Introduction
The implantation of a mammalian embryo is a crucial
step in the establishment of a normal pregnancy. To prepare for
implantation, the uterus undergoes dynamic and tightly regulated
proliferation and differentiation following stimulation by changes
in the levels of the ovarian hormones estrogen and progesterone
(1). Implantation comprises three
stages: Apposition, adhesion and penetration. In the apposition
stage, the blastocyst unstably adheres to the endometrial surface
(2). Following apposition, the
trophoblast and luminal epithelium adhere sufficiently strongly to
prevent the displacement of the blastocyst (3). The embryo then invades through the
luminal epithelium into the stroma to associate with the maternal
vasculature (4).
In rats, implantation occurs at a specific time,
during a brief 24-h period 5 days after mating (5,6).
Prior to implantation, the endometrium is unresponsive to the
blastocyst or to other decidualizing signals. In mammals,
implantation is regulated through the normal reproductive cycle
and, in numerous mammalian species, may be timed to coincide with
conditions that are more favorable for the support of embryonic
growth (7). The control of
implantation is primarily maternal and achieved through
hormone-mediated changes in the expression of adhesion molecules
and vessel-related factors.
Osteopontin (OPN) is an adhesion protein (8). Amino acid analysis of OPN has
revealed the existence of a conserved cell-binding
arginine-glycine-aspartic acid sequence, which has been
demonstrated to be involved in adherence to cell surface receptors
(9). In the reproductive tract,
OPN, which is expressed by secretory-phase endometrial cells,
invading trophoblasts, decidual glands and the placenta, is
temporally involved in blastocyst invasion and placentation
(10,11).
Vascular endothelial growth factor (VEGF) is an
endothelial cell-specific mitogen in vitro and is known to
be the key factor responsible for vasculogenesis and angiogenesis
in a variety of models (12). In
rodents, VEGF acts in embryo-endometrium interactions by regulating
endometrial vascular permeability and endothelial cell
proliferation at implantation sites (13,14).
Furthermore, VEGF receptor 1 (VEGFR1) and VEGFR2 have been observed
in microvessels during the midsecretory period, emphasizing a
possible association between the increased microvascular density
(MVD) and vascular permeability (15). These studies have determined two
basic roles for VEGF in endometrial tissue: a) The regulation of
endometrial vascularization and vascular permeability; b) the
establishment of a receptive endometrium to support blastocyst
implantation and trophoblast invasion.
Assisted reproductive technology (ART) is now
available to select high-quality embryos; however, despite such
technological advances, implantation rates remain relatively low
(16). Uterine receptivity has a
key role in the establishment of successful pregnancies, and
impaired receptivity may limit the success of ART. Controlled
ovarian hyperstimulation (COH) is a frequently used therapeutic
strategy in the treatment of infertility; however, the effect of
COH on implantation remains controversial (17–19).
The use of gonadotropin-releasing hormone agonist (GnRHa) is
associated with advanced endometrial maturation of 2–4 days on the
day of oocyte retrieval, and no pregnancy occurs when the
advancement is >3 days (20).
It has additionally been suggested that COH results in
supraphysiological levels of estrogen and progesterone, which may
impair endometrial receptivity (21).
The use of herbal medicines for the treatment of
infertility has been well documented in China for numerous years.
In contrast to target-oriented Western medicine, Traditional
Chinese Medicine (TCM) uses a holistic and synergistic approach to
restore the balance of body energy and to maintain the normal
functioning of the body (22,23).
According to the theory of TCM, we have developed Zi Dan Yin (ZDY)
(Table I) to be used to prepare
the endometrium for implantation.
 | Table IComposition of Zi Dan Yin. |
Table I
Composition of Zi Dan Yin.
| Components | Ratio |
|---|
| Sheng Di
[Rehmannia glutinosa (Gaertn.) Libosch., root)] | 15 |
| Dan Shen
(Salviae miltiorrhizae Bge., root) | 10 |
| Dang gui
[Angelica sinensis (Oliv.) Diels., root] | 12 |
| Chuan Duan
(Dipsacus asperoides C.Y. Cheng et T.M. Ai., root) | 15 |
| Du Zhong
(Eucommia ulmoides Oliv., cortex) | 12 |
| Shan Yao
(Dioscorea opposita Thunb., rhizome) | 15 |
| Mei Gui-hua
(Rosa rugosa Thunb., flower) | 6 |
| Chuan Xiong
(Ligusticum Chuanxiong Hort., rhizome) | 6 |
| Yi Yi-ren [Coix
lacryma-jobi L. var. ma-yuen (Roman.) Stapf., seed] | 12 |
Although there have been marked increased in the
understanding of implantation, therapeutic options remain poor.
Further studies are required to provide clinical treatment options
for patients experiencing implantation failure-related infertility.
The incidence of early pregnancy loss during or immediately
subsequent to implantation is high (25–40%) (24). Failed implantation is also a major
limiting factor in assisted reproduction (25). The aim of the present study was to
investigate the effects of COH on implantation biology and
pregnancy outcome, and to explore the potential therapeutic role of
the TCM Zi Dan Yin (ZDY).
Materials and methods
Ethics statement
All of the experimental protocols utilized in the
present study were approved by the Ethics Committee of the Beijing
University of Chinese Medicine Animal Care and Use Committee (no.
2013-015-A).
ZDY preparation
All the crude drugs involved in the composition of
ZDY were obtained from the Department of Pharmacy, Dongfang
Hospital of Beijing University of Chinese Medicine (Beijing,
China). The quality of the raw herbs was controlled according to
the requirement of the Pharmacopoeia of the People’s Republic of
China (26). Aqueous extract of
ZDY was prepared in accordance with the following procedure. In
brief, nine medicinal materials were mixed in proportion and
macerated for 1 h with eight volumes of distilled water and then
decocted for 2 h. The cooled extract was subsequently filtered. The
extraction procedure was repeated twice. The extracts were then
combined and concentrated by boiling to a final volume of 100 ml
(4.12 g/ml). This dilution was used in the following preliminary
experiments in a range of concentrations from 1.03 to 4.12
g/ml.
Treatment
Mature, female Sprague Dawley rat virgins aged seven
to eight weeks (weight, 200–220 g) were maintained in the Research
Centre, Beijing University of Chinese Medicine on a 12-h light/dark
regimen with free access to water and a standard diet. The estrous
cycle was identified by vaginal smear. Only the female rats with
regular cycles were used. Suitable rats were randomly allocated
into one of four groups: Control, COH, ZDY and COH + ZDY.
Rats in the COH group were administered 1 ml/100 g
distilled water for 12 days and then treated with the GnRHa-long
protocol. In brief, the GnRHa (Diphereline™; Ipsen,
Boulogne-Billancourt, France) was injected intraperitoneally at 1.5
μg/100 g body weight/day between days 3 and 9 of the estrous cycle.
Pregnant mare’s serum gonadotropin (Inner Mongolia Chifeng Bo En
Pharmaceutical Co., Ltd., Chifeng, China) was injected
intraperitoneally at 5 IU/100 g body weight on the ninth day of the
estrous cycle followed by the injection of human chorionic
gonadotropin (hCG; Yantai North Pharmaceutical Co., Ltd., Yantai,
China) at 10 IU/100 g 28 h later. In the ZDY group, the animals
were administered 1 ml ZDY/100 g body weight/day for 12 days
followed by saline injections at the same time and volume as the
COH group. In the COH + ZDY group, the animals were administered
the 1 ml ZDY/100 g daily for 12 days and were then subjected to the
same GnRHa-long protocol as the COH group. In the control group,
the rats were administered distilled water for 12 days, followed by
injections with saline at the same time and volume as the
treatments used in the COH group. The female rats were housed
overnight with males (1:1) subsequent to being given hCG or saline.
Successful mating was checked daily by the presence of vaginal
plugs. The morning when the plug was found was designated the day 1
of gestation (D1).
In rats, implantation occurs on the fifth day after
coitus. During this specific, temporary period, known as the
‘receptive phase’ and ‘window of implantation’, the endometrium
undergoes marked changes in structure and function induced by
ovarian steroids, which prepares the endometrium to be receptive to
the embryo (5). Thus, to
demonstrate the characteristics of the endometrium in a COH rat
model during implantation, the rats were sacrificed using 10%
chloral hydrate (302-17-0; Solabrio, Beijing, China) prior to
implantation (D3 and D4) and during the implantation window (D5)
(n=6/day). Whole uteri were collected promptly without excess fat
and connective tissue. One-third of each sample was fixed in 4%
paraformaldehyde, and the remaining part of each sample was stored
at −80°C until protein and mRNA extraction.
MVD
For the immunohistochemical detection of cluster of
differentiation (CD) 34, uteri were fixed for 12 h at 4°C in
buffered paraformaldehyde and were then routinely processed for
paraffin embedding. The paraffin-fixed tissues were cut into 4-μm
sections. Briefly, the slides were dewaxed, rehydrated, blocked and
incubated with the primary antibody: Anti-CD34 goat polyclonal
antibody (cat. no. sc-1336; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) at a 1:200 dilution overnight at 4°C. Subsequent to
rinsing three times with phosphate-buffered saline (PBS), the
tissues were incubated with secondary antibody (PV-0003; ZSGB-BIO,
Beijing, China) for 25 min, followed by incubation with a
3,3′-diaminobenzidine kit (ZLI-9018; ZSGB-BIO, Beijing, China). As
a control, normal PBS was used and the primary antibody was
omitted. Finally, the sections were dehydrated, counterstained and
mounted for observation.
MVDs were viewed at ×400 magnification (40X
objective lens and 10X ocular lens; 0.24 mm2/field).
Tissue images were captured with a digital camera (Olympus, Inc.,
Tokyo, Japan). For each section, at least five randomly selected
fields were counted to determine the density of the microvessels
within the uterus. The number of CD34-positive vessels was
quantified using Diagnostic Instruments SPOT imaging software
(Diagnostic Instruments, Inc., Sterling Heights, MI, USA). The MVD
was calculated as the number of CD34-positive vessels/(40×0.24
mm2).
Western blot analysis
Uterine slices previously frozen at −80°C were
incubated and lysed in radioimmunoprecipitation assay lysis buffer
(cat. no. C1053; Applygen Technologies, Inc., Beijing, China)
supplemented with protease inhibitor cocktail (cat. no. P1265;
Applygen Technologies, Inc.). The protein concentration was
quantified using a bicinchoninic acid assay (cat. no. P1511;
Applygen Technologies, Inc.). Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) using a 10% polyacrylamide gel was
performed and the samples were transferred to nitrocellulose
membranes (Bio-Rad, Hercules, CA, USA). The membranes were blotted
with rabbit polyclonal anti-VEGF (cat. no. ab46154; Abcam,
Cambridge, UK) or rabbit polyclonal anti-OPN (cat. no. ab8448;
Abcam) primary antibodies at a dilution of 1:2,000 and incubated
overnight at 4°C. Following incubation, the membranes were washed
three times with Tris-buffered saline/Tween 20 and then incubated
with goat anti-rabbit immunoglobulin G secondary antibodies (cat.
nos. P1308 and P1309; Applygen Technologies, Inc.) at a dilution of
1:2,000 at room temperature for 1 h. The blots were visualized with
Super ECL Plus Detection Reagent (cat. no. P1010; Applygen
Technologies, Inc.). The enhanced chemiluminescence signals were
detected with Quantity One® software (Bio-Rad). β-actin
(cat. no. ab8226; Abcam) was used as the reference protein to
validate the amount of protein loaded onto the gel.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
VEGF and OPN gene expression was validated using
qPCR. Total RNA was extracted from the uteri of rats in the
control, COH, ZDY and COH + ZDY groups using TRIzol®
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RNA was thawed on ice and quantified
spectrophotometrically, and the quality was then assessed using
SDS-PAGE. RT was performed with 8 μl total RNA per 20 μl reaction
using the standard cDNA Synthesis kit (Takara Bio, Inc., Shiga,
Japan). The qPCR primer sequences for the target genes were as
follows: GAPDH forward primer, 5′-TGC TGA GTA TGT CGT GGA G-3′ and
reverse primer, 5′-GTC TTC TGA GTG GCA GTG AT- 3′ (288 bp); VEGF
forward primer, 5′-GGC TCA CTT CCA GAA ACA CG-3′ and reverse
primer, 5′-GTG CTC TTG CAG AAT CTA GTG G-3′ (165 bp); OPN forward
primer, 5′-GAG GTG ATA GCT TGG CTT ACG G-3′ and reverse primer,
5′-CGC TGG GCA ACT GGG ATG-3′ (154 bp).
For each qPCR reaction, the typical thermal cycling
conditions included an initial activation step at 95°C for 5 min,
40 cycles of amplification and a final melting curve (65–95°C). PCR
reactions were performed using an ABI Prism® 7700
Sequence Detection system (Applied Biosystems, Foster City, CA,
USA). Experiments were carried out in triplicate, and cDNA
concentrations were normalized with the GAPDH PCR products. Gene
expression was analyzed using the 2−ΔΔCt algorithm.
Average number of implantation sites and
live births
On D10, uteri were collected from each group
immediately subsequent to sacrifice (n=6). Whole uteri were
collected promptly without excess fat and connective tissue, and
the conceptuses were removed from the uteri. The number of
implantation sites in the uterine horn was recorded. The average
number of implantation sites was calculated as the total number of
implantation sites/number of rats. Subsequent to conceiving and
bearing newborn rats, another sample of rats (n=6) were used to
conceive and bear newborn rats to determine the number of live
births from each group. The average number of live births was
calculated as the total number of newborn rats/number of rats.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance and least significant
difference tests were used with the SPSS 17.0 statistical software
package (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. Graphs of the data
were produced using Microsoft Excel software (Microsoft Corp.,
Redmond, WA, USA).
Results
MVD results
A summary of the MVD results is shown in Table II. Through the immunohistochemical
analysis, the MVD was found to be significantly increased in the
COH group compared with that in the control, ZDY and COH + ZDY
groups (P<0.01), with a maximal value on D5. No significant
differences were found among the control, ZDY and COH + ZDY groups
(P>0.05).
 | Table IIMicrovascular density. |
Table II
Microvascular density.
| Group | D3 (n) | D4 (n) | D5 (n) |
|---|
| Control | 1.79±0.06 | 2.88±0.19 | 3.56±0.11 |
| COH | 2.24±0.09a | 3.91±0.07a | 4.34±0.11a |
| ZDY | 1.86±0.03 | 2.88±0.09 | 3.34±0.10 |
| COH + ZDY | 1.74±0.07 | 2.97±0.08 | 3.32±0.09 |
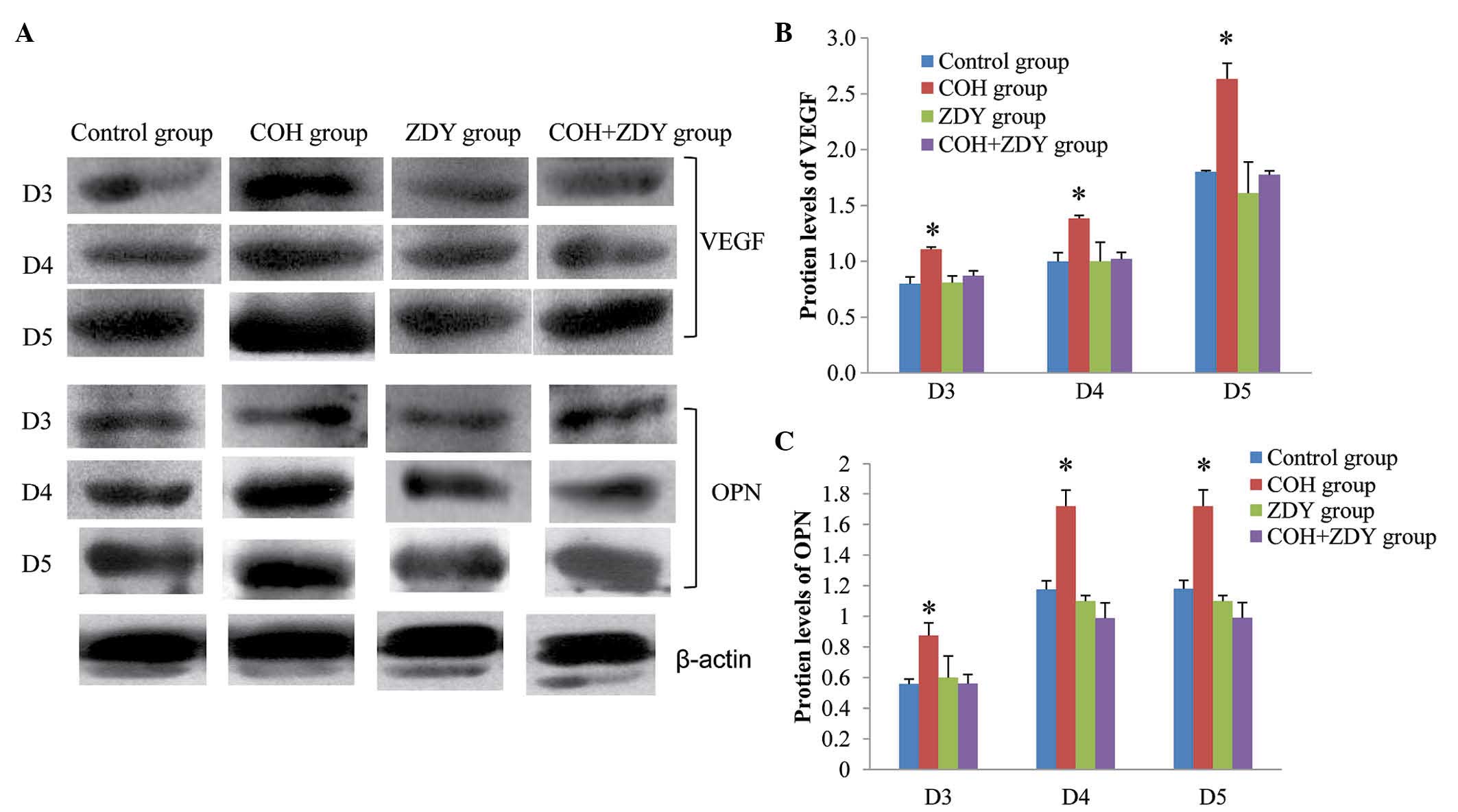
Western blot analysis results
The protein expression of endometrial VEGF and OPN
during implantation was confirmed by western blotting (Fig. 1). The protein levels of VEGF and
OPN were normalized by β-actin. Among the four groups, the VEGF and
OPN expression levels in the COH group were significantly higher
than those in the other groups on D3, D4 and D5 (P<0.05). No
significant differences were found among the control, ZDY and COH +
ZDY groups (P>0.05).
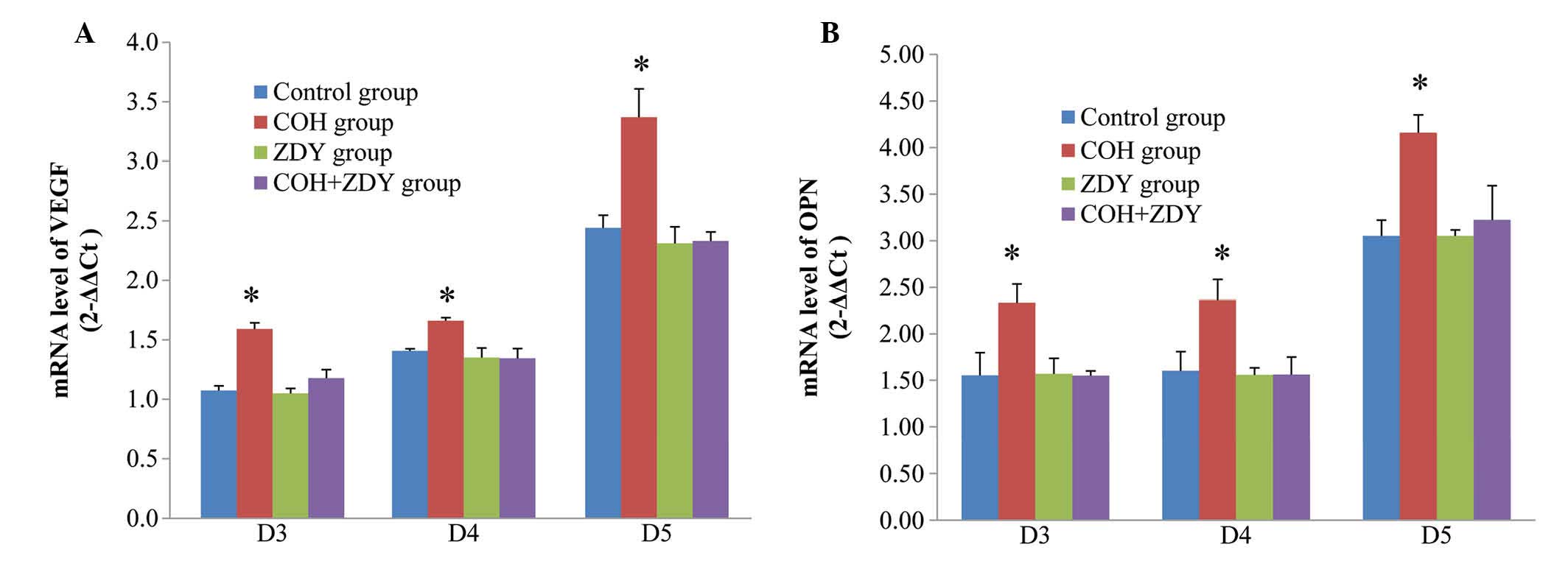
RT-qPCR results
To demonstrate whether the VEGF and OPN mRNA
expression results were consistent with the results of protein
expression, RT-qPCR analysis was used (Fig. 2). VEGF and OPN mRNA were found to
be expressed in the endometrium of the rats during the implantation
window. Compared with the control, ZDY and COH + ZDY groups, the
VEGF and OPN mRNA expression levels, normalized by GAPDH, in the
COH group were significantly increased (P<0.05). During the
implantation window, no significant differences were found among
the control, ZDY and COH + ZDY groups (P>0.05).
Measurements of the number of
implantation sites and live births
The effects of COH and/or ZDY treatment on the
number of implantation sites and live births are summarized in
Table III. The number of
implantation sites and live births in the COH group was
significantly lower than that in the other groups. No significant
differences were found among the control, ZDY and COH + ZDY
groups.
 | Table IIINumber of implantation sites and live
births. |
Table III
Number of implantation sites and live
births.
| Parameter | Control | COH | ZDY | COH + ZDY |
|---|
| Implantation sites
(n) | 10.33±0.84 | 3.67±0.49a | 10.00±0.73 | 8.67±0.33 |
| Live births
(n) | 11.00±0.63 | 4.33±0.42a | 10.67±0.71 | 9.33±0.61 |
Discussion
The early stages of embryo implantation in rats are
characterized by two endometrial vascular events: A localized
increase in endometrial vascular permeability, which is the
earliest indicator of the implantation, and increased endothelial
cell proliferation (13). VEGF
exhibits a potent vascular permeability-inducing action and has
been revealed to be the factor responsible for implantation-related
increases in vascular permeability in the rat endometrium.
Furthermore, the role of VEGF in vascular beds has been
demonstrated in other mammalian species, including humans, pigs and
rabbits (27,28). A previous study showed that
endothelial cell proliferation was increased from the third day of
pregnancy and remained elevated throughout the entire endometrium
up to the fifth day of pregnancy (29). The present study indicated that
VEGF can be found in the rat endometrium on D3, D4 and D5.
One of the commonest methods of indirectly assessing
vasculogenic and angiogenic activity is to examine and compare the
net gain or loss in the number of microvessels between different
tissues. This is most frequently used to count the number of vessel
cross-sections or profiles present in a specified area of a
histological section. Following the invasion of the maternal
endometrium, embryonic development is characterized by a marked
growth of blood vessels occurring concurrently with decidualization
and the development of vascular membranes (30). Previous studies have demonstrated
the presence of VEGF mRNA in trophoblast cells in rats (31) and mice (32). These findings suggest that VEGF may
be a key factor in the induction of vascular growth in the decidua
and vascular membranes; however, there is little knowledge
concerning the direct association between the MVD and VEGF
expression. To the best of our knowledge, this study is the first
to measure the MVD, as well as VEGF expression, in a COH rat model.
The findings showed that the changes in MVD and VEGF expression are
consistent in the rat endometrium throughout implantation.
Furthermore, OPN showed the same expression trend at the same time.
We suggest the existence of an association between the MVD and the
levels of VEGF and OPN.
Implantation is a complex process involving
proliferation and tissue remodeling. Adhesion molecules and
cytokines have been suggested to play key roles in this process
(33), and OPN has the capacity to
act as both a cytokine and an adhesion molecule. A previous study
has shown that OPN expression is increased in the secretory-phase
endometrium and decidua of pregnant females (34). Furthermore, microarray studies have
consistently found a 4.9- to 20-fold upregulation of OPN during
this period in humans (33–38).
In mice, OPN induces blastocysts to activate their adhesion
competence through the formation of integrin adhesion complexes at
the trophectoderm cell surface (39). By contrast, OPN-null mice remain
fertile (40,41). In the present study, OPN protein
levels were low on D3 and D4 and markedly increased on D5. OPN mRNA
levels were low on D3, and markedly increased on D4 and D5. OPN
expression therefore increased throughout the implantation process.
It has been suggested that angiogenesis and vascular remodeling is
facilitated by OPN and αvβ3 (42). This is one possible explanation for
the increased OPN expression associated with increased MVD.
COH was observed to have a negative effect on the
endometrium, as the COH rat model exhibited a significant higher
MVD and VEGF and OPN expression compared with the control group
rats. Previous studies have reported increased pre-implantation
mortality following superovulation in mice (43,44),
hamsters (45) and rats (46). Furthermore, impaired development
and reduced implantation rates were found with embryos from
superovulated donors, although the weight of the live fetuses
obtained following transfer from superovulated donors was not
significantly different from that of control embryos; this may
suggest that superovulation has certain negative effects even on
viable embryos (47). Failed
implantation is a significant limiting factor in assisted
reproduction (25). Since ovarian
stimulation generates a cascade of hormonal and physiological
events, the embryos mature in an environment that exhibits
differences from the environment in which embryos mature naturally
(48). Furthermore, variations may
also be apparent in the timing of ovulation and implantation
(49). The relative contribution
of the endometrium to the rate of successful reception is not
currently known, and no accepted criteria exist for the evaluation
of endometrial implantation; however, preparations of the
endometrium-produced cytokines may mediate these precisely defined
morphological changes (21,50).
In the present study, the expression of VEGF and OPN in the COH
group was higher than that in the control, ZDY and COH + ZDY
groups, indicating that COH may impair the synchronization of
embryonic development and endometrial maturation; however, the
adverse effects of COH cannot only be attributed to asynchrony, as
an early study showed that superovulation following synchronization
also resulted in increased embryonic loss (43).
In the present study, ZDY suppressed the COH-induced
increases in MVD and VEGF and OPN expression in rats. No
significant differences were found between the control and ZDY
groups. These results therefore suggest that a TCM such as ZDY
could prove to be of clinical use in patients with impaired
endometrial receptivity subsequent to COH; however, TCM cannot
translate the normal state into a super-normal state, which
accounts for a lack of significant difference between the control
and ZDY groups. A further clinical study is required to confirm the
proposed approach in this aspect.
In combination, the present results indicated that
COH treatment increased the expression of MVD, VEGF and OPN during
implantation. The study showed that VEGF and OPN may play important
roles during implantation, and that an association exists between
changes in the MVD and VEGF and OPN expression. In addition to the
molecular and structural markers of endometrial receptivity, MVD
could be an alternative approach to identify this period of
receptivity in rats. The present study also revealed that high
levels of VEGF and OPN were apparent subsequent to COH treatment.
These findings confirmed the adverse effects of COH with GnRHa on
implantation, which have been documented in previous studies
(47,51). ZDY markedly restored the
endometrial MVD and VEGF and OPN expression during implantation in
the COH rat model. The present study provides novel insight into
TCM approaches for infertility treatment and ART.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81173292).
References
|
1
|
Finn CA and Martin L: The control of
implantation. J Reprod Fertil. 39:195–206. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabibzadeh S and Babaknia A: The signals
and molecular pathways involved in implantation, a symbiotic
interaction between blastocyst and endometrium involving adhesion
and tissue invasion. Hum Reprod. 10:1579–1602. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cakmak H and Taylor HS: Implantation
failure: molecular mechanisms and clinical treatment. Hum Reprod
Update. 17:242–253. 2011. View Article : Google Scholar :
|
|
4
|
Sharkey AM and Smith SK: The endometrium
as a cause of implantation failure. Best Pract Res Clin Obstet
Gynaecol. 17:289–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Psychoyos A: Hormonal control of
ovoimplantation. Vitam Horm. 31:201–256. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Psychoyos A: Hormonal control of uterine
receptivity for nidation. J Reprod Fertil Suppl. 17–28.
1976.PubMed/NCBI
|
|
7
|
Renfree MB and Shaw G: Diapause. Annu Rev
Physiol. 62:353–375. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oldberg A, Franzén A and Heinegård D:
Cloning and sequence analysis of rat bone sialoprotein
(osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence.
Proc Natl Acad Sci USA. 83:8819–8823. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruoslahti E and Pierschbacher MD: New
perspectives in cell adhesion: RGD and integrins. Science.
238:491–497. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown LF, Berse B, Van de Water L, et al:
Expression and distribution of osteopontin in human tissues:
widespread association with luminal epithelial surfaces. Mol Biol
Cell. 3:1169–1180. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nomura S, Wills AJ, Edwards DR, Heath JK
and Hogan BL: Developmental expression of 2ar (osteopontin) and
SPARC (osteonectin) RNA as revealed by in situ hybridization. J
Cell Biol. 106:441–450. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maglione D, Guerriero V, Viglietto G, et
al: Two alternative mRNAs coding for the angiogenic factor,
placenta growth factor (PlGF), are transcribed from a single gene
of chromosome 14. Oncogene. 8:925–931. 1993.PubMed/NCBI
|
|
13
|
Rabbani M and Rogers PA: Role of vascular
endothelial growth factor in endometrial vascular events before
implantation in rats. Reproduction. 122:85–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rockwell LC, Pillai S, Olson CE and Koos
RD: Inhibition of vascular endothelial growth factor/vascular
permeability factor action blocks estrogen-induced uterine edema
and implantation in rodents. Biol Reprod. 67:1804–1810. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krüssel JS, Casañ EM, Raga F, et al:
Expression of mRNA for vascular endothelial growth factor
transmembraneous receptors Flt1 and KDR, and the soluble recetor
sflt in cycling human endometrium. Mol Hum Reprod. 5:452–458. 1999.
View Article : Google Scholar
|
|
16
|
de Mouzon J, Goossens V, Bhattacharya S,
et al; European IVF-monitoring (EIM) Consortium, for the European
Society of Human Reproduction and Embryology (ESHRE). Assisted
reproductive technology in Europe, 2006: results generated from
European registers by ESHRE. Hum Reprod. 25:1851–1862. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levi AJ, Drews MR, Bergh PA, Miller BT and
Scott RT Jr: Controlled ovarian hyperstimulation does not adversely
affect endometrial receptivity in in vitro fertilization cycles.
Fertil Steril. 76:670–674. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pattinson HA, Greene CA, Fleetham J and
Anderson-Sykes SJ: Exogenous control of the cycle simplifies thawed
embryo transfer and results in a pregnancy rate similar to that for
natural cycles. Fertil Steril. 58:627–629. 1992.PubMed/NCBI
|
|
19
|
Pellicer A, Valbuena D, Cano F, Remohí J
and Simón C: Lower implantation rates in high responders: evidence
for an altered endocrine milieu during the preimplantation period.
Fertil Steril. 65:1190–1195. 1996.PubMed/NCBI
|
|
20
|
Bourgain C and Devroey P: The endometrium
in stimulated cycles for IVF. Hum Reprod Update. 9:515–522. 2003.
View Article : Google Scholar
|
|
21
|
Dey SK, Lim H, Das SK, et al: Molecular
cues to implantation. Endocr Rev. 25:341–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Efferth T, Li PC, Konkimalla VSB and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen Z, Wang Z, Wang S, et al: Discovery of
molecular mechanisms of traditional Chinese medicinal formula
Si-Wu-Tang using gene expression microarray and connectivity map.
PLoS One. 6:e182782011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilcox AJ, Weinberg CR, O’Connor JF, et
al: Incidence of early loss of pregnancy. New Engl J Med.
319:189–194. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norwitz ER, Schust DJ and Fisher SJ:
Implantation and the survival of early pregnancy. N Engl J Med.
345:1400–1408. 2001. View Article : Google Scholar
|
|
26
|
Zhong-zhi Q, Yang D, Yan-ze L, et al:
Pharmacopoeia of the People’s Republic of China (2010 Edition): A
Milestone in Development of China’s Healthcare. Chinese Herbal
Medicines. 2:157–160. 2010.
|
|
27
|
Collins PD, Connolly DT and Williams TJ:
Characterization of the increase in vascular permeability induced
by vascular permeability factor in vivo. Br J Pharmacol.
109:195–199. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hippenstiel S, Krüll M, Ikemann A, et al:
VEGF induces hyperpermeability by a direct action on endothelial
cells. Am J Physiol. 274:L678–L684. 1998.PubMed/NCBI
|
|
29
|
Goodger AM and Rogers PA: Uterine
endothelial cell proliferation before and after embryo implantation
in rats. J Reprod Fertil. 99:451–457. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramsey EM and Donner MW: Placental
Vasculature and Circulation: Anatomy, Physiology, Radiology,
Clinical Aspects: Atlas and Textbook. Thieme; Stuttgart: 1980
|
|
31
|
Jakeman LB, Armanini M, Phillips HS and
Ferrara N: Developmental expression of binding sites and messenger
ribonucleic acid for vascular endothelial growth factor suggests a
role for this protein in vasculogenesis and angiogenesis.
Endocrinology. 133:848–859. 1993.PubMed/NCBI
|
|
32
|
Breier G, Albrecht U, Sterrer S and Risau
W: Expression of vascular endothelial growth factor during
embryonic angiogenesis and endothelial cell differentiation.
Development. 114:521–532. 1992.PubMed/NCBI
|
|
33
|
Giudice LC: Genes associated with
embryonic attachment and implantation and the role of progesterone.
J Reprod Med. 44(2 Suppl): 165–171. 1999.
|
|
34
|
Young MF, Kerr JM, Termine JD, et al: cDNA
cloning, mRNA distribution and heterogeneity, chromosomal location,
and RFLP analysis of human osteopontin (OPN). Genomics. 7:491–502.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carson DD, Lagow E, Thathiah A, et al:
Changes in gene expression during the early to mid-luteal
(receptive phase) transition in human endometrium detected by
high-density microarray screening. Mol Hum Reprod. 8:871–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao L, Tulac S, Lobo SA, et al: Global
gene profiling in human endometrium during the window of
implantation. Endocrinology. 143:2119–2138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Borthwick JM, Charnock-Jones DS, Tom BD,
et al: Determination of the transcript profile of human
endometrium. Mol Hum Reprod. 9:19–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Talbi S, Hamilton A, Vo K, et al:
Molecular phenotyping of human endometrium distinguishes menstrual
cycle phases and underlying biological processes in normo-ovulatory
women. Endocrinology. 147:1097–1121. 2006. View Article : Google Scholar
|
|
39
|
Chaen T, Konno T, Egashira M, et al:
Estrogen-dependent uterine secretion of osteopontin activates
blastocyst adhesion competence. PLoS One. 7:e489332012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liaw L, Birk DE, Ballas CB, Whitsitt JS,
Davidson JM and Hogan BL: Altered wound healing in mice lacking a
functional osteopontin gene (spp1). J Clin Invest. 101:1468–1478.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rittling SR, Matsumoto HN, Mckee MD, et
al: Mice lacking osteopontin show normal development and bone
structure but display altered osteoclast formation in vitro. J Bone
Miner Res. 13:1101–1111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liaw L, Almeida M, Hart CE, Schwartz SM
and Giachelli CM: Osteopontin promotes vascular cell adhesion and
spreading and is chemotactic for smooth muscle cells in vitro. Circ
Res. 74:214–224. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beaumont HM and Smith AF: Embryonic
mortality during the pre- and post-implantation periods of
pregnancy in mature mice after superovulation. J Reprod Fertil.
45:437–448. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ertzeid G and Storeng R: Adverse effects
of gonadotrophin treatment on pre- and postimplantation development
in mice. J Reprod Fertil. 96:649–655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McKiernan SH and Bavister BD:
Gonadotrophin stimulation of donor females decreases
post-implantation viability of cultured one-cell hamster embryos.
Hum Reprod. 13:724–729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miller BG and Armstrong DT: Effects of a
superovulatory dose of pregnant mare serum gonadotropin on ovarian
function, serum estradiol, and progesterone levels and early embryo
development in immature rats. Biol Reprod. 25:261–271. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ertzeid G and Storeng R: The impact of
ovarian stimulation on implantation and fetal development in mice.
Hum Reprod. 16:221–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Foote R and Ellington J: Is a
superovulated oocyte normal? Theriogenology. 29:111–123. 1988.
View Article : Google Scholar
|
|
49
|
Allen J and McLaren A: Cleavage rate of
mouse eggs from induced and spontaneous ovulation. J Reprod Fertil.
27:137–140. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Makrigiannakis A, Minas V, Kalantaridou
SN, Nikas G and Chrousos GP: Hormonal and cytokine regulation of
early implantation. Trends Endocrinol Metab. 17:178–185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ruan HC, Zhu XM, Luo Q, et al: Ovarian
stimulation with GnRH agonist, but not GnRH antagonist, partially
restores the expression of endometrial integrin beta3 and
leukaemia-inhibitory factor and improves uterine receptivity in
mice. Hum Reprod. 21:2521–2529. 2006. View Article : Google Scholar : PubMed/NCBI
|