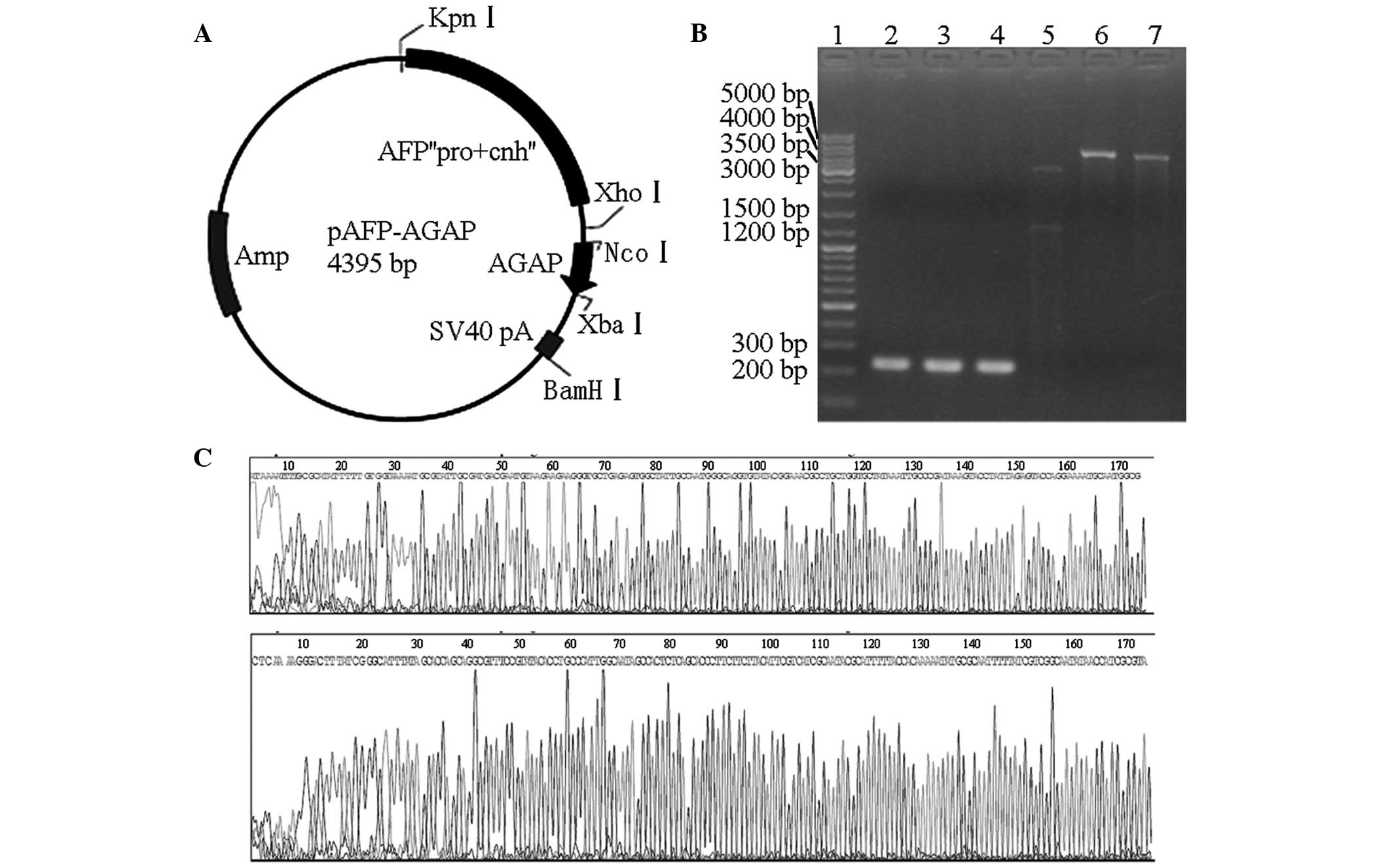
pAFP plasmid, producing the pAFP-AGAP plasmid (Fig. 1A). In this resulting construct, the
synthesis of the AGAP protein largely depended on the human AFP
promoter (217 bp) and enhancer (785 bp) to mediate transcription.
The pAFP-AGAP plasmid was transformed into Escherichia coli
DH5α cells, and then screened and verified by PCR and restriction
enzyme digestion (Thermo Fisher Scientific, Vilnius, Lithuania).
The sequence of the AGAP gene in the pAFP-AGAP plasmid was
confirmed at Takara Bio, Inc.
Cell culture
HepG2 (Bogoo, Inc., Shanghai, China) is a human
AFP-producing hepatoma cell line. The cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) that was supplemented with 10% fetal
bovine serum (FBS; HyClone).
Transfection and mRNA expression of
AGAP
At one day prior to transfection, 2×105
cells were plated in 2 ml growth medium without antibiotics and
seeded into six-well plates (Costar®; Corning Life
Sciences, Cambridge, MA, USA). After overnight cultivation, the
cells were divided into two groups: pAFP-AGAP group and pAFP group.
The medium was then removed and the cells were transfected with a
DNA-lipid complex. The complex consisted of a 250-μl solution
containing 4 μg pAFP-AGAP (or pAFP), a 250-μl
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) solution containing 10 μl stock solution (1:1),
and 2 ml DMEM without FBS. The cells were incubated with the
DNA-lipid complex at 37°C for 6 h, washed and supplemented with 2
ml complete medium containing 10% FBS. Cells were harvested at 24,
48 and 72 h after cultivation in an incubator with 95% humidified
air and 5% CO2 at 37°C.
Total RNA was isolated from the cells using RNAiso
Reagent (Takara Bio, Inc.), according to the manufacturer’s
instructions. The mRNA expression of AGAP was analyzed by RT-PCR
using the forward and reverse primers of AGAP, as described
previously. The final product was visualized by 10% agarose gel
electrophoresis.
Cytotoxicity assay
HepG2 cells were maintained and transfected with the
plasmids using Lipofectamine® 2000 as follows. A 100-μl
cell suspension was dispensed in a 96-well plate (5,000
cells/well). Following overnight cultivation, the medium was
removed and the cells were divided into two groups: pAFP-AGAP group
and pAFP group. The cells were then transfected with a DNA-lipid
complex. The DNA-lipid complex was freshly obtained by adding a
25-μl solution of 0.2 μg pAFP-AGAP (or pAFP) to 25 μl
Lipofectamine® 2000 solution, containing 0.5 μl stock
solution (1:1). The cells from both groups were then diluted in 100
μl DMEM without FBS. The cells were incubated with the DNA complex
at 37°C for 6 h, washed and supplemented with 100 μl complete
medium containing 10% FBS. The plates were incubated for 24, 48 or
72 h in 95% humidified air with 5% CO2 at 37°C.
Cell Counting Kit 8 (CCK-8) solution (10 μl;
Dojindo, Kyushu, Japan) was added to each well of the plate and the
cells were further cultured for 1 h at 37°C. The color reaction was
quantitated using an automatic plate reader (ELx800G; DIALAB GmbH,
Inc., Vienna, Austria) at 450 nm with a reference filter of 630 nm.
The results were expressed as the mean value of three wells. The
percentage of growth inhibition was calculated as follows: Growth
inhibition = (A − B)/(A − C) × 100%, where A is the absorbance from
the cells incubated with the medium containing pAFP, B is the
absorbance from the cells incubated with the medium containing
pAFP-AGAP and C is the absorbance from the cells incubated with
medium alone.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analyses were conducted using SPSS version
12.0 software (SPSS Inc., Chicago, IL, USA). Data from all the
experiments were statistically analyzed using the Student’s t-test,
where P<0.05 was considered to indicate a statistically
significant difference.
Results
Verification of the recombinant
eukaryotic expression vector
Through RT-PCR analysis of the scorpion genetic
material, the AGAP gene sequence was determined to be ~220 bp. The
final products of the AGAP DNA fragments following RT-PCR were
consistent with the expected length, as shown by electrophoresis
(Fig. 1B). Thus, the AGAP gene
sequence was cloned into the pAFP plasmid to obtain the eukaryotic
expression vector, pAFP-AGAP (Fig.
1A). Subsequently, the pAFP-AGAP plasmid was digested with
XbaI to produce a 4,395-bp fragment (lane 6). The plasmid
was digested with KpnI and XbaI to produce 3,118 bp
and 1,277 bp fragments (lane 5). The plasmid was also digested with
NcoI and XbaI to produce 220 bp and 4,175 bp
fragments (lane 7). Each fragment was detected on the
electropherogram, and indicated that the construction of the
pAFP-AGAP vector was correct by multi-enzyme digestion (Fig. 1B). In addition, the sequence of the
AGAP gene in the pAFP-AGAP plasmid was confirmed (Fig. 1C). The forward and reverse
sequences of the recombinant plasmid exhibited 98% similarity with
the GenBank sequences. The difference may have been caused by gene
mutation or the mismatch of gene cloning.
Transfection and mRNA expression of
AGAP
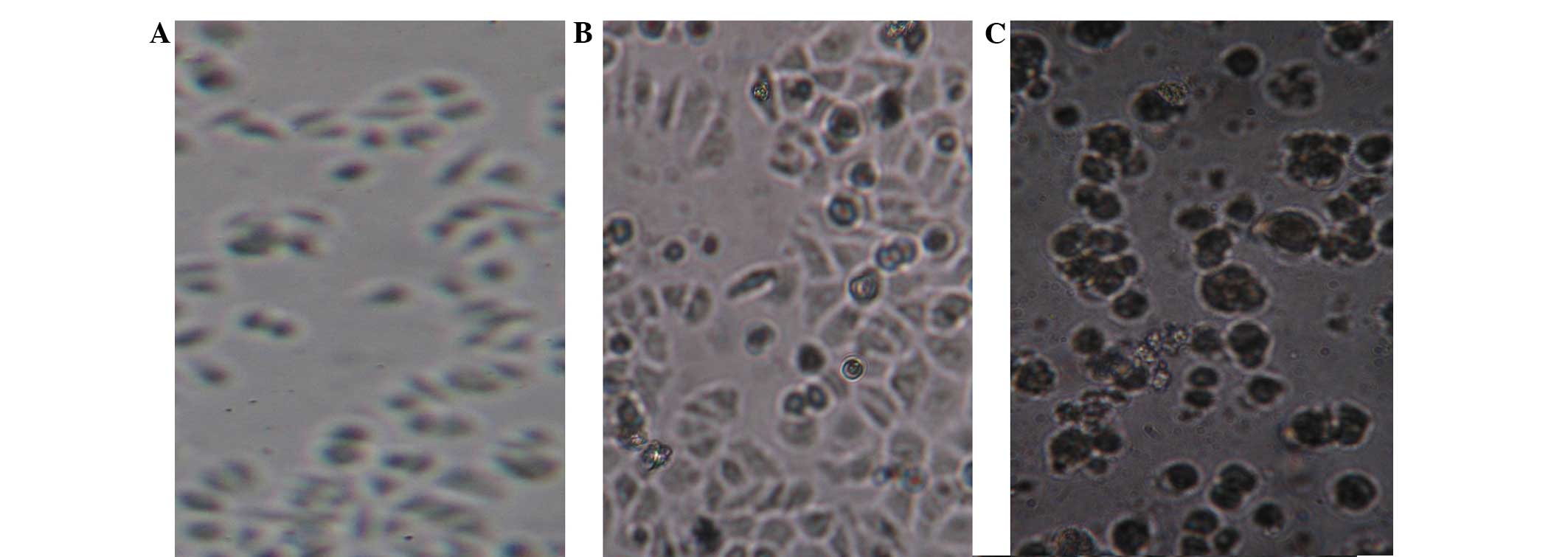
HepG2 cells were transfected with the pAFP-AGAP
complex. The cells grew normally after 24 h (Fig. 2A); however, cellular swelling and
cell suspension was observed 48 h after cultivation (Fig. 2B). The presence of floating cells,
cytoplasmic granulations and vacuolation was observed after 72 h
(Fig. 2C). This phenomenon
indicated that the cytotoxicity increased gradually over time. The
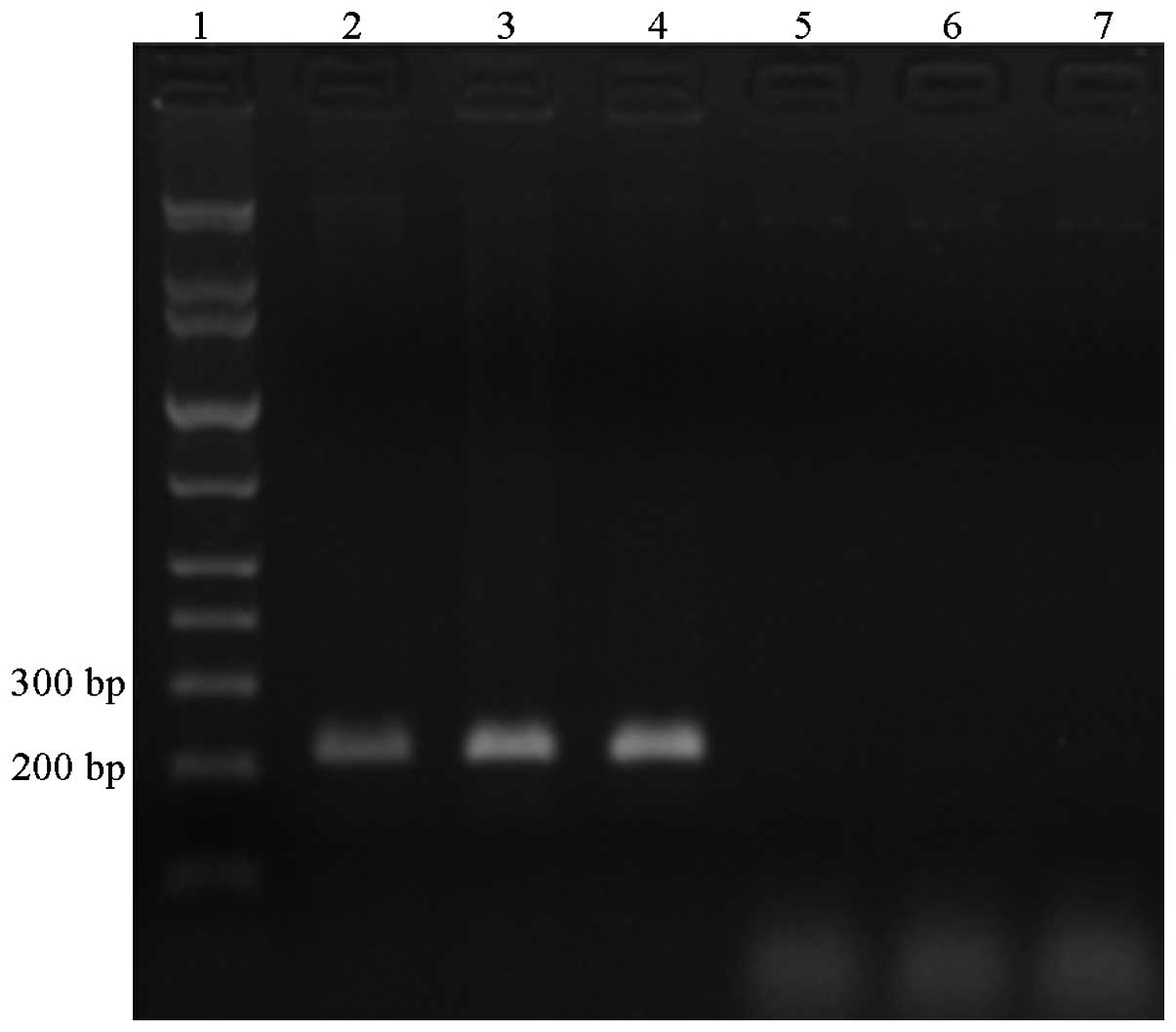
AGAP gene was amplified using RT-PCR from the RNA of the cells
simultaneously. The products following RT-PCR of the HepG2 cells
transfected with the pAFP-AGAP plasmid for 24, 48 and 72 h were
confirmed to be the correct size by gel electrophoresis. However,
the same product was not found on the electrophoresis gel following
RT-PCR of the HepG2 cells transfected with the pAFP plasmid only
(control; Fig. 3).
CCK-8 analysis
Cytotoxicity was detected in the HepG2 cells
following transfection with the pAFP-AGAP plasmid or the pAFP
complex for 24, 48 and 72 h. The percentage of growth inhibition
was found to be 2.4, 44.4 and 74.6% at 24, 48 and 72 h,
respectively. Thus, growth inhibition was shown to increase
gradually over time (Table I).
 | Table ICCK-8 analysis showing the OD values
of the HepG2 cells transfected with the various plasmids. |
Table I
CCK-8 analysis showing the OD values
of the HepG2 cells transfected with the various plasmids.
| Parameter | 24 h | 48 h | 72 h |
|---|
| Transfection with
pAFP | 0.42±0.05 | 0.81±0.07 | 1.50±0.13 |
| Transfection with
pAFP-AGAP | 0.41±0.04 | 0.45±0.02 | 0.38±0.04 |
| P-value | 0.97 | <0.001 | <0.001 |
Discussion
Scorpions have been used in traditional Chinese
medicine for a number of years (12), with scorpion venom applied for the
treatment of convulsions and epilepsy since the Sung Dynasty
(960-1279 A.D.) (13). AGAP has
been purified from the venom of Buthus martensii Karsch,
which is a widely distributed scorpion species in China. Previous
studies have demonstrated that AGAP exhibits analgesic and
antitumor activities (14,15). Gu et al (14) demonstrated that AGAP could inhibit
colon cancer cell growth. In addition, AGAP has been shown to
prolong the survival times of mice that have undergone Ehrlich
ascites tumor cell engraftment, whilst effectively inhibiting S-180
fibrosarcoma growth (5).
Therefore, the present study investigated AGAP as a suicide gene
for use in gene therapy.
The AFP gene encodes the major serum protein in the
developing mammalian fetus, with AFP gene expression observed in
the visceral yolk sac endoderm, the fetal liver, and to a much
lower degree, in the fetal gut and kidney (16). Under physiological conditions, the
AFP gene is expressed at extremely low levels in the adult liver;
however, gene expression can be reactivated during periods of
renewed cell growth, including during liver regeneration and in HCC
(6). A previous study demonstrated
that AFP expression is frequently upregulated in liver cancer cells
(17). In addition, Marrero et
al (18) showed that
diptherotoxin inhibited hepatocellular carcinoma under the control
of the AFP promoter; however, this approach was limited by the weak
activity of the AFP promoter. It is known that effective control of
downstream genes is dependent on the promoter and enhancer working
together. Thus, the present study utilized the AFP promoter and
enhancer to construct a gene-modified HCC-specific AGAP expression
vector.
In conclusion, the results of the present study
indicated that the AGAP gene was successfully integrated into the
pAFP plasmid and expressed. The AGAP gene was specifically
expressed at very high levels in human HCC tumor cells. Therefore,
there is a potential industrial application of inducing AGAP
expression through eukaryotic expression vectors. Similarly to the
use of the AFP promoter and enhancer for HCC, prostate-specific
antigen promoters have been used for prostate cancer (19), while E2F and telomerase reverse
transcriptase promoters are used for various types of tumors
(20,21). These additional promoters may be
used in place of AFP to adapt this novel strategy to different
types of tumors.
However, future studies are required and should
focus on the detection of expression at the protein level, in
addition to in vivo analysis.
Acknowledgements
The authors thank Professor Cheng Qian and Dr
Cristian Smerdou at the Spanish Institute of Fundación para la
Investigación Médica Aplicada (Pamplona, Spain) for the supply of
the pAFP plasmid.
References
|
1
|
Farmer DG and Busuttil RW: The role of
multimodal therapy in the treatment of hepatocellular carcinoma.
Cancer. 73:2669–2670. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levin B and Amos C: Therapy of
unresectable hepatocellular carcinoma. N Engl J Med. 332:1294–1296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gutierrez AA, Lemoine NR and Sikora K:
Gene therapy for cancer. Lancet. 339:715–721. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YF, Hu J, Zhang JH, Wang SL and Wu CF:
Isolation purification, and N-terminal partial sequence of an
antitumoral peptide from the venom of the Chinese scorpion Buthus
martensii Karsch. Prep Biochem Biotechnol. 32:317–327. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abelev GI: Alpha-fetoprotein in
ontogenesis and its association with malignant. Adv Cancer Res.
14:295–358. 1971. View Article : Google Scholar
|
|
7
|
Kang JH, Toita R and Murata M: Liver
cell-targeted delivery of therapeutic molecules. Crit Rev
Biotechnol. Jul 15–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Li DM, Chen K, et al: Development of
a gene therapy strategy to target hepatocellular carcinoma based
inhibition of protein phosphatase 2A using the α-fetoprotein
promoter enhancer and pgk promoter: an in vitro and in vivo study.
BMC Cancer. 12:5472012. View Article : Google Scholar
|
|
9
|
Moolten FL: Drug sensitivity (‘suicide’)
genes for selective cancer chemotherapy. Cancer Gene Ther.
1:279–287. 1994.PubMed/NCBI
|
|
10
|
Hu Y, Shen Y, Ji B, et al: Liver-specific
gene therapy of hepatocellular carcinoma by targeting human
telomerase reverse transcriptase with pegylated
immuno-lipopolyplexes. Eur J Pharm Biopharm. 78:320–325. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaun M, Rodriguez-Madoz JR, Alzuguren P,
et al: Increased efficacy and safety in the treatment of
experimental liver cancer with a novel adenovirus-alphavirus hybrid
vector. Cancer Res. 66:1620–1629. 2006. View Article : Google Scholar
|
|
12
|
Tan YH and Guo JS: Research advances in
chemical component and analgesic effect of Buthus martensii Karsch.
Hunan Zhong Yi Yao Daobao. 7:210–212. 2001.
|
|
13
|
Zhou XH, Yang D, Zhang JH, Liu CM and Lei
KJ: Purification and N-terminal partial sequence of anti-epilepsy
peptide from venom of the scorpion Buthus martensii Karsch. Biochem
J. 257:509–517. 1989.PubMed/NCBI
|
|
14
|
Gu Y, Liu SL, Ju WZ, Li CY and Cao P:
Analgesic-antitumor peptide induces apoptosis and inhibits the
proliferation of SW480 human colon cancer cells. Oncol Lett.
5:483–488. 2013.PubMed/NCBI
|
|
15
|
Mao Q, Ruan J, Cai X, et al:
Antinociceptive effects of analgesic-antitumor peptide (AGAP), a
neurotoxin from the scorpion Buthus martensii Karsch, on
formalin-induced inflammatory pain through a mitogen-activated
protein kinases-dependent mechanism in mice. PLoS One.
8:e782392013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tilghman SM: The structure and regulation
of the alpha-fetoprotein and albumin genes. Oxf Surv Eukaryot
Genes. 2:160–206. 1985.PubMed/NCBI
|
|
17
|
Selten GC, Princen HM, Selten-Versteegen
AM, Mol-Backx GP and Yap SH: Sequence content of alpha-fetoprotein,
albumin and fibrinogen polypeptide mRNAs in different organs,
developing tissues and in liver during carcinogenesis in rats.
Biochim Biophys Acta. 699:131–137. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marrero JA, Su GL, Wei W, et al: Des-gamma
carboxyprothrombin can differentiate hepatocellular carcinoma from
nonmalignant chronic liver disease in American patients.
Hepatology. 37:1114–1121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Matherly J, Smallwood A, Adams JY,
Billick E, Belldegrun A and Carey M: Chimeric PSA enhancers exhibit
augmented activity in prostate cancer gene therapy vectors. Gene
Ther. 8:1416–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parr MJ, Manome Y, Tanaka T, Wen P, Kufe
DW, Kaelin WG Jr and Fine HA: Tumor-selective transgene expression
in vivo mediated by an E2F-responsive adenoviral vector. Nat Med.
3:1145–1149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang TG, Savontaus MJ, Shinozaki K,
Sauter BV and Woo SL: Telomerase-dependent oncolytic adenovirus for
cancer treatment. Gene Ther. 10:1241–1247. 2003. View Article : Google Scholar : PubMed/NCBI
|