Introduction
Globally, lung cancer has the highest rates of
morbidity and mortality of all malignancies (1). Non-small cell lung cancer (NSCLC)
accounts for 80–85% of lung cancer cases worldwide (2).
Human epidermal growth factor receptor (EGFR)
belongs to the type I receptor family. This family has four cognate
family members, including EGFR (HER1), HER2, HER3 and HER4, which
mediate the following signal transduction pathways:
Ras-Raf-mitogen-activated protein kinase kinase-extracellular
signal-regulated kinase-mitogen-activated protein kinase,
phospholipase C-γ,
phosphatidylinositol-3-kinase/phosphoinositide-dependent kinase 1
and Janus kinase/signal transducers and activators of transcription
(3). These receptors regulate cell
proliferation, differentiation and apoptosis (4). EGFR mutations in patients with
NSCLC occur in the intracellular tyrosine kinase (TK) region above
the first four exons (18 to 21). A total of 30 different types of
mutations have been identified in the TK region (5), the most common occurring in exons 19
and 21, which account for ~85% of all the mutations (6).
Excision repair cross-complementing gene 1
(ERCC1) is the key gene in two DNA repair pathways:
nucleotide excision repair (NER) and chain crosslink repair
(7). Overexpression of
ERCC1 can rapidly repair damaged DNA arrest at the
G2/M phase and cause cells to be resistant to platinum
(8). Ribonucleotide reductase
subunit M1 (RRM1) is involved in DNA synthesis and repair
(9). Results from the
Iressa® Pan-Asia Study (10) clinical trial indicated that, in
Asian populations, patients with EGFR mutations were more
responsive to chemotherapy than patients with wild-type
EGFR. Patients with lung cancer with low expression of
ERCC1 and RRM1 are more responsive to gemcitabine-
and platinum-based chemotherapy, respectively (11,12).
Patients with NSCLC are generally treated with chemotherapy drugs,
including cisplatin and gemcitabine. In the present study, 257
patients with stages I-IV NSCLC from multiple hospitals were
analyzed for the presence of EGFR mutations and expression
levels of ERCC1 and RRM1 mRNA. The data were
statistically analyzed to determine significant correlations
between EGFR mutations and expression of these two
chemotherapy resistance genes in patients with NSCLC. These data
may prove useful in further identifying more effective
individualized treatment plans for patients with EGFR
mutations, particularly for patients with small-molecule
EGFR-tyrosine kinase inhibitor (EGFR-TKI) primary or secondary
resistance.
Materials and methods
Specimens
For the detection of EGFR mutations, as well
as ERCC1 and RRM1 mRNA expression levels, paraffin
tissue specimens were collected from 257 patients from the General
Military Hospital of Beijing PLA (Beijing, China; 103 cases), the
Affiliated Zhongshan Hospital of Dalian University (Dalian, China;
58 cases) and the People’s Hospital of Weifang (Weifang, China; 96
cases). The patients had undergone surgery between 2004 and 2013;
the pathological diagnosis was adenocarcinoma, and patients had not
received preoperative chemotherapy, radiotherapy or biological
immunotherapy. All protocols in the present study were approved by
the Human Clinical and Research Ethics Committees of the General
Military Hospital of Beijing PLA, the Affiliated Zhongshan Hospital
of Dalian University (Dalian, China) and the People’s Hospital of
Weifang (Weifang, China). Written informed consent was obtained
from all of the patients.
Reagents and instruments
The DNA and RNA extraction kits were purchased from
Qiagen (Hilden, Germany). The human EGFR mutation
qualitative detection kit and the tumor-related gene expression
relative quantification detection kit (ERCC1 and
RRM1) were obtained from Amoy Diagnostics Co., Ltd. (Xiamen,
China). The B-500 instrument to measure nucleic acid protein
concentrations was purchased from Shanghai Chong Meng Biotechnology
Co. Ltd. (Shanghai, China) and the ABI 7500 quantitative polymerase
chain reaction (qPCR) instrument was purchased from Applied
Biosystems® (Life Technologies, Foster City, CA,
USA).
qPCR to detect EGFR mutations in NSCLC
tissues
Between four and eight 4-μm-thick paraffin tissue
sections were obtained and dewaxed. The genomic RNA extraction kit
was used to extract RNA from the tissue samples according to the
manufacturer’s instructions. A spectrophotometer was used to
determine the purity and concentration of the extracted RNA, which
was used as a template to synthesize the corresponding DNA. The
human EGFR mutation qualitative detection kit, which
contains 29 different fusion mutant primers and probes to amplify
EGFR exons 18, 19, 20 and 21, was used (ADx-EG09; Amoy
Diagnostics Co., Ltd.); the DNA was amplified in an ABI 7500 qPCR
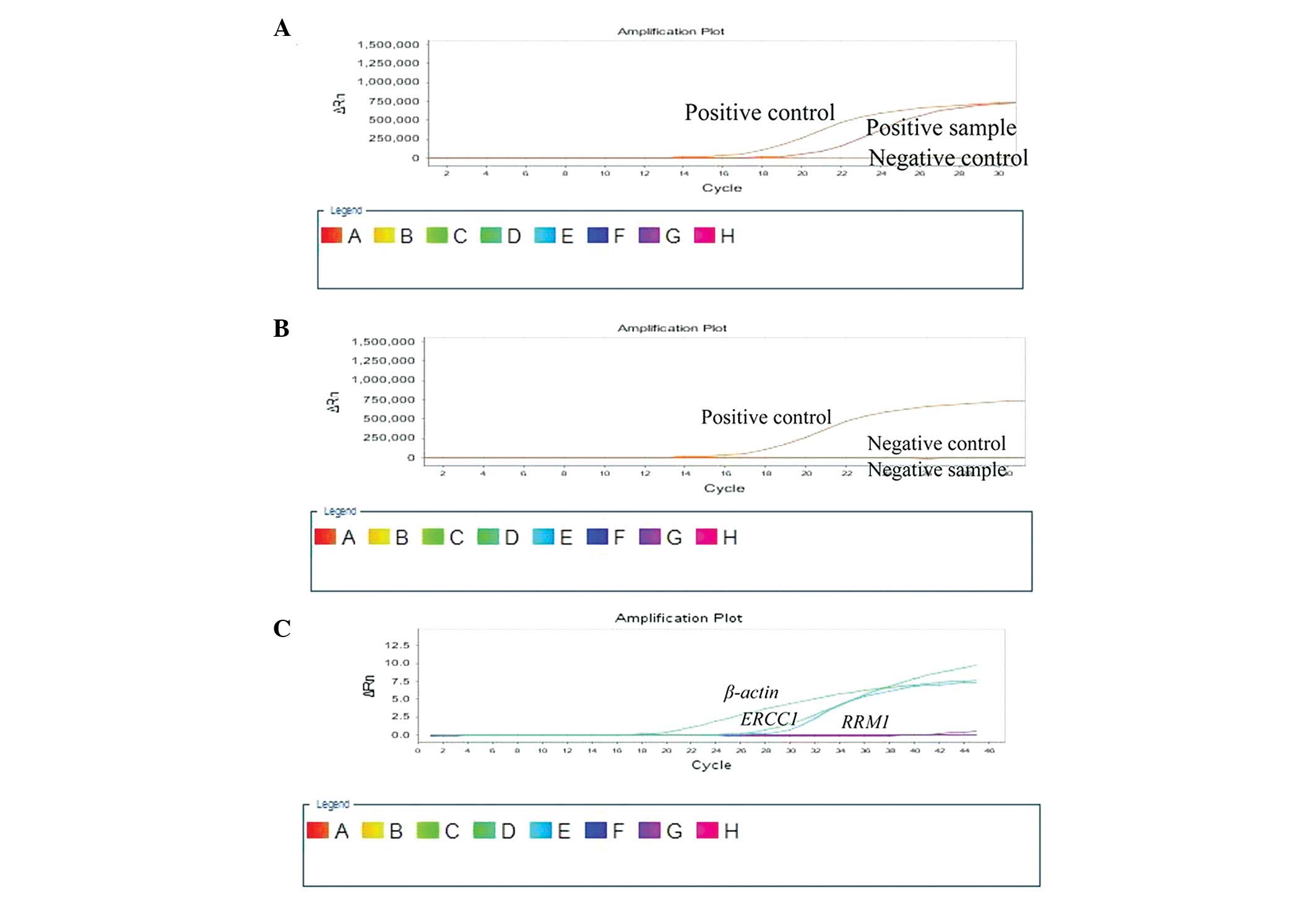
instrument (Fig. 1A and B). The
qPCR cycling conditions were set as follows: 95°C for 5 min,
follwed by 45 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 45 sec.
qPCR to detect the expression of ERCC1
and RRM1 mRNA in NSCLC tissue
RNA was extracted from 4-μm-thick paraffin tissue
sections in accordance with the aforementioned methods. The
tumor-associated gene expression detection kit for ERCC1
(ADx-ER01) and RRM1 (ADx-RR01) (Amoy Diagnostics Co., Ltd.)
was used to determine the mRNA expression level of these genes
using the absolute quantitative method; β-actin was used as the
reference gene. ERCC1 had a standard mean of
4.29×10−3, and RRM1 had a standard mean of
11.37×10−3 (Fig.
1C).
Statistical analysis
The data were analyzed using the statistical
software SPSS (version 19.0; IBM SPSS, Armonk, NY, USA) using the
χ2 and Fisher’s exact test with a test level α=0.05. The
P-value was set to bilateral distribution and P<0.05 was
considered to indicate a statistically significant difference.
Results
Association between EGFR mutations,
expression of ERCC1 and RRM1 mRNA, and patient clinical
characteristics
Of the 257 cases of NSCLC, EGFR mutations
were present in 126 cases (49.03%). The EGFR mutation rate
was higher in non-smoking patients (92/158, 58.23%; P<0.001);
however, the EGFR mutation rate was not associated with the
age, tumor size, lymph-node metastasis or clinical stage of the
patient. High expression of ERCC1 mRNA was observed in
122/257 cases (47.47%). ERCC1 mRNA expression levels were
not associated with the gender, age, smoking status, tumor size,
lymph-node metastasis or clinical stage of the patient. High
expression of RRM1 mRNA was observed in 159/257 cases
(61.87%). RRM1 mRNA expression levels were not associated
with the gender, age, smoking status, tumor size, lymph-node
metastasis or clinical stage of the patient (Table I).
 | Table IAssociation between EGFR
mutations, ERCC1 and RRM1 mRNA expression, and
patient clinical characteristics. |
Table I
Association between EGFR
mutations, ERCC1 and RRM1 mRNA expression, and
patient clinical characteristics.
| EGFR | | ERCC1 | | RRM1 | |
|---|
|
| |
| |
| |
|---|
| Clinical
features | Mutant (n) | Wild-type (n) | P-value | High (n) | Low (n) | P-value | High (n) | Low (n) | P-value |
|---|
| Gender | | | <0.001 | | | 0.898 | | | 0.088 |
| Male | 52 | 86 | | 65 | 73 | | 92 | 46 | |
| Female | 74 | 45 | | 57 | 62 | | 67 | 52 | |
| Age, years | | | 0.287 | | | 0.943 | | | 0.714 |
| ≥59 | 68 | 62 | | 62 | 68 | | 79 | 51 | |
| <59 | 58 | 69 | | 60 | 67 | | 80 | 47 | |
| Smoking
history | | | <0.001 | | | 0.199 | | | 0.129 |
| Yes | 34 | 65 | | 52 | 47 | | 67 | 32 | |
| No | 92 | 66 | | 70 | 88 | | 92 | 66 | |
| Tumor diameter,
cm | | | 0.282 | | | 0.687 | | | 0.292 |
| ≥5 | 56 | 67 | | 60 | 63 | | 72 | 51 | |
| <5 | 70 | 64 | | 62 | 72 | | 87 | 47 | |
| Lymph node
metastasis | | | 0.903 | | | 0.470 | | | 0.188 |
| Yes | 51 | 54 | | 47 | 58 | | 70 | 35 | |
| No | 75 | 77 | | 75 | 77 | | 89 | 63 | |
| Clinical stage | | | 0.203 | | | 0.339 | | | 0.577 |
| I | 50 | 42 | | 40 | 52 | | 59 | 33 | |
| II+III+IV | 76 | 89 | | 82 | 83 | | 100 | 65 | |
Association between EGFR mutations and
expression levels of ERCC1 mRNA
Of the 126 patients with NSCLC with an EGFR
mutation, 79 (62.70%) showed low expression of ERCC1. Of the
131 patients with NSCLC with the wild-type EGFR gene, 56
(42.75%) had low expression of ERCC1 mRNA. These data
indicate that patients with NSCLC with an EGFR mutation had
significantly lower expression of ERCC1 mRNA (P<0.05)
(Table II).
 | Table IIAssociation between EGFR
mutations and the expression level of ERCC1 mRNA. |
Table II
Association between EGFR
mutations and the expression level of ERCC1 mRNA.
| ERCC1 mRNA
expression | |
|---|
|
| |
|---|
| EGFR | High (n) | Low (n) | Total (n) |
|---|
| Mutant | 47 | 79 | 126 |
| Wild-type | 75 | 56 | 131 |
| Total (n) | 122 | 135 | 257 |
Association between EGFR mutations and
the expression level of RRM1 mRNA
Of the 126 patients with NSCLC with an EGFR
gene mutation, 51 (40.48%) had low expression of RRM1 mRNA,
while 47 out of the 131 patients (35.88%) with the wild-type
EGFR gene had low expression of RRM1 mRNA. These data
indicate that there was no correlation between EGFR and
RRM1 mutations in patients with NSCLC (P>0.05) (Table III).
 | Table IIIAssociation between EGFR
mutations and the expression level of RRM1 mRNA. |
Table III
Association between EGFR
mutations and the expression level of RRM1 mRNA.
| RRM1 mRNA
expression | |
|---|
|
| |
|---|
| EGFR | High (n) | Low (n) | Total (n) |
|---|
| Mutant | 75 | 51 | 126 |
| Wild-type | 84 | 47 | 131 |
| Total (n) | 159 | 98 | 257 |
Association between the expression levels
of ERCC1 and RRM1 mRNA
Of the 122 patients with NSCLC with high expression
of ERCC1 mRNA, 72 cases (59.02%) had high expression of
RRM1 mRNA. Of the 135 patients with NSCLC with low
expression of ERCC1 mRNA, 48 cases (35.56%) had low
expression of RRM1 mRNA. A total of 120 cases had low or
high expression of both ERCC1 and RRM1 mRNA, and 137
cases had contrasting expression levels of ERCC1 and
RRM1 mRNA. These data indicate that the expression of
ERCC1 and RRM1 mRNA was not significantly correlated
(χ2=0.800, P=0.371) in NSCLC tissue (Table IV).
 | Table IVAssociation between the expression
levels of ERCC1 and RRM1 mRNA. |
Table IV
Association between the expression
levels of ERCC1 and RRM1 mRNA.
| RRM1 mRNA
expression | |
|---|
|
| |
|---|
| ERCC1 mRNA
expression | High (n) | Low (n) | Total (n) |
|---|
| High | 72 | 50 | 122 |
| Low | 87 | 48 | 135 |
| Total (n) | 159 | 98 | 257 |
Discussion
EGFR mutations and the responsiveness of
NSCLC to the molecular targeted drugs gefitinib (trade name,
Iressa) and erlotinib (trade name, Tarceva®) have a
close association (13,14). Small-molecule TKIs have been shown
to have a high efficiency in patients with an exon 19 deletion in
the EGFR gene (15);
however, patients with NSCLC with EGFR mutations in exon 20
are resistant to drug treatment with TKIs (16). Other studies have reported that the
NER complexes prognosis is good but not suitable for receiving
platinum-based chemotherapy (17,18).
The results of the present study showed that the
EGFR mutation rate was 48.03% (126/257) in patients with
stages I-IV NSCLC, which is consistent with previously reported
data (14,19). A higher percentage of patients with
NSCLC (47.47%; 122/257) showed high mRNA expression levels of
ERCC1 compared with data from a previous study (20), while the percentage of patients
with NSCLC with a high RRM1 mRNA expression level (61.87%;
159/257) was consistent with data presented in a previous study
(21). The expression levels of
ERCC1 and RRM1 mRNA were not associated with the
gender, age, smoking status, tumor size, lymph node metastasis,
pathological staging or other clinical characteristics of the
patient.
The current study found that the mutational status
of EGFR was associated with ERCC1 mRNA expression
levels in patients with NSCLC; patients with EGFR mutations
had a significantly lower expression of ERCC1 mRNA
(P<0.05). EGFR mutations did not, however, significantly
correlate with RRM1 expression levels (P>0.05) in
patients with NSCLC. A previous study has shown that EGFR
mutations in patients with NSCLC were correlated with expression
levels of ERCC1 (P<0.001). Furthermore, EGFR
mutations in the adenocarcinoma subgroup were correlated with
ERCC1 expression levels (P=0.001) (22). It has also been shown that NER
enzymes in cells can lead to cell damage, resulting in genomic
instability and an increased mutation rate (23). Cancer cells with low expression of
ERCC1 have a decreased ability to repair DNA damage, an
increase in the number of EGFR mutations and increased
sensitivity to platinum-based chemotherapy, which may be why
patients with NSCLC with EGFR mutations have a higher
response rate to chemotherapy.
The present study also found that, in NSCLC tissues,
mRNA expression levels of ERCC1 and RRM1 were not
correlated (P>0.05), which is inconsistent with data presented
by Reynolds et al (24).
The association between the expression of ERCC1 and
RRM1 mRNA remains controversial and warrants further
research. At present, numerous studies have confirmed that therapy
can be selected based on patient expression levels of ERCC1
and RRM1, and this can be extended to patients with NSCLC
(25,26).
In conclusion, the current study has demonstrated
that patients with EGFR mutations tend to have lower
expression levels of ERCC1 mRNA. We hypothesize that
patients with EGFR mutations may be more responsive to
cisplatin-based chemotherapy, although the molecular mechanisms
require further study. No difference in the expression levels of
RRM1 mRNA was observed between patients with NSCLC with
EGFR mutations and those without mutations. In the present
study, we have determined the first-line chemotherapy for tumors
involving platinum and gemcitabine drug resistance genes and
EGFR mutations but not the microtubule drug resistance gene
TUBB3 or the thymidylate synthase resistance gene
TYMS. Future studies, therefore, are likely to focus on
EGFR, TUBB3 and TYMS mutations to better
identify effective individualized treatment plans, particularly
individualized treatment plans for EGFR-TKI (e.g. imatinib) primary
or secondary resistance observed in certain patients.
Acknowledgements
This study was supported by the Wu Jieping Medical
Foundation Clinical Research Special Project Fund
(320.6750.1360).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sher T, Dy GK and Adjei AA: Small cell
lung cancer. Mayo Clin Proc. 83:355–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao C, Lu S, Sowa A, et al: Priming with
EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer
cells to respond to chemotherapeutical drugs. Cancer Lett.
266:249–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS: Review of epidermal growth
factor receptor biology. Int J Radiat Oncol Biol Phys. 59(2 Suppl):
21–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan SK, Gullick WJ and Hill ME: Mutations
of the epidermal growth factor receptor in non-small cell lung
cancer - search and destroy. Eur J Cancer. 42:17–23. 2006.
View Article : Google Scholar
|
|
7
|
Simon GR, Ismail-Khan R and Bepler G:
Nuclear excision repair-based personalized therapy for non-small
cell lung cancer: from hypothesis to reality. Int J Biochem Cell
Biol. 39:1318–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosell R, Lord RV, Taron M and Reguart N:
DNA repair and cisplatin resistance in non-small-cell lung cancer.
Lung Cancer. 38:217–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su C, Zhou S, Zhang L, et al: ERCC1, RRM1
and BRCA1 mRNA expression levels and clinical outcome of advanced
non-small cell lung cancer. Med Oncol. 28:1411–1417. 2011.
View Article : Google Scholar
|
|
10
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bepler G, Kusmartseva I, Sharma S, et al:
RRM1 modulated in vitro and in vivo efficacy of gemcitabine and
platinum in non-small-cell lung cancer. J Clin Oncol. 24:4731–4737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riely GJ, Pao W, Pham D, et al: Clinical
course of patients with non-small cell lung cancer and epidermal
growth factor receptor exon 19 and exon 21 mutations treated with
gefitinib or erlotinib. Clin Cancer Res. 12:839–844. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi S, Boggon TJ, Dayaram T, et al:
EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ceppi P, Volante M, Novello S, et al:
ERCC1 and RRM1 gene expressions but not EGFR are predictive of
shorter survival in advanced non-small-cell lung cancer treated
with cisplatin and gemcitabine. Ann Oncol. 17:1818–1825. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cobo M, Isla D, Massuti B, et al:
Customizing cisplatin based on quantitative excision repair
cross-complementing 1 mRNA expression: a phase III trial in
non-small-cell lung cancer. J Clin Oncol. 25:2747–2754. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pao W, Miller V, Zakowski M, et al: EGF
receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar
|
|
20
|
Deng QH, Qiu Y, Mo MC, et al: EGFR gene
copy number, ERCC1 and BRCA1 protein expression and their
relationship in non-small cell lung cancer. Zhonghua Zhong Liu Za
Zhi. 33:508–512. 2011.(In Chinese). PubMed/NCBI
|
|
21
|
Lin XY, Chen Y and Chen ZZ: Expression of
ERCC1 and RRM1 in non-small cell lung cancer (NSCLC) and clinical
prognosis. Fujian Yike Daxue Xuebao. 45:10–14. 2011.(In
Chinese).
|
|
22
|
Gandara DR, Grimminger P, Mack PC, et al:
Association of epidermal growth factor receptor activating
mutations with low ERCC1 gene expression in non-small cell lung
cancer. J Thorac Oncol. 5:1933–1938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsudomi T, Morita S, Yatabe Y, et al:
West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): an
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar
|
|
24
|
Reynolds C, Obasaju C, Schell MJ, et al:
Randomized phase III trial of gemcitabine-based chemotherapy with
in situ RRM1 and ERCC1 protein levels for response prediction in
non-small-cell lung cancer. J Clin Oncol. 27:5808–5815. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simon GR, Schell MJ, Begum M, et al:
Preliminary indication of survival benefit from ERCC1 and
RRM1-tailored chemotherapy in patients with advanced nonsmall cell
lung cancer: evidence from an individual patient analysis. Cancer.
118:2525–2531. 2012. View Article : Google Scholar :
|
|
26
|
Hu YY, Zhang DB, Dong YG, et al: The
protein expression and the significance of RRM1 and ERCC1 in
non-small cell lung cancer. Shandong Yi Yao. 52:1–3. 2012.(In
Chinese).
|