Introduction
Preeclampsia is a multi-system disorder of pregnancy
characterized by hypertension, proteinuria and systemic
vasoconstriction. The disorder is diagnosed in the latter half of
pregnancy, affects ~5% of pregnant females and accounts for
considerable mortality and morbidity (1). However its cause and pathogenesis are
unclear. In previous years, vascular remodeling disorders of the
uterine and placenta and placenta hypoperfusion have been generally
recognized (2). It appears that
when trophoblasts invade into spiral arteries insufficiently in the
early stages of pregnancy, this impacts the process of vascular
remodeling, resulting in ischemia and hypoxia of the placenta and
causing hypertensive disease in pregnancy. The role of matrix
metalloproteinases (MMPs) in the pathogenesis of hypertensive
disorders in pregnancy has been the focus of much attention
(3–6). Further studies have detected that
MMP-1 is secreted from trophoblasts, which is an important factor
in the regulation of trophoblast invasion (4,7–9).
MMPs are a type of proteinase, which can degrade the
extracellular matrix (ECM) and remodel normal structure. Tissue
inhibitors of metalloproteinases (TIMPs) are specific endogenous
inhibitors that bind MMPs in a 1:1 stoichiometry, and their
expression is regulated during development and tissue remodeling.
TIMPs (21–29 kDa) have an N-terminal domain (125 amino acids) and a
C-terminal domain (65 amino acids). The N-terminal domain folds as
a separate unit and is capable of inhibiting MMPs (10). MMP-1 is an important member of the
MMP family. The zymolytes of MMP-1 are collagen and metagelatin,
which play major roles in trophoblast invasion (7). TIMP-1 is a natural inhibitor of MMP-1
(11).
However, there are few studies regarding MMP-1 and
hypertensive disorders in pregnancy (1). This study explored whether reduced
MMP-1 expression is associated with shallow trophoblast invasion
and the pathogenesis of preeclampsia. MMP-1 and TIMP-1 protein
expression in maternal umbilical serum, placenta and decidua from
cases and controls were compared by ELISA and immunohistochemical
analysis.
Materials and methods
Patient selection
Following approval by the ethics committee of Taihe
Hospital (Shiyan, China) and informed consent from each patient, 73
pregnant females were recruited as the test subjects, including 43
inpatients with hypertensive disorders in pregnancy and 30 normal
pregnant females as the control. The 43 inpatients with
hypertensive disorders in pregnancy included 18 patients with
gestational hypertension, nine with mild preeclampsia and 16 with
severe preeclampsia. They all delivered in the obstetrical
department of the Taihe Hospital between July 2011 and August 2012.
All cases were single pregnancies where the patients were healthy
and did not exhibit complications including hypertension, diabetes
and heart, kidney or liver diseases prior to the study.
Gestational hypertension was defined by hypertension
with systolic blood pressure ≥140 mmHg and/or diastolic blood
pressure ≥90 mmHg, appearing for the first time after
mid-pregnancy, without proteinuria. Mild preeclampsia was defined
as hypertension with a systolic blood pressure ≥140 mmHg and/or a
diastolic blood pressure ≥90 mmHg in association with proteinuria
[24 h urinary protein >300 mg per 24 h or persistent 30 mg/dl
(1+ on dipstick testing) in random urine samples] with or without
edema. Severe preeclampsia was defined as hypertension with a
systolic blood pressure ≥160 mmHg and/or a diastolic blood pressure
≥110 mmHg in association with proteinuria [24 h urinary protein
>2 g per 24 h or persistent 200 mg/dl (2+ on dipstick testing)
in random urine samples] with or without edema.
Experimental methods
Hemostatic umbilical vein (5 ml) was removed rapidly
following delivery, solidified under room temperature, then
centrifuged at 5,000 × g for 10 min at 4°C and the serum was
preserved at −80°C. Subsequent to delivery of the placenta, the
maternal side of the placenta (1×1×1 cm) and the decidua were
promptly removed. These were then rinsed three times with
physiological saline and fixed immediately with formalin for 24–48
h, imbedded in paraffin, then cut into 3-μm slices.
Total MMP-1 and TIMP-1 levels in umbilical serum
were measured using Human MMP-1/TIMP-1 PicoKine™ ELISA kits (rabbit
anti-human; Boster Biological Engineering, Wuhan, China), according
to the manufacturer’s instructions. Optical density was measured at
450 nm. The levels of MMP-1 and TIMP-1 in the placenta and decidua
were detected by immunohistochemistry (streptavidin-biotin
complex). Polyclonal (rabbit anti-human) antibodies against MMP-1
and TIMP-1 (diluted 1:200, Boster Biological Engineering) were used
to assess the cellular expression of the protein. Double
immunostaining was performed in an automated slide stainer
following deparaffination in xylene, rehydration and heat-induced
antigen retrieval at 37°C for 10 min in Tris-buffered saline at pH
6 (Dako, Glostrup, Denmark). Bovine serum albumin (2%) was added
for 10 min to inhibit non-specific binding. A primary antibody
mixture was added and the slides were incubated overnight at 4°C.
The slides were incubated with secondary immunoglobulin G
antibodies [tetramethyl rhodamine isothiocyanate-conjugated (goat
anti-rabbit) antibody and fluorescein isothiocyanate-conjugated
(goat anti-rabbit) antibody] at 1:200 dilutions for 30 min in a
dark chamber. All sections were counterstained with DAPI and
examined using an electron microscope (BX51; Olympus Corporation,
Tokyo, Japan) at ×400 magnification. Negative controls were
performed by omission of the primary antibodies as well as their
substitution by isotype-matched rabbit serum.
The proportions of trophoblasts and deciduas
expressing the MMP-1 protein were independently assessed by two
pathologists, blinded to group status, subsequent to reaching an
agreement on the inclusion criteria for positively stained cells.
The positive or negative results were judged through the dye area,
and the dye strength in the observed area. The dye area was
evaluated on a scale of 0–3: 0, none; 1, ≤25%; 2, 26–49% and 3,
≥50%. The dye strength was evaluated on a scale of 0–2: 0, no
dyeing; 1, moderate dyeing and 2, strong dyeing. The two points
were added to assess the grades as follows 0 points is (−), 1–2
points is (+), 3–4 points is (++) and 5 points is (+++).
Statistical analysis
All results were processed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses
of clinical data were performed using the unpaired two-sample
Student’s t-test (two-sided) for continuous variables after testing
for Gaussian distribution. The measurement data were examined by
variance analysis or Fisher’s exact test. The enumeration data were
examined by the χ2 test. A level of P<0.05 was
considered to indicate statistical significance.
Results
Patient characteristics
The mean ages, gestational ages and infant birth
weights of all subjects are listed in Table I. The difference in the age and
gestational age of the subjects between the two groups revealed no
statistical significance (t=1.589, P=0.116 and t=1.064, P=0.294,
respectively). The differences in the birth weight among the two
groups indicated significant differences (t=3.008, P=0.004).
 | Table IAge, gestational age and birth weight
in the two groups. |
Table I
Age, gestational age and birth weight
in the two groups.
| Group | Age (years) | Gestational age
(weeks) | Birth weight (g) |
|---|
| Normal | 27.77±4.09 | 37.27±2.27 | 3293.33±343.09 |
| Experimental | 29.51±4.94 | 36.78±1.25 | 2965.65±586.98 |
| t | 1.589 | 1.064 | 3.008 |
| P-value | 0.116 | 0.294 | 0.004 |
Serum MMP-1 and TIMP-1
The levels of MMP-1 and TIMP-1 in the serum of the
umbilical cord are listed in Tables
II and III, respectively.
The levels of MMP-1 in the umbilical serum of the normal,
gestational hypertension, mild preeclampsia and severe preeclampsia
groups were 294.33±11.53, 247.78±20.32, 177.67±12.63 and
124.68±15.41 pg/ml, respectively, and there were significant
differences between each two groups (P<0.05). However, the
levels of TIMP-1 in the umbilical serum of the four groups were
1,304.20±69.66, 1,326.20±329.86, 1,340.11±547.05 and 1,363.00±71.50
pg/ml, respectively, and no significant difference was identified
between each two groups regarding the level of TIMP-1 in the
umbilical serum (P>0.05).
 | Table IILevels of MMP-1 in serum of the
umbilical cord. |
Table II
Levels of MMP-1 in serum of the
umbilical cord.
| Group | n | MMP-1 (pg/ml) |
|---|
| Normal | 30 | 294.33±11.53 |
| Gestational
hypertension | 18 | 247.78±20.32 |
| Mild
preeclampsia | 9 | 177.67±12.63 |
| Severe
preeclampsia | 16 | 124.68±15.41 |
 | Table IIILevels of TIMP1 in serum of the
umbilical cord. |
Table III
Levels of TIMP1 in serum of the
umbilical cord.
| Group | n | TIMP-1 (pg/ml) |
|---|
| Normal | 30 | 1304.20±69.66 |
| Gestational
hypertension | 18 | 1326.20±329.86 |
| Mild
preeclampsia | 9 | 1340.11±547.05 |
| Severe
preeclampsia | 16 | 1363.00±71.50 |
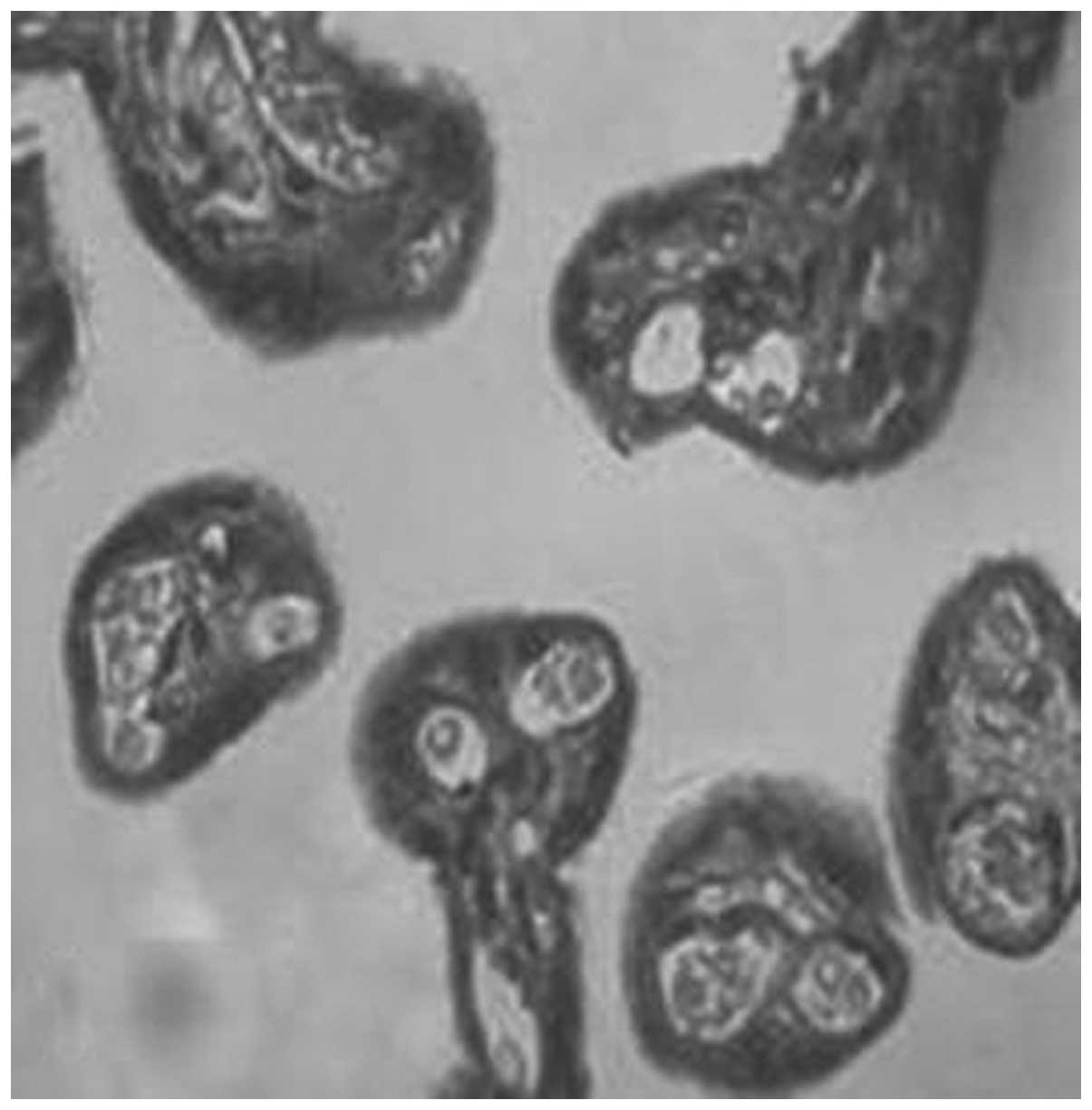
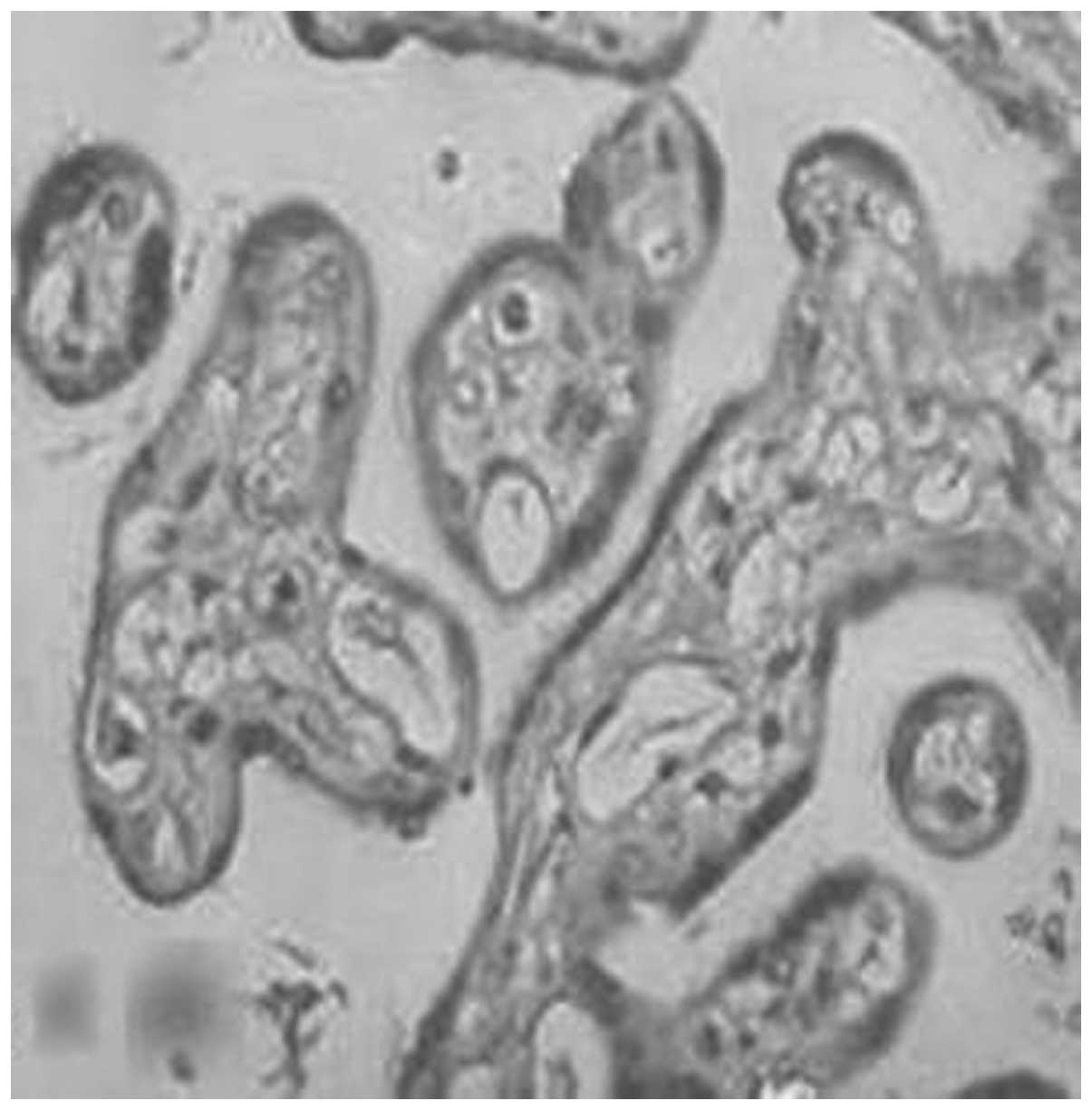
MMP-1 and TIMP-1 expression in the
placenta and decidua
The expression of MMP-1 and TIMP-1 was mainly
located in the cytomembrane and the cytoplasm of the placental
trophoblasts. They were weakly dyed in the endothelial cells of the
capillaries and the stromal fibroblasts, moderately dyed in the
cytomembrane and the cytoplasm of the decidua, and weakly dyed in
the cytoplasm of the spiral artery. The positive rates of
expression of MMP-1 in the placenta of the normal, gestational
hypertension, mild preeclampsia and severe preeclampsia groups were
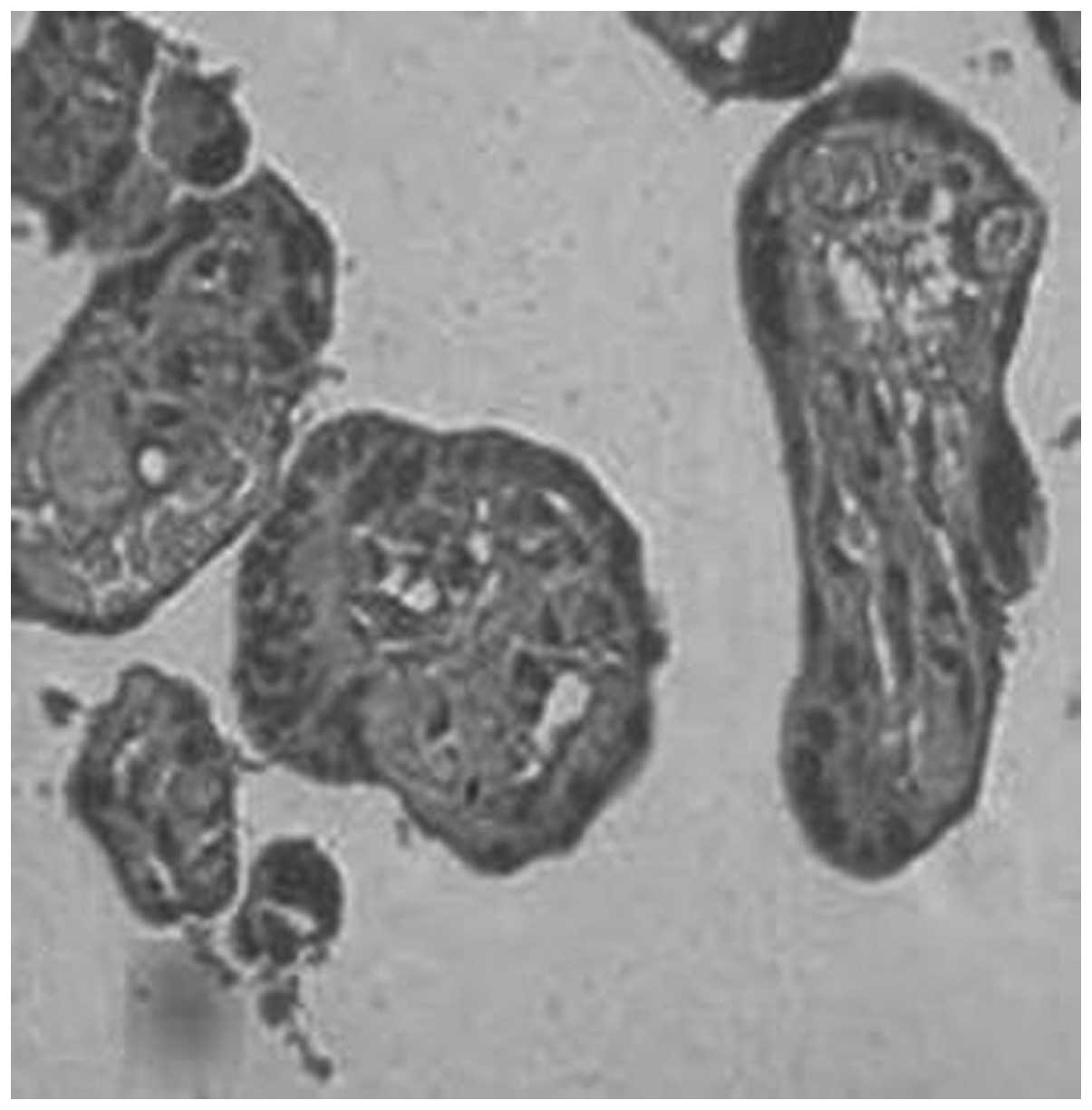
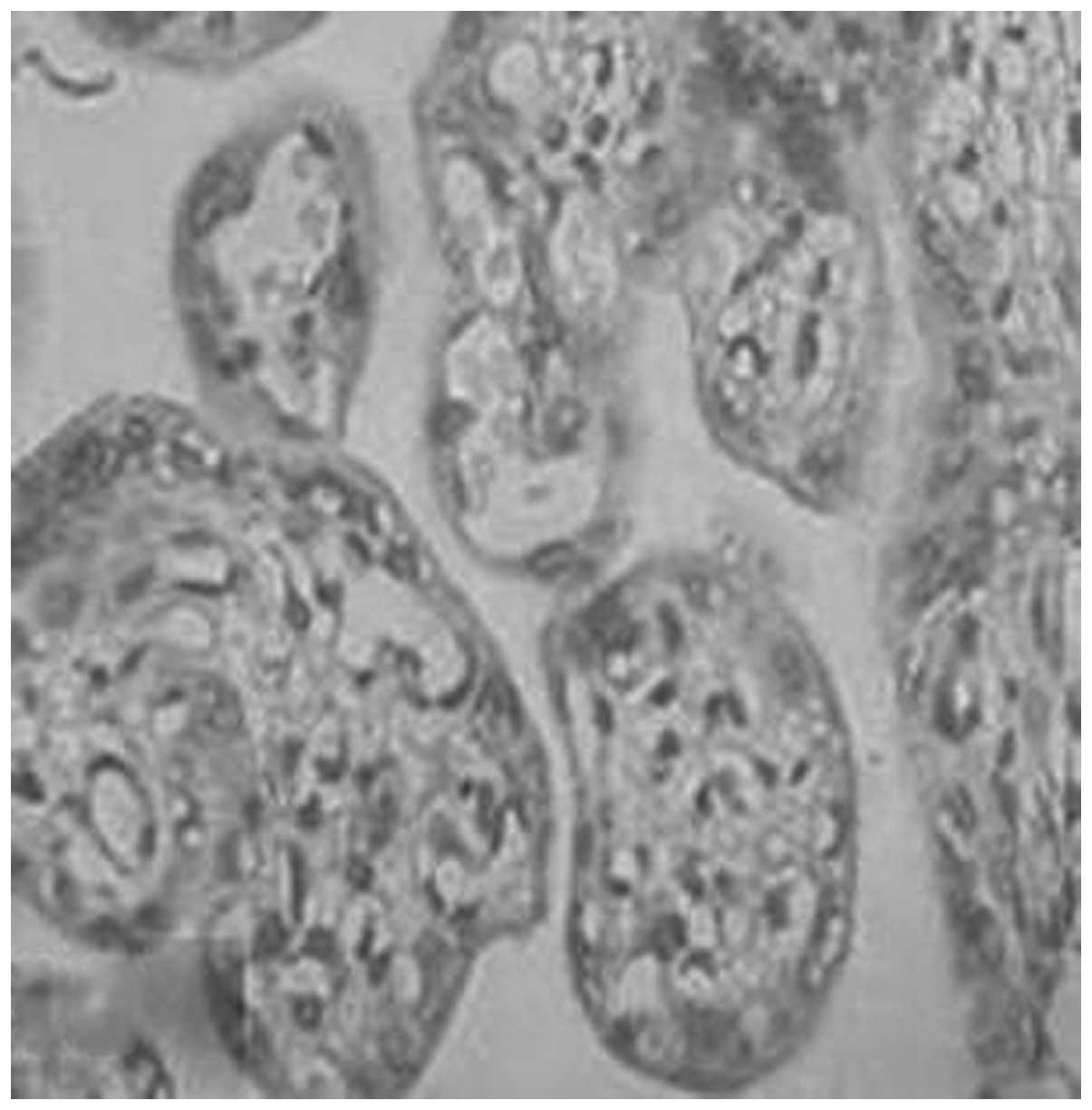
96.7, 77.8, 66.7 and 23.1%, respectively (Figs. 1–4, Table
IV). There were significant differences in MMP-1 expression
between each two groups (P<0.05). The positive rates of
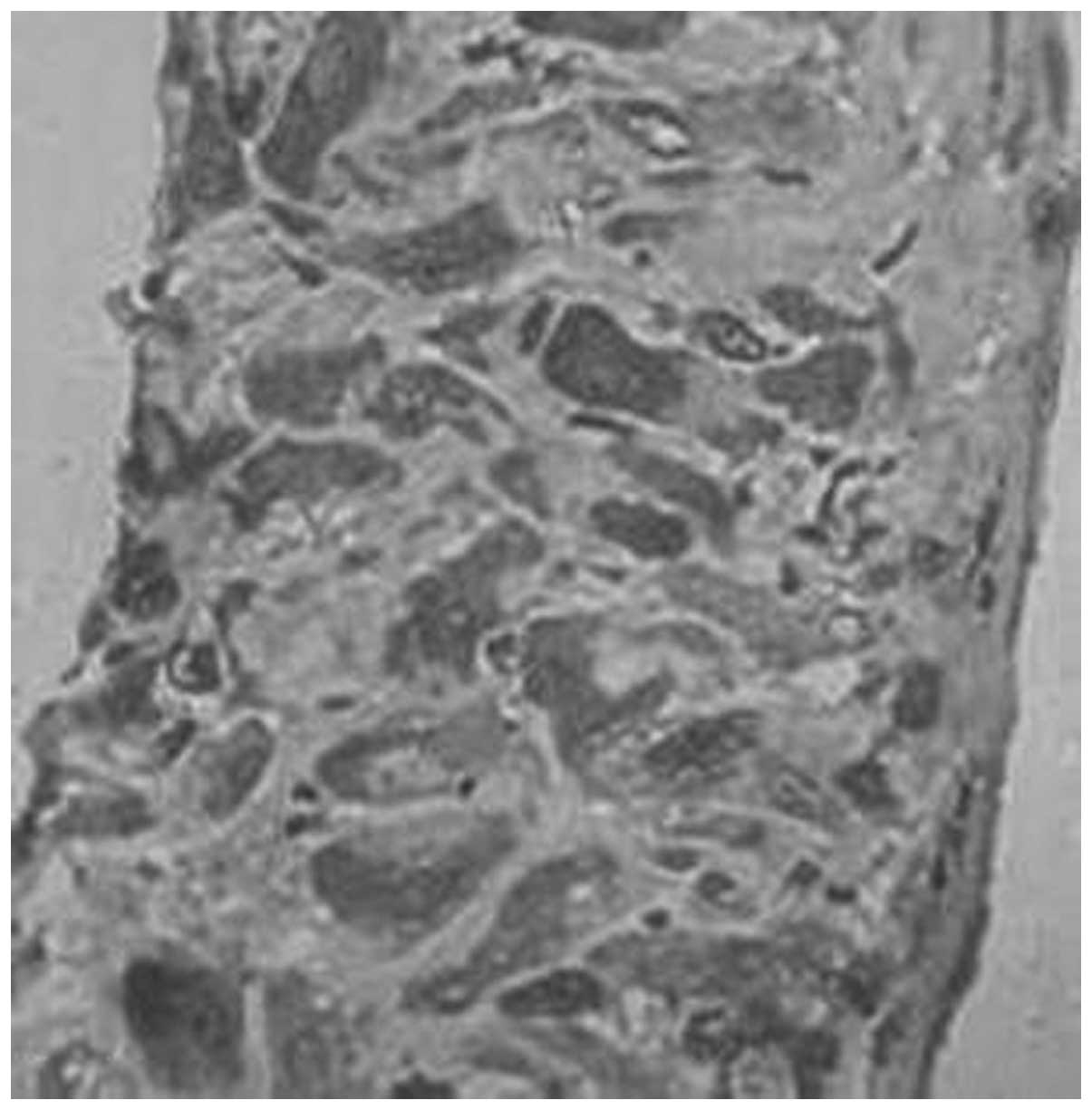
expression of MMP-1 in the decidua were 93.3, 77.8, 55.6 and 12.5%,
respectively (Figs. 5–8, Table
V). There were significant differences between each two groups
(P<0.05).
 | Table IVImmunocytochemical distribution of
matrix metalloproteinase-1 in the placenta. |
Table IV
Immunocytochemical distribution of
matrix metalloproteinase-1 in the placenta.
| Group | n | Matrix
metalloproteinase-1 | Positive rate
(%) |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Normal | 30 | 1 | 7 | 10 | 12 | 96.7 |
| Gestational
hypertension | 18 | 4 | 6 | 5 | 3 | 77.8a |
| Mild
preeclampsia | 9 | 3 | 3 | 2 | 1 | 66.7b |
| Severe
preeclampsia | 16 | 13 | 2 | 1 | 0 | 23.1c |
 | Table VImmunocytochemical distribution of
matrix metalloproteinase-1 in the decidua. |
Table V
Immunocytochemical distribution of
matrix metalloproteinase-1 in the decidua.
| Group | n | Matrix
metalloproteinase-1 | Positive rate
(%) |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Normal | 30 | 2 | 6 | 9 | 9 | 93.3 |
| Gestational
hypertension | 18 | 4 | 6 | 4 | 4 | 77.8a |
| Mild
preeclampsia | 9 | 4 | 2 | 2 | 1 | 55.6b |
| Severe
preeclampsia | 16 | 14 | 1 | 1 | 0 | 12.5c |
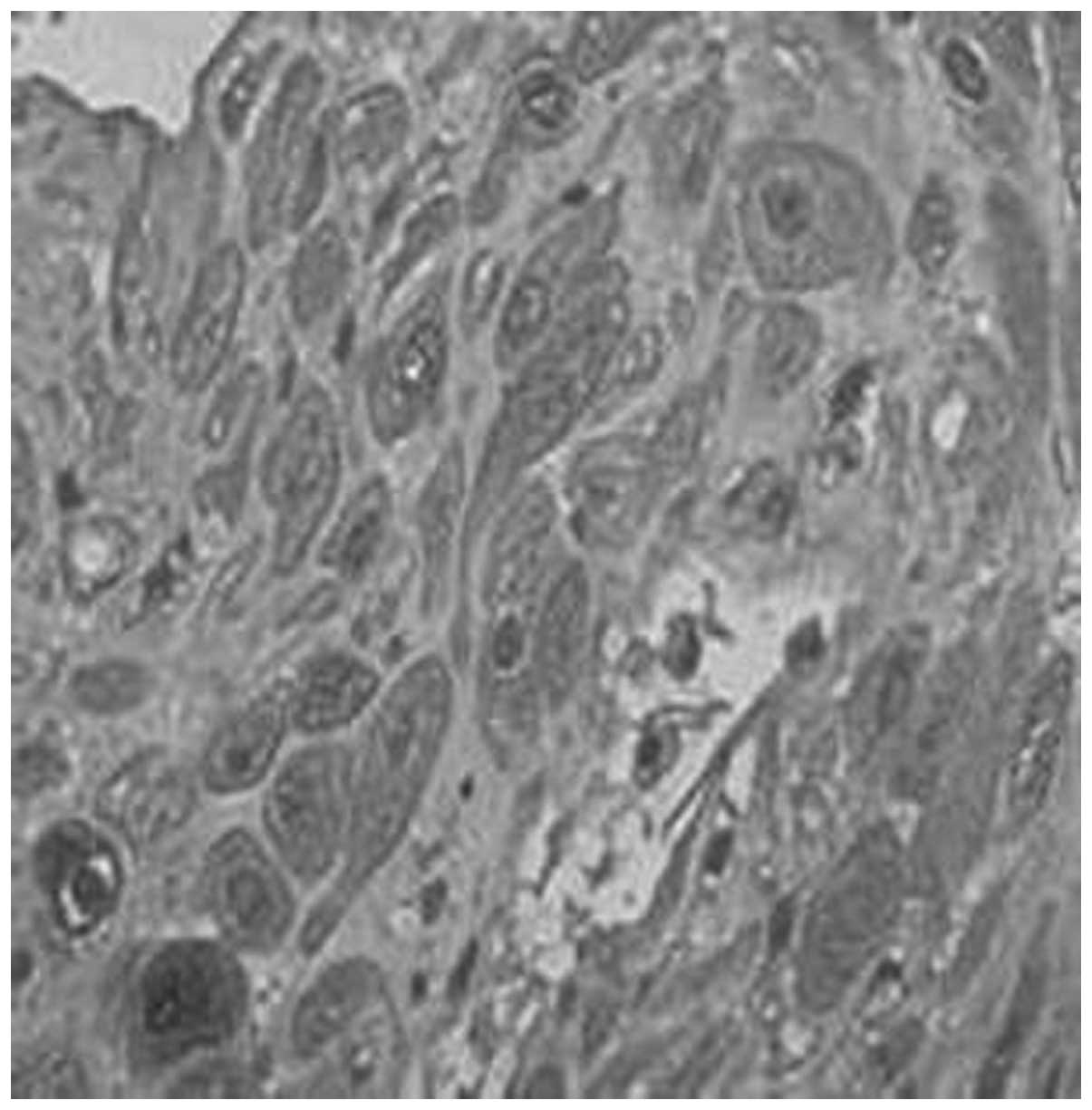
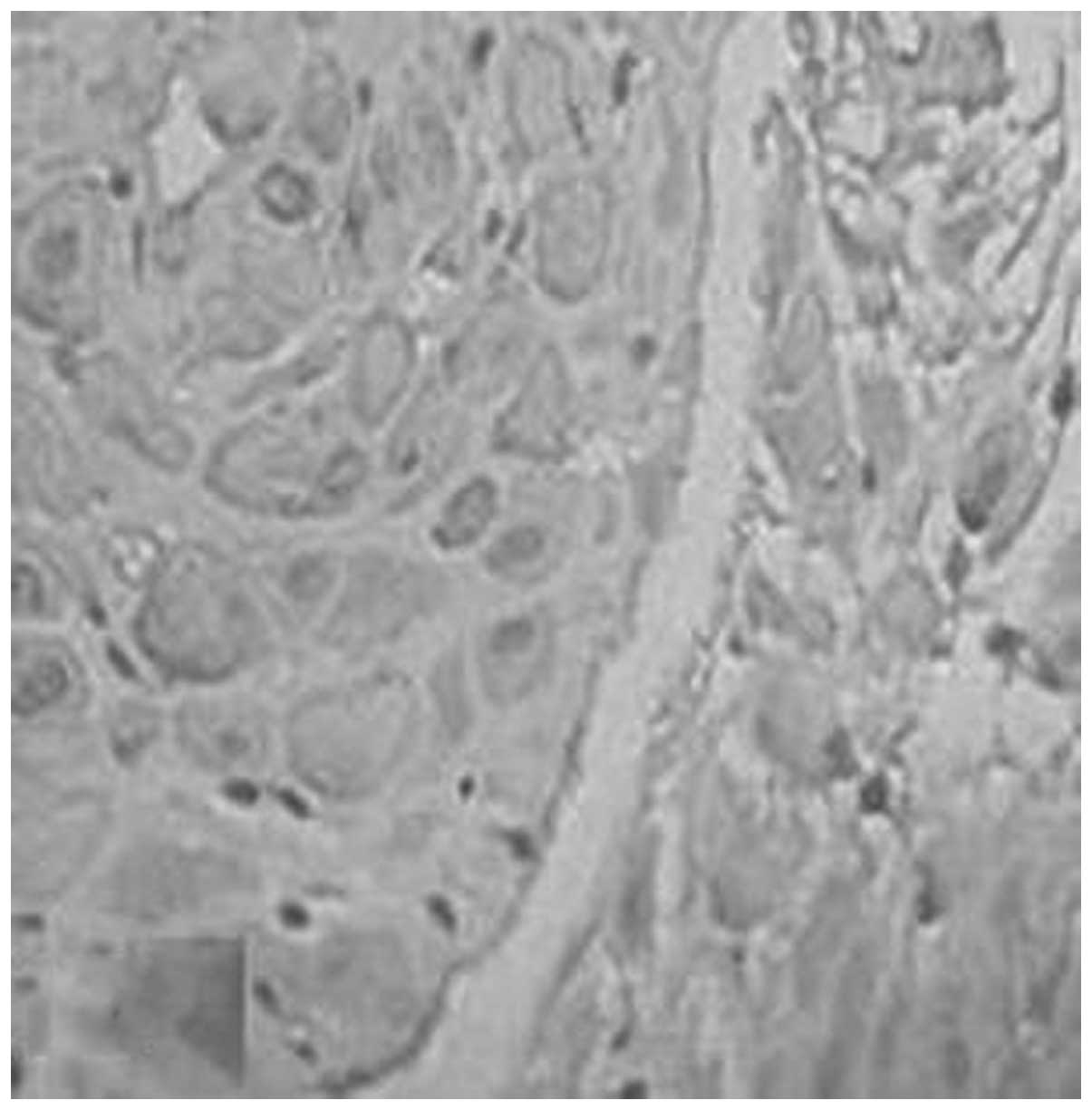
However, the positive rates of expression of TIMP-1
in the placenta of the normal, gestational hypertension, mild
preeclampsia and severe preeclampsia groups were 56.7, 61.1, 66.7
and 75.0%, respectively (Figs. 9
and 10, Table VI). No significant differences
were identified between each two groups (P>0.05). The positive
rates of expression of TIMP-1 in the decidua of the four groups
were 60.0, 61.1, 66.7 and 68.8%, respectively (Figs. 11 and 12, Table
VII). No significant differences were identified between each
two groups (P>0.05).
 | Table VIImmunocytochemical distribution of
tissue inhibitor of metalloproteinase-1 in the placenta. |
Table VI
Immunocytochemical distribution of
tissue inhibitor of metalloproteinase-1 in the placenta.
| Group | n | Tissue inhibitor of
metalloproteinase-1 | Positive rate
(%) |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Normal | 30 | 13 | 8 | 6 | 3 | 56.7 |
| Gestational
hypertension | 18 | 7 | 3 | 4 | 4 |
61.1a |
| Mild
preeclampsia | 9 | 3 | 2 | 2 | 2 |
66.7b |
| Severe
preeclampsia | 16 | 4 | 3 | 3 | 6 |
75.0c |
 | Table VIIImmunocytochemical distribution of
tissue inhibitor of metalloproteinase-1 in the decidua. |
Table VII
Immunocytochemical distribution of
tissue inhibitor of metalloproteinase-1 in the decidua.
| Group | n | Tissue inhibitor of
metalloproteinase-1 | Positive rate
(%) |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Normal | 30 | 12 | 10 | 6 | 2 | 60.0 |
| Gestational
hypertension | 18 | 7 | 4 | 4 | 3 |
61.1a |
| Mild
preeclampsia | 9 | 3 | 3 | 2 | 1 |
66.7b |
| Severe
preeclampsia | 16 | 5 | 4 | 3 | 3 |
68.8c |
Correlation
The levels of MMP-1 in the hypertensive disorders in
the pregnancy and control groups exhibited positive correlations
with the MMP-1 levels in the placenta (r=0.921, P<0.05), and
also in the decidua (r=0.885, P<0.05). The levels of TIMP-1 in
the hypertensive disorders in pregnancy and control groups
exhibited positive correlations with the MMP-1 levels in the
placenta (r=0.891, P<0.05) and the decidua (r=0.914,
P<0.05).
Discussion
In this study, investigation of protein expression
at the maternal-fetal interface revealed that MMP-1 was decreased
in the umbilical serum, placenta and decidua of the patients with
preeclampsia compared with the controls. The proportions of MMP-1
to TIMP-1 in the umbilical serum, placenta and decidua were also
all decreased. MMPs are a family of proteolytic enzymes that
degrade various components of the ECM. MMP-1 is an important
member, which particularly degrades interstitial collagen (12) and is abundant in tissues of the
placenta and decidua. The invasive capacity of trophoblasts has
been associated with their secretion of MMP-1 (13). TIMPs are specific endogenous
inhibitors that bind MMPs in a 1:1 stoichiometry.
The present study revealed that MMP-1 and TIMP-1
were mainly expressed in the cytotrophoblasts and
syncytiotrophoblasts of the placenta and decidua (Figs. 1–12). This was consistent with the
aforementioned studies. In the process of embryo implantation and
placentation, trophoblast invasion demands that they secrete
hydrolysis enzymes effectively and degrade the major components of
the ECM, including collagen, glycoproteins and proteoglycans. MMPs
are effective hydrolyzing enzymes that are secreted by
trophoblasts, and their expression is accurately regulated in time
and space. In this process, trophoblasts invade the spiral arteries
of the uterus and replace vascular muscle elastic membrane with
fibrin, resulting in hemangiectasis, decreased vascular resistance
and significantly increased blood flow. These physiological changes
are termed vascular remodeling (14). From the present study, it appeared
that MMP-1 secretion and subsequent ECM degradation occurred in the
direction of invasion.
MMP-1 expression has also been shown to be crucial
for the migratory capacity of mesenchymal stem cells (8). The present study has provided
evidence suggesting that impaired trophoblast invasion in
hypertensive disorders in pregnancy is associated with reduced
MMP-1 levels in trophoblasts and decidual cells. There are at least
three potential pathogenetic mechanisms by which MMP1 promotes
trophoblast invasion (15).
Firstly, MMP-1 could hydrolyze the basement membrane, interstitial
decidua and vascular cavity surface, rendering these clear of
physical barriers for trophoblast invasion. Secondly, MMP-1 may be
associated with the apoptosis of decidual cells. Thirdly, MMP1
could also play a biological role through other proteins.
The results of the present study demonstrated that
the expression levels of MMP-1 in the umbilical cord blood,
placenta and decidua of patients with hypertension disorder in
pregnancy were clearly lower than those in patients with normal
pregnancy (P<0.05). With the aggravation of illness, MMP-1
expression reduced more markedly and the positive rates of MMP-1
and TIMP-1 dropped. It is hypothesized that in hypertensive
disorders in pregnant patients, the trophoblasts were dysplastic,
and the invasion ability was lower than that in patients at normal
late pregnancy. The trophoblasts invaded the spiral arteries and
uterine smooth muscle insufficiently (shallow placenta
implantation). Therefore, the spiral arteries could not adapt to
the physiological changes in pregnancy, which caused a reduction of
the blood flow of the placenta, reduction of the oxygen content of
the villi, villous ischemia and anoxia. The conclusion of the
present study is consistent with the findings of Jurajda et
al (16). All these factors
may be associated with hypertension disorder in pregnancy. It is
speculated that MMP-1 and TIMP-1 may be involved in the occurrence
and development of hypertension disorders in pregnancy in every
part of the maternal-fetal interface. However, this study was the
first step in exploring the association between MMP-1 and
preeclampsia. Further investigations at the RNA and DNA molecular
level are required to provide more evidence regarding this
association.
Acknowledgements
The authors would like to acknowledge the support of
the Department of Obstetrics and the Central Laboratory in Taihe
Hospital, Hubei University of Medicine, as well as the guidance of
their teachers.
References
|
1
|
Roberts JM and Cooper DW: Pathogenesis and
genetics of pre-eclampsia. Lancet. 357:53–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Lu S, Liu C, et al: Expressional
and epigenetic alterations of placental matrix metalloproteinase 9
in preeclampsia. Gynecol Endocrinol. 26:96–102. 2010. View Article : Google Scholar
|
|
3
|
Merchant SJ, Narumiya H, Zhang Y, Guilbert
LJ and Davidge ST: The effects of preeclampsia and oxygen
environment on endothelial release of matrix metalloproteinase-2.
Hypertens Pregnancy. 23:47–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galewska Z, Bańkowski E, Romanowicz L and
Jaworski S: Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s
content in the umbilical cord artery. Clin Chim Acta. 335:109–115.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narumiya H, Zhang Y, Fernandez-Patron C,
Guilbert LJ and Davidge ST: Matrix metalloproteinase-2 is elevated
in the plasma of women with preeclampsia. Hypertens Pregnancy.
20:185–194. 2001. View Article : Google Scholar
|
|
6
|
Mahameed S, Goldman S, Gabarin D, Weiss A
and Shalev E: The effect of serum from women with preeclampsia on
JAR (trophoblast-like) cell line. J Soc Gynecol Investig.
12:e45–e50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lian IA, Toft JH, Olsen GD, et al: Matrix
metalloproteinase 1 in pre-eclampsia and fetal growth restriction:
reduced gene expression in decidual tissue and protein expression
in extravillous trophoblasts. Placenta. 31:615–620. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakano M, Hara T, Hayama T, et al:
Membrane-type 1 matrix metalloproteinase is induced in decidual
stroma without direct invasion by trophoblasts. Mol Hum Reprod.
7:271–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurskainen T, Seiki M, Apte SS, et al:
Production of membrane-type matrix metalloproteinase-1 (MT-MMP-1)
in early human placenta. A possible role in placental implantation?
J Histochem Cytochem. 46:221–229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raffetto JD and Khalil RA: Matrix
metalloproteinases and their inhibitors in vascular remodeling and
vascular disease. Biochem Pharmacol. 75:346–359. 2008. View Article : Google Scholar
|
|
12
|
Hulboy DL, Rudolph LA and Matrisian LM:
Matrix metalloproteinases as mediators of reproductive function.
Mol Hum Reprod. 3:27–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Librach CL, Werb Z, Fitzgerald ML, et al:
92-kD type IV collagenase mediates invasion of human
cytotrophoblasts. J Cell Biol. 113:437–449. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mishra B, Kizaki K, Koshi K, et al:
Expression of extracellular matrix metalloproteinase inducer
(EMMPRIN) and its related extracellular matrix degrading enzymes in
the endometrium during estrous cycle and early gestation in cattle.
Reprod Biol Endocrinol. 8:60–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tayebjee MH, Karalis I, Nadar SK, et al:
Circulating matrix metalloproteinase-9 and tissue inhibitors of
metalloproteinases-1 and-2 levels in gestational hypertension. Am J
Hypertens. 18:325–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jurajda M, Kanková K, Muzik J, et al: Lack
of an association of a single nucleotide polymorphism in the
promoter of the matrix metalloproteinase-1 gene in Czech women with
pregnancy-induced hypertension. Gynecol Obstet Invest. 52:124–127.
2001. View Article : Google Scholar : PubMed/NCBI
|