Introduction
Renal ischemia and reperfusion (I/R) injury is one
of the main causes of acute kidney injury (AKI) and often occurs in
such surgeries as kidney transplantation, partial nephrectomy,
renal artery angioplasty, accidental or iatrogenic trauma,
hydronephrosis and elective urological operations (1,2). AKI
is characterized by a rapid decline in renal function, resulting in
a failure to maintain fluid, electrolyte and acid-base homeostasis
in clinical practice (3). Over the
past decades, the incidence of AKI in adults and children has been
continuously increasing, and attention paid to it has been growing
due to its high morbidity and mortality rates as well as poor
prognosis (4).
Renal I/R injury is a pathological process involving
oxidative stress, inflammation reaction, calcium ion overloading
and apoptosis. Cell apoptosis has been considered one of the most
serious consequences of renal I/R injury in previous studies and
determines the outcome of renal damage (5,6). As
apoptosis is extremely important in renal I/R injury, the ideal
therapeutic approach is to target its processes.
Picrorhiza scrophulariiflora belongs to the
plant family, Scrophulariaceae. The roots of this plant are of
benefit to health and often used in traditional Chinese medicine to
treat a number of conditions (7).
Extracts of the roots contain various terpenoids and glycosides
(8) and picroside II is one of the
main active constituents of the extracts. Numerous published
studies have shown that picroside II has a wide range of
pharmacological effects, including neuroprotective,
hepatoprotective, anti-apoptosis, anti-cholestatic,
anti-inflammatory and immune-modulating activities (9–11).
However, to the best of our knowledge, it has not been demonstrated
whether picroside II can protect renal tissue against apoptosis
induced by renal I/R injury. Therefore, the major purpose of this
study was to determine whether picroside II is able to attenuate
apoptosis following renal I/R injury and its possible
mechanism.
Materials and methods
Animal model of I/R
The present study was approved by the Institutional
Animal Care and Use Committee of Wuhan University (Wuhan, China).
Adult healthy male Sprague-Dawley rats, specific pathogen-free
grade, weight 220–250 g, were supplied by the Experimental Animals
Center of the Medical College of Wuhan University (Wuhan, China).
This project was approved by the committee of experimental animals
of Wuhan University, and the procedures were carried out according
to routine animal-care guidelines. All experimental procedures
complied with the Guide for the Care and Use of Laboratory Animals
(1996). Briefly, rats were anesthetized with pentobarbital (45
mg/kg) and placed on a homeothermic table in order to maintain the
core body temperature at 37°C. A midline laparotomy was made and
right nephrectomy was performed. Subsequently, the left kidney was
subjected to 45 min of ischemia followed by 24 h of reperfusion.
All animals were randomly separated into three groups:
Sham-operated (sham), I/R and picroside II groups. Each group
contained eight rats. In the I/R and picroside II groups, after the
right kidneys were removed, the left kidney vessels were clamped
for 45 min followed by 24 h reperfusion. Rats in the sham group
were only subjected to resection of the right kidney. The
interventions were performed as described below.
Intervention study
Picroside II (CAS No: 39012-20-9, purity >98%,
molecular formula C23H28O13) was
purchased from Tianjin Kuiqing Medical Technology Co. Ltd.
(Tianjin, China). It was diluted to form a 10 g/l solution with 1
mol/l phosphate-buffered saline (PBS). Picroside II (10 mg/kg) 250
μl was administered via the tail vein to the rats in the picroside
II group with a micro-syringe as described in a previous study
(10), at the end of the 45 min of
ischemia and prior to reperfusion. The rats in the I/R and sham
groups were simultaneously injected with 1 mol/l PBS 250 μl. All
mice were sacrificed following the 24 h reperfusion period with an
overdose of pentobarbital sodium (Sigma-Aldrich, St. Louis, MO,
USA), and the left kidneys were removed for the following
experiments and the blood samples were collected for the detection
of blood urea nitrogen (BUN) and creatinine (Cr) levels.
Preservation of kidneys
The left kidney was removed under fully maintained
anesthesia. After removal, the kidney was fixed in 10%
phosphate-buffered formalin or immediately frozen, and stored at
−80°C for subsequent experiments.
Serum assays
At 24 h after I/R injury in every group, 1-ml blood
samples were taken and analyses were performed according to the
instructions of the Creatinine Assay and Urea Assay kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). The absorbance
was measured using a spectrophotometer (UV-1700; Shimadzu
Corporation, Tokyo, Japan) and then the concentrations of BUN and
Cr were calculated.
Histological examinations
After the kidney fixed in 10% phosphate-buffered
formalin, it was embedded with paraffin and sectioned to 4-μm
thickness. The sections were deparaffinized and hydrated gradually,
and stained with hematoxylin and eosin (H&E) and terminal
deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate-biotin nick end labeling (TUNEL) techniques,
respectively. Morphologic assessments were observed by an
experienced renal pathologist who was unaware of the treatments. An
established grading scale of 0–4, outlined by Jablonski et
al (12), was used for the
histopathologic assessment of I/R-induced damage.
Apoptosis detection by the TUNEL
method
The cell apoptosis was detected with a TUNEL assay
according to the manufacturer’s instructions (Roche, Basel,
Switzerland). Five fields were randomly selected and the number of
positive cells was counted. The percentage of positive cells served
as the apoptosis index.
Immunohistochemistry
The expression of cleaved caspase-3 was conducted by
immunohistochemical staining. Briefly, 4-μm sections were
deparaffinized, and endogenous peroxidase activity was blocked with
3% hydrogen peroxide at 37°C for 10 min. Then the sections were
treated with 10% normal goat serum in Tris-buffered saline (TBS)
for 30 min at 37°C. Subsequently, they were incubated overnight at
4°C with polyclonal rat anti-cleaved caspase-3 (1:1,000; cat. no.
#9661, Cell Signaling Technology, Boston, MA, USA). After washing
three times with PBS, these sections were incubated with the
secondary antibody from the UltraVision™ Quanto Detection System
HRP DAB (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min at
room temperature, followed by color reagent 3,3′-diaminobenzidine
(DAB). In the negative control group, the experiments were
routinely performed.
Reverse transcription-polymerase chain
reaction (RT-qPCR)
Total RNA was isolated from the rat tissue from each
group using TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) and the RNA concentration was obtained using a
spectrophotometer. Single-stranded cDNA was synthesized using a
cDNA synthesis kit (Takara, Kyoto, Japan) according to the
instructions provided by the manufacturer. qPCR was performed with
the Applied Biosystems SYBR Green mix kit (Applied Biosystems,
Foster City, CA, USA) using the SLAN-96s Real-Time PCR system
(Shanghai Hongshi Medical Technology Co., Ltd., Shanghai, China).
The reaction composition contained: 2 μl cDNA, 12.5 μl 2X SYBR
Green mix, 1 μl forward primer and 1 μl reverse primer and 8.5 μl
ddH2O in a final volume of 25 μl. The primers used were
as follows: Bax forward, 5′-TGAACTGGACAACAACATGGAG-3′, and reverse,
5′-AGCAAAGTAGAAAAGGGCAACC-3′ (GenBank accession number NM_017059);
Bcl-2 forward, 5′-TTTGATTTCTCCTGGCTGTCT-3′ and reverse,
5′-CTGATTTGACCATTTGCCTG-3′ (GenBank accession number NM_016993);
PARP-1 forward 5′-TCTCCAATCGCTTCTACACCCT-3′ and reverse,
5′-TACTGCTGTCATCAGACCCACC-3′ (GenBank accession number NM_013063).
β-actin was used as a housekeeping gene. The data are presented as
a ratio of gene to β-actin mRNA [sense: 5′-TGCTATGTTGCCCTAGAC
NM_017059 TTCG-3′ and antisense: 5′-GTTGGCATAGAGGTCTTTACGG-3′
(GenBank accession number NM_031144).
Western blot analysis
Total proteins were extracted, and quantified using
bicinchoninic acid method. Then, equivalent weights of protein (40
μg/lane) was separated on 10% SDS-PAGE gels and then transferred to
a nitrocellulose membrane. The membranes were blocked with 5%
non-fat milk in Tris-buffered saline and Tween 20 (TBST) buffer and
then incubated with the following rabbit polyclonal primary
antibodies: Bax (1:1,000 dilution; 2772s, Cell Signaling
Technology, Boston, MA, USA), Bcl-2 (1:1,000 dilution; 2870s, Cell
Signaling Technology) and poly(ADP-ribose) polymerase-1 (PARP-1)
(1:1,000 dilution; sc-7150, Santa Cruz Biotechnology, Santa Cruz,
CA, USA) at 4°C overnight. Subsequently, after being washed twice
with PBS, the membranes were incubated with goat anti-rabbit
horseradish peroxidase-conjugated immunoglobulin G secondary
antibody (1:2,000; ZDR-5306, ZSGB-BIO, Beijing, China) at room
temperature for 1 h. Specific bands were visualized using Immobilon
Western Chemiluminescence HRP substrate (Merck Millipore,
Darsmtadt, Germany). Optical densities were detected using Quantity
One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were conducted using SPSS version
17.0 (SPSS Inc., Chicago, IL, USA). The means of the different
groups were compared using one-way analysis of variance and
Student-Newman-Keuls test. Differences were considered
statistically significant when P<0.05.
Results
Renal function
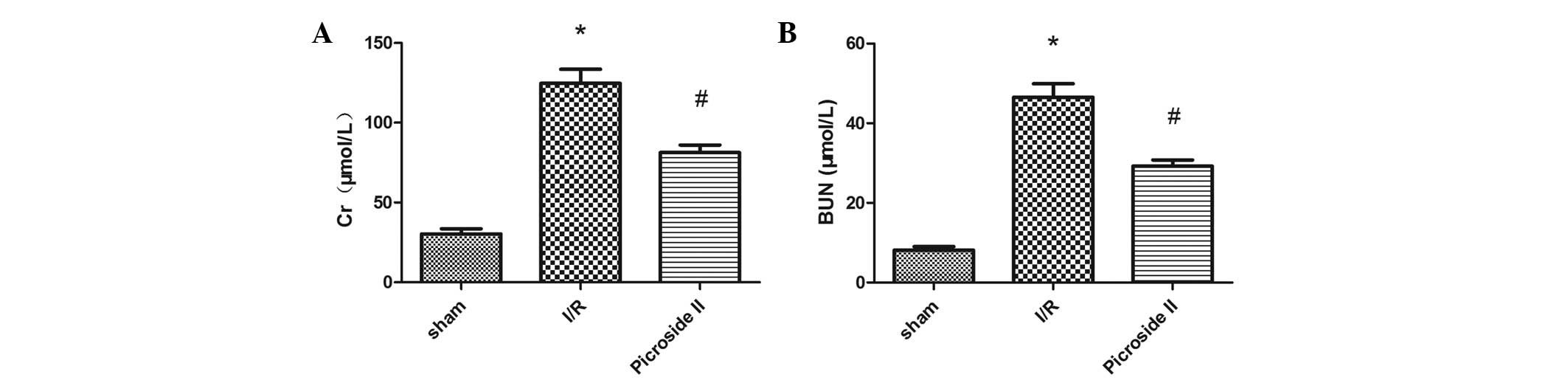
It was evident from the results that rats subjected
to I/R injury showed significant increases in BUN and Cr levels
compared with rats in the sham group. The renal function damage
induced by I/R was significantly improved by the treatment with
picroside II (P<0.05; Fig.
1).
Histopathology
Renal I/R resulted in significant renal injury, as
evidenced by tubular necrosis, medullary hemorrhage and congestion.
However, treatment with picroside II reduced these types of severe
renal damage (Fig. 2A–C).
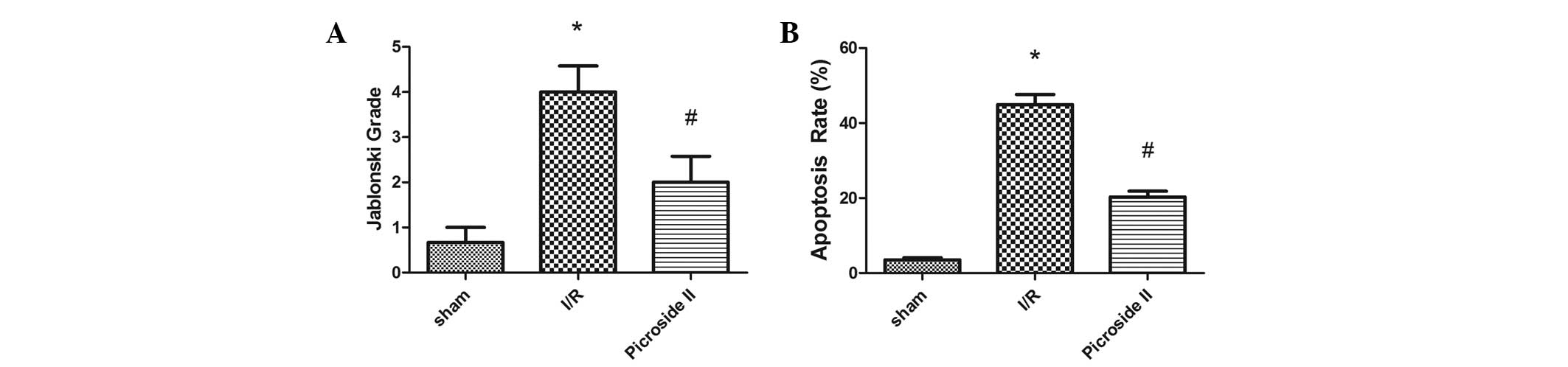
According to the Jablonski scores, 45 min of renal ischemia
followed by 24 h of reperfusion resulted in severe acute tubular
necrosis. Quantitative analysis showed an clearly decreased score
in the picroside II group compared with the I/R group (Fig. 3A).
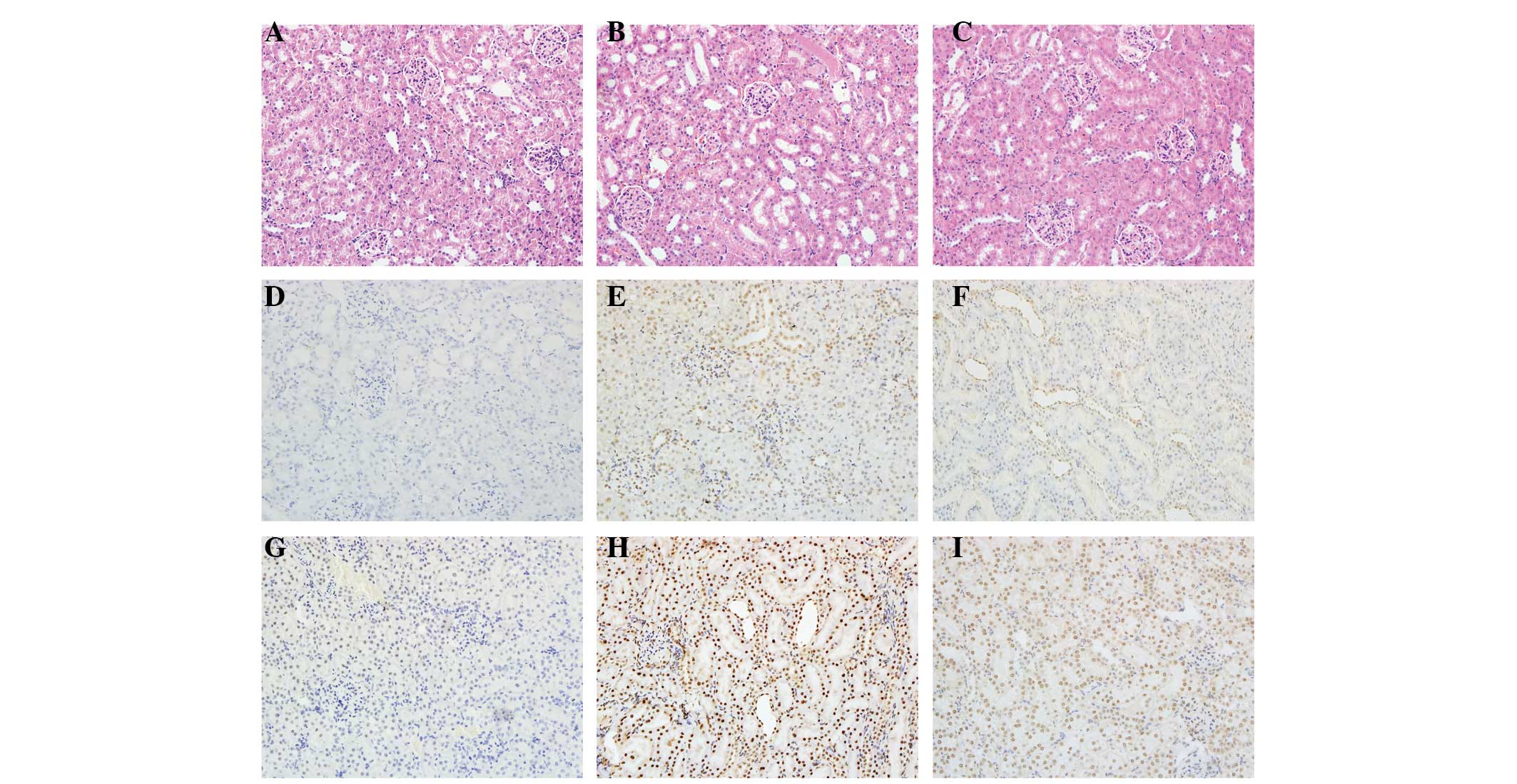 | Figure 2Histological features were evaluated
by H&E and TUNEL staining and immunohistochemistry was
performed to evaluate the expression of cleaved caspase-3.
Representative kidney sections stained with (A-C) H&E, (D-F)
TUNEL and (G-I) cleaved caspase-3 (brown nuclear staining) in
kidneys at the end of the 24 h reperfusion period. Sections from
(A,D,G) a sham-operated rat, (B,E,H) a rat subjected to I/R and
(C,F,I) a rat subjected to picroside II treatment. All images of
H&E, TUNEL and immunohistochemical staining, original
magnification ×200. H&E, hematoxylin and eosin; TUNEL, terminal
deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate-biotin nick end labeling; I/R, ischemia and
reperfusion. |
TUNEL staining
At 24 h after I/R, no TUNEL-positive cells were
present in the sham group. In the I/R group, positive cells were
easily observed. However, there were clearly fewer TUNEL-positive
cells in the picroside II group compared with the I/R group
(Fig. 2D–F). The mean percentage
of TUNEL positive cells in the I/R group was greater than that in
the sham and picroside II groups (Fig.
3B).
Immunohistochemistry
In this study, cleaved caspase-3 was detected by
immunohistochemical techniques. Staining revealed that cleaved
caspase-3 was rarely found in the kidneys of the rats in the sham
group. However, in the I/R group, the rat renal tissues were
strongly positive for cleaved caspase-3 expression. Compared with
the I/R group, the expression of cleaved caspase-3 was reduced in
the picroside II group (Fig.
2G–I).
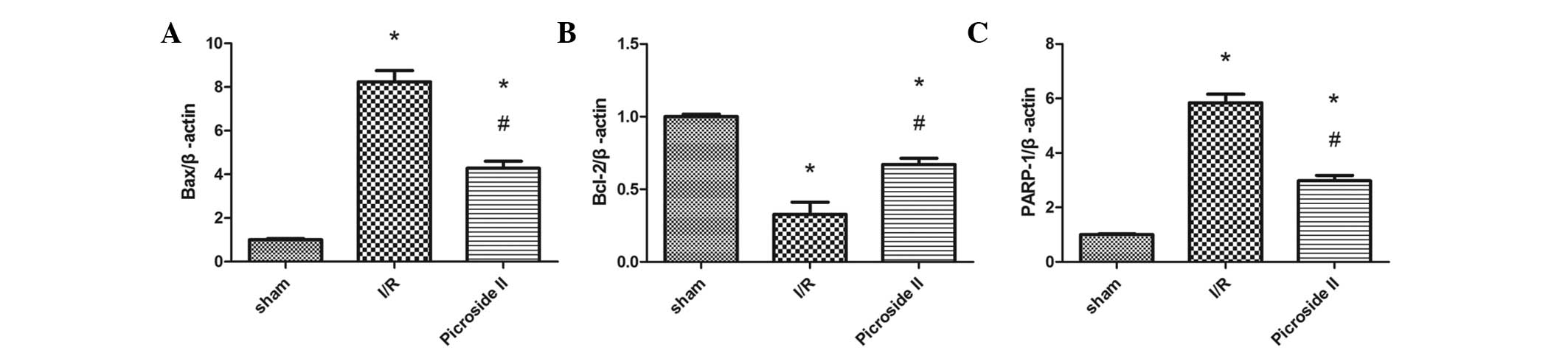
RT-qPCR analysis
The relative mRNA expression levels of Bax, Bcl-2
and PARP-1 to β-actin were determined. The mRNA levels of Bax and
PARP-1 were significantly greater in the I/R group than in the sham
group. However, the treatment with picroside II was found to
significantly reduce the mRNA expression levels of Bax and PARP-1
following I/R (Fig. 4).
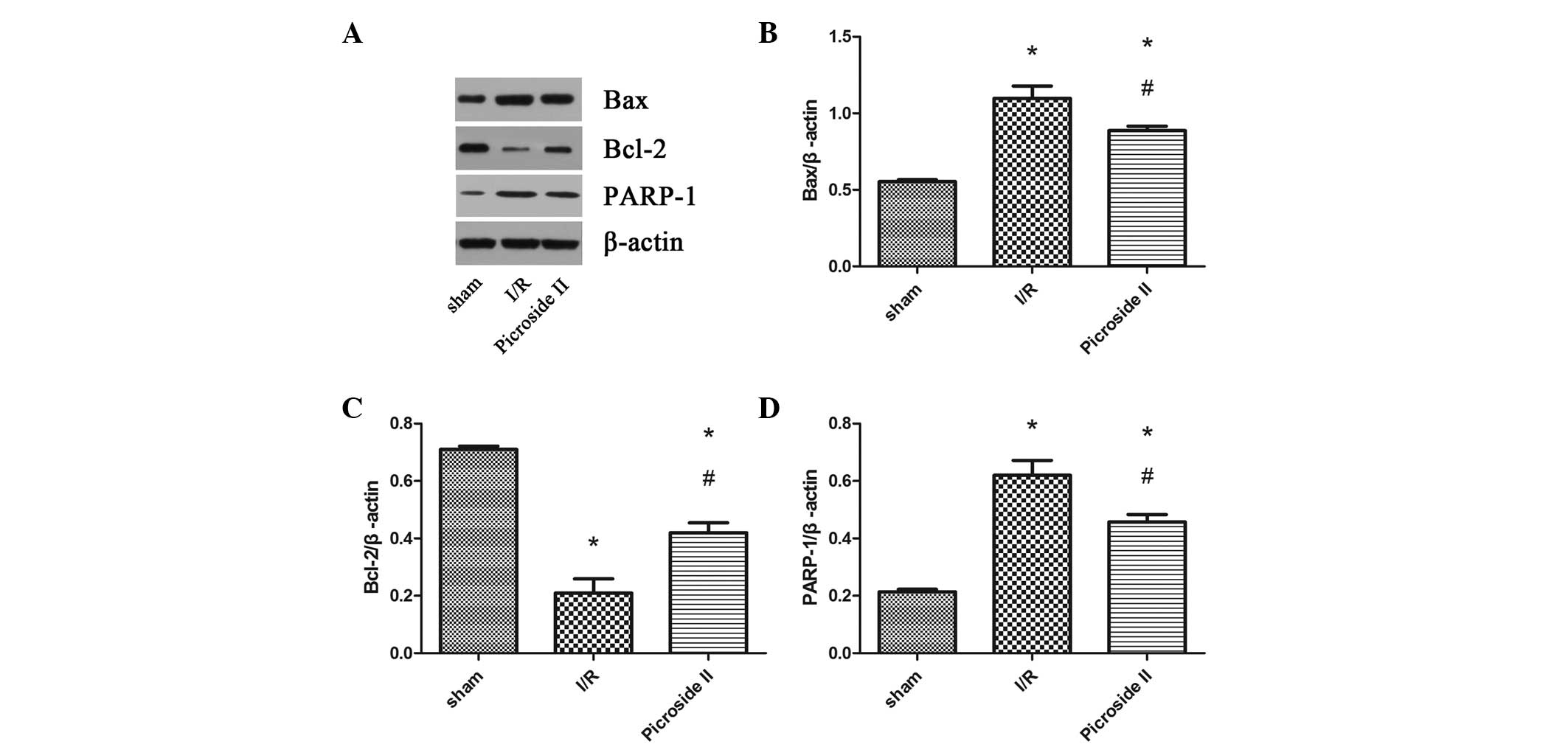
Western blot analysis
To investigate the different levels of protein
expression, the expression of Bax, Bcl-2 and PARP-1 was analyzed by
western blotting (Fig. 5). It was
evident from the results that the expression levels of Bax and
PARP-1 were upregulated in the I/R and picroside II groups compared
with the levels in the sham group. However, picroside II attenuate
these increases in expression induced by I/R. Bcl-2 levels were
downregulated in rats subjected to I/R compared with those in the
sham group, but in the picroside II group, the expression level was
clearly greater than that observed in the I/R group.
Discussion
AKI is a common clinical complication, with a rapid
reduction in the glomerular filtration rate. Although there are
numerous renal replacement therapies, the morbidity and mortality
rates associated with AKI remain high (13). Currently, the main therapy is
focused on nutritional and supportive care and there is no specific
therapy that can be used to significantly improve the clinical
outcome of patients with AKI (14). Therefore, it is necessary to seek
new therapeutic strategies for AKI.
Picroside II is one of the main active constituents
of the extracts of Picrorhiza scrophulariiflora Pennell and
it has been shown to possess a wide range of pharmacological
effects, including neuroprotective, hepatoprotective,
anti-apoptosis, anti-cholestatic, anti-inflammatory and
immune-modulating activities (15–17).
In a previous study, it was demonstrated that picroside II can
inhibit apoptosis in rats subjected to focal cerebral I/R injury
(10). Another study reported that
picroside II protects hepatocytes against injury and counteracts
apoptosis by maintaining the integrity of the mitochondrial
membrane and enhancing the activity of ATPase in mitochondria
(11). However, to the best of our
knowledge, it has not been demonstrated whether picroside II is
able to protect renal tissue against renal I/R injury. In the
present study, an investigation of whether picroside II could
attenuate the apoptosis induced by renal I/R injury in rats was
conducted.
It is generally accepted that apoptosis leads to
renal dysfunction subsequent to ischemia (18–20).
This is based on studies using various approaches (including
caspase-3 activity, Bax activation, cytochrome c release and
changes in the Bcl-2/Bax ratio) that have demonstrated that
apoptosis is a consequence of ischemia (21). Apoptotic mechanisms are complex and
the caspase enzyme cascade plays a key role among the multiple
mediators of the complex process of apoptosis. Caspase-3 is an
important enzyme among the cysteine proteases that exist as
inactive zymogens. It is the most crucial downstream apoptosis
protease in the caspase cascade ‘waterfall’ (22). Its activation is determined by a
series of signal transduction cascades, among which the interaction
between Bcl-2 and Bax plays a key role (23). The results of the present study
indicated that picroside II significantly reduced apoptosis caused
by I/R injury, which was demonstrated by TUNEL staining.
Immunohistochemical staining revealed that the expression of
cleaved caspase-3 was clearly greater in the I/R group than in the
sham group, and that treatment with picroside II significantly
reduced its expression, which was consistent with the results of
TUNEL staining.
To clarify the protective mechanisms of picroside
II, the expression levels of certain important apoptotic molecules
were evaluated. The proteins of the Bcl-2 family, which are crucial
regulatory factors, promote either cell survival (e.g., Bcl-2) or
cell death by apoptosis (e.g., Bax). The ratio of Bcl-2/Bax is a
pivotal factor that determines whether or not apoptosis occurs in
cells exposed to injury (24).
Also, activated caspase-3 can hydrolyze PARP, which is a type of
catalytic protease. RT-qPCR and western blotting indicated that the
expression levels of Bax and PARP-1 were upregulated and the
expression level of Bcl-2 was downregulated in the I/R group, and
that picroside II treatment was clearly able to ameliorate these
changes in expression induced by I/R. In addition, the Bcl-2/Bax
expression ratio decreased markedly in the I/R group compared with
that in the sham group, but increased significantly with picroside
II treatment, indicating that picroside II attenuated apoptosis by
affecting the Bcl-2/Bax expression ratio.
In conclusion, the present study demonstrated for
the first time that picroside II is able to protect renal tissue
against I/R injury. This protective effect may be achieved through
the inhibition of apoptosis. Therefore, these findings reveal the
potential role of picroside II as a therapeutic option for AKI.
Acknowledgements
This study was supported by the Natural Science
Foundation of Hubei Province (grant no. 2013CFB226).
Abbreviations:
|
AKI
|
acute kidney injury
|
|
I/R
|
ischemia and reperfusion
|
References
|
1
|
Sagiroglu T, Torun N, Yagci M, Yalta T,
Sagiroglu G and Oguz S: Effects of apelin and leptin on renal
functions following renal ischemia/reperfusion: An experimental
study. Exp Ther Med. 3:908–914. 2012.PubMed/NCBI
|
|
2
|
Yun Y, Duan WG, Chen P, Wu HX, Shen ZQ,
Qian ZY and Wang DH: Ischemic postconditioning modified renal
oxidative stress and lipid peroxidation caused by ischemic
reperfusion injury in rats. Transplant Proc. 41:3597–3602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang S, Chou WP and Pei L: Effects of
propofol on renal ischemia/reperfusion injury in rats. Exp Ther
Med. 6:1177–1183. 2013.PubMed/NCBI
|
|
4
|
Uchino S, Bellomo R, Morimatsu H, Morgera
S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, et al:
Continuous renal replacement therapy: a worldwide practice survey.
The beginning and ending supportive therapy for the kidney (BEST
kidney) investigators. Intensive Care Med. 33:1563–1570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang ZX, Shek K, Wang S, Huang X, Lau A,
Yin Z, Sun H, Liu W, Garcia B, Rittling S and Jevnikar AM:
Osteopontin expressed in tubular epithelial cells regulates NK
cell-mediated kidney ischemia reperfusion injury. J Immunol.
185:967–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JX, Li P, Tezuka Y, Namba T and Kadota
S: Three phenylethanoid glycosides and an iridoid glycoside from
Picrorhiza scrophulariiflora. Phytochemistry. 48:537–542. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou LC, Zhu TF, Xiang H, Yu L, Yan ZH, Gan
SC, Wang DC, Zeng S and Deng XM: New secoiridoid glycosides from
the roots of Picrorhiza scrophulariiflora. Molecules. 13:2049–2057.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng FJ, Jiao SM and Yu B: Picroside II
protects cardiomyocytes from hypoxia/reoxygenation-induced
apoptosis by activating the PI3K/Akt and CREB pathways. Int J Mol
Med. 30:263–270. 2012.PubMed/NCBI
|
|
10
|
Li Q, Li Z, Xu XY, Guo YL and Du F:
Neuroprotective properties of picroside II in a rat model of focal
cerebral ischemia. Int J Mol Sci. 11:4580–4590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao H and Zhou YW: Anti-lipid peroxidation
and protection of liver mitochondria against injuries by picroside
II. World J Gastroenterol. 11:3671–3674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jablonski P, Howden BO, Rae DA, Birrell
CS, Marshall VC and Tange J: An experimental model for assessment
of renal recovery from warm ischemia. Transplantation. 35:198–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueda N, Kaushal GP and Shah SV: Apoptotic
mechanisms in acute renal failure. Am J Med. 108:403–415. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yaklin KM: Acute kidney injury: an
overview of pathophysiology and treatments. Nephrol Nurs J.
38:13–18. 2011.PubMed/NCBI
|
|
15
|
Cao Y, Liu JW, Yu YJ, Zheng PY, Zhang XD,
Li T and Guo MC: Synergistic protective effect of picroside II and
NGF on PC12 cells against oxidative stress induced by
H2O2. Pharmacol Rep. 59:573–579.
2007.PubMed/NCBI
|
|
16
|
Smit HF, Kroes BH, van den Berg AJ, van
der Wal D, van den Worm E, Beukelman CJ, van Dijk H and Labadie RP:
Immunomodulatory and anti-inflammatory activity of Picrorhiza
scrophulariiflora. J Ethnopharmacol. 73:101–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He LJ, Liang M, Hou FF, Guo ZJ, Xie D and
Zhang X: Ethanol extraction of Picrorhiza scrophulariiflora
prevents renal injury in experimental diabetes via
anti-inflammation action. J Endocrinol. 200:347–355. 2009.
View Article : Google Scholar
|
|
18
|
Saikumar P and Venkatachalam MA: Role of
apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol.
23:511–521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonegio R and Lieberthal W: Role of
apoptosis in the pathogenesis of acute renal failure. Curr Opin
Nephrol Hypertens. 11:301–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kinaci MK, Erkasap N, Kucuk A, Koken T and
Tosun M: Effects of quercetin on apoptosis, NF-κB and NOS gene
expression in renal ischemia/reperfusion injury. Exp Ther Med.
3:249–254. 2012.PubMed/NCBI
|
|
21
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prabhakar G, Vona-Davis L, Murray D,
Lakhani P and Murray G: Phosphocreatine restores high-energy
phosphates in ischemic myocardium: implication for off-pump cardiac
revascularization. J Am Coll Surg. 197:786–791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li
L, Xu XN, Gong LL, Qin HL, Fang LH, et al: Coptisine protects rat
heart against myocardial ischemia/reperfusion injury by suppressing
myocardial apoptosis and inflammation. Atherosclerosis.
231:384–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang H, Yu F, Tong Z, Yuan B and Wang C:
Effect of ischemia post-conditioning on skeletal muscle oxidative
injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1α/β-actin
mRNA, IL-6/β-actin mRNA and caveolin-3/β-actin mRNA expression in
ischemia-reperfusion rabbits. Mol Biol Rep. 40:507–514. 2013.
View Article : Google Scholar
|