Introduction
Numerous teenagers in Korea are under severe social
and psychological stresses, particularly due to the extremely
competitive system of college admission. Various types of mental
conditions, including lack of social skills, violent tendencies,
attempted suicide, internet game addiction, smartphone dependence
syndrome, smoking, alcohol and drug abuse, and abnormal sexual
attitudes and behaviors are emerging as serious social problems.
Recently, teenage pregnancy has become an increasing problem
(Christian Union Newspaper & Kookmin Ilbo, Korea, 03/17/2010).
Pregnancy is considered normal for married couples; however, being
an unwed mother at a young age is considered shameful in Korea. In
many cases, rather than visit a doctor, young women try to purchase
drugs that will induce abortion through internet sites due to their
ease of access (KBS, Korea, 12/20/2013). However, such drugs are
generally understood to be hazardous compounds due to the severe
reproductive damage they cause (i.e., due to their teratogenicity).
Therefore, the use of these drugs without a doctor’s prescription
is an unsafe practice.
In this study, we have examined several drugs,
including isotretinoin, misoprostol, methotrexate, mifepristone and
levonorgestrel, that are known to be abortion-inducing compounds.
Isotretinoin, an orally active retinoic acid derivative, has been
used for the treatment of severe refractory nodulocystic acne for
more than 30 years (1,2). Misoprostol, a synthetic prostaglandin
E1 methyl ester analog, has potent antisecretory and cytoprotective
effects in the treatment of gastric and duodenal ulcers (3,4).
Methotrexate has long been used for the treatment of cancers
(5). Mifepristone is a
progesterone receptor antagonist used as an abortifacient in the
early stage of pregnancy and has occasionally been used in the
treatment of refractory Cushing’s syndrome (6,7).
However, mifepristone is not approved for use in Korea.
Levonorgestrel is a second-generation synthetic progestogen used as
an active ingredient in certain hormonal contraceptives (8,9). All
of these drugs are classified as ‘category X’. Drugs in this
category have a high risk of causing permanent damage to the fetus
and should not be used during pregnancy. This study was designed to
compare the potential toxicity associated with the oral
administration of abortion-related compounds for a short time
period using ICR mice.
Materials and methods
Animal maintenance
Sixty-five female ICR mice (aged 6 weeks) were
purchased from Nara Biotech (Seoul, Korea) and were used following
a 1-week quarantine and acclimatization period. Four animals per
cage were housed in a room maintained at a temperature of 23±3°C
with a relative humidity of 50±10%. The animals were provided tap
water and commercial rodent chow (NIH-31 Open Formula Auto, Ziegler
Bros Inc., Gardners, PA, USA) ad libitum. The procedures
used in the animal experiment were approved by the Institutional
Animal Care and Use Committee, Konkuk University (Seoul,
Korea).
Chemicals
Isotretinoin, misoprostol, methotrexate,
mifepristone and levonorgestrel were purchased from Sigma-Aldrich
(St. Louis, MO, USA; Table I). The
test drugs were dissolved/suspended in 0.75% Tween-80 solution
(Sigma-Aldrich), and dosing solutions were prepared daily prior to
treatment. The application volume of the drugs was 10 ml/kg body
weight and was calculated based on the most recently recorded body
weight of each individual animal. The test mixture was administered
daily by gavage to female mice for 3 days. Control mice received an
equivalent volume of 0.75% Tween-80 solution alone. The oral
administration method was selected for this study since these drugs
are administered orally in a clinical setting.
 | Table IDrug information. |
Experimental groups and dose
selection
Healthy female mice were randomly assigned to
sixteen experimental groups, comprising one control group and
fifteen drug treatment groups (4–5 mice per group). Each drug was
administered at three doses: low, medium and high. Isotretinoin was
administered once daily at doses of 1, 10 and 100 μg/kg;
misoprostol was administered at 6.7, 67 and 670 μg/kg; methotrexate
was administered at 83, 830 and 8,300 μg/kg; mifepristone was
administered at 3.3, 33 and 330 μg/kg; and levonorgestrel was
administered at 25, 250 and 2,500 μg/kg. The approximate clinical
dose in humans was selected as the low dose, the medium dose was
ten times the low dose, and the high dose was ten times the medium
dose.
Clinical observation and mortality
The rats were observed daily for clinical signs as
well as physiological and behavioral changes throughout the period
of dosing. Toxic manifestations and mortality were also monitored
once daily. The body weight of each mouse was measured at the
beginning of drug exposure.
Hematology
The animals were fasted overnight prior to necropsy
and blood collection. Blood samples were drawn from the posterior
vena cava using a syringe with a 26-gauge needle under ether
anesthesia. Blood samples were collected into complete blood count
bottles containing EDTA-2 K (Green Cross Medical Industry, Seoul,
Korea) and analyzed using an automatic hematology analyzer (Abaxis
VetScan® HM2 Hematology System, Union City, CA, USA).
The following parameters were determined: red blood cell count,
hemoglobin concentration, hematocrit, mean corpuscular volume, mean
corpuscular hemoglobin, mean corpuscular hemoglobin concentration,
platelet count, total white blood cell (WBC) count, and
differential WBC count: lymphocytes, monocytes, granulocytes,
percentage of lymphocytes, percentage of monocytes and percentage
of granulocytes.
Serum biochemistry
Blood samples were centrifuged at 3,000 rpm for 10
min within 1 h of collection. The sera were stored at −80°C prior
to analysis. The following serum biochemistry parameters were
evaluated using an autoanalyzer (Cobas c111 System, Roche
Diagnostics Ltd, Rotkreuz, Switzerland): aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase,
blood urea nitrogen, creatinine, creatine kinase, lactate
dehydrogenase, lipase, glucose, total cholesterol, total bilirubin,
total protein, albumin, calcium, inorganic phosphorus and uric
acid.
Gross findings
All surviving animals were anesthetized with ether
to collect blood samples at the end of the experiment. The mice
were sacrificed by exsanguination from the abdominal aorta.
Complete gross postmortem examinations were performed on all
terminated animals.
Statistical analysis
All results are presented as the mean value ±
standard deviation (SD). Within-group comparisons were performed
using analysis of variance. Significant differences between the
control and experimental groups were assessed by Student’s t-test.
Gross necropsy findings are represented as frequencies. P<0.05,
P<0.01 and P<0.001 were considered to indicate different
levels of statistical significance.
Results
One animal in the high-dose methotrexate (8,300
μg/kg) group was found dead on day 2. The animal did not
demonstrate any notable clinical signs prior to death; however,
there was a high incidence of decreased activity among the other
animals in the high-dose methotrexate group. No abnormal changes in
general behavior or other physiological activities were observed in
the groups receiving the other drugs.
All animals that survived until necropsy were
subjected to hematological examination. As shown in Table II, most of the animals treated
with isotretinoin, misoprostol, methotrexate, mifepristone and
levonorgestrel demonstrated a significant decrease (P<0.001) in
granulocytes count and differentiation percentage compared with the
control group. Conversely, lymphocyte differentiation was
significantly increased by all drugs with the exception of
methotrexate. Misoprostol at a dose of 670 μg/kg/day significantly
decreased the total WBC count, and the high dose of mifepristone
(330 μg/kg/day) significantly increased the hematocrit level.
Certain hematological parameters were also altered by drug
treatment; however, a dose-related response was not observed.
 | Table IIHematological parameters following 3
days of oral treatment of the drugs in female mice (mean ± SD,
n=3–5 mice). |
Table II
Hematological parameters following 3
days of oral treatment of the drugs in female mice (mean ± SD,
n=3–5 mice).
| | Isotretinoin
(μg/kg/day) | Misoprostol
(μg/kg/day) | Methotrexate
(μg/kg/day) | Mifepristone
(μg/kg/day) | Levonorgestrel
(μg/kg/day) |
|---|
| |
|
|
|
|
|
|---|
| Control | 1 | 10 | 100 | 6.7 | 67 | 670 | 83 | 830 | 8300 | 3.3 | 33 | 330 | 25 | 250 | 2500 |
|---|
| WBC
(K/μgl) | 2.87 | 2.10 | 2.64 | 2.17 | 2.55 | 2.51 | 1.53 | 2.23 | 2.56 | 1.75 | 2.54 | 1.96 | 3.37 | 2.07 | 1.29 | 2.30 |
| ±0.69 | ±1.00 | ±0.55 | ±1.02 | ±0.88 | ±0.79 | ±0.74a | ±0.30 | ±1.34 | ±0.29 | ±0.45 | ±0.42 | ±0.89 | ±0.77 | ±0.50a | ±1.39 |
| LYM
(K/μgl) | 1.60 | 1.72 | 2.08 | 1.82 | 2.02 | 1.62 | 1.14 | 1.69 | 1.91 | 1.18 | 2.05 | 1.52 | 2.91 | 1.71 | 0.92 | 1.75 |
| ±0.55 | ±0.80 | ±0.60 | ±0.84 | ±1.06 | ±0.68 | ±0.45 | ±0.42 | ±1.10 | ±0.12 | ±0.49 | ±0.20 | ±0.76a | ±0.64 | ±0.39 | ±1.08 |
| MON
(K/μgl) | 0.11 | 0.11 | 0.14 | 0.08 | 0.10 | 0.14 | 0.07 | 0.14 | 0.13 | 0.13 | 0.38 | 0.06 | 0.13 | 0.07 | 0.06 | 0.10 |
| ±0.07 | ±0.02 | ±0.07 | ±0.05 | ±0.05 | ±0.05 | ±0.05 | ±0.09 | ±0.04 | ±0.03 | ±0.51 | ±0.04 | ±0.15 | ±0.04 | ±0.02 | ±0.07 |
| GRA
(K/μgl) | 1.17 | 0.28 | 0.43 | 0.27 | 0.43 | 0.76 | 0.33 | 0.40 | 0.53 | 0.44 | 0.36 | 0.37 | 0.33 | 0.29 | 0.32 | 0.46 |
| ±0.48b | ±0.21b | ±0.15b | ±0.16b | ±0.30b | ±0.18a | ±0.34b | ±0.22b | ±0.26b | ±0.19b | ±0.31b | ±0.23b | ±0.32b | ±0.15b | ±0.19b | ±0.28b |
| LY% (%) | 55.38 | 82.43 | 78.03 | 84.25 | 75.43 | 53.03 | 77.40 | 75.05 | 70.45 | 68.30 | 80.95 | 79.00 | 85.57 | 83.03 | 70.68 | 75.03 |
| ±15.65 | ±3.20c | ±9.60a | ±3.35c | ±23.84a | ±30.09 | ±14.35a | ±11.07 | ±14.35 | ±9.40 | ±12.05a | ±10.03a | ±15.62c | ±2.11a | ±11.64 | ±8.16 |
| MO% (%) | 4.08 | 6.53 | 5.45 | 3.05 | 4.35 | 5.33 | 4.50 | 6.90 | 6.10 | 7.30 | 5.33 | 3.08 | 3.77 | 3.40 | 5.08 | 4.03 |
| ±3.35 | ±4.70 | ±3.59 | ±1.62 | ±3.09 | ±1.20 | ±2.68 | ±5.06 | ±4.00 | ±1.31 | ±1.26 | ±1.36 | ±2.86 | ±1.57 | ±2.13 | ±0.74 |
| GR% (%) | 90.58 | 11.05 | 16.50 | 12.68 | 20.25 | 31.60 | 18.13 | 18.05 | 23.43 | 24.40 | 13.73 | 17.93 | 10.63 | 13.57 | 24.25 | 21.00 |
| ±91.27b | ±6.11b | ±6.78b | ±2.86b | ±20.84b | ±9.69c | ±16.79b | ±10.89b | ±10.80b | ±8.11c | ±11.38b | ±9.72b | ±30.10b | ±3.67b | ±10.06b | ±8.80b |
| RBC
(M/μgl) | 8.72 | 7.62 | 8.65 | 8.26 | 8.06 | 9.13 | 9.14 | 8.77 | 7.97 | 8.14 | 9.33 | 9.08 | 9.41 | 8.63 | 7.07 | 9.28 |
| ±0.48 | ±2.72 | ±0.44 | ±1.46 | ±0.52 | ±0.39 | ±0.29 | ±0.50 | ±1.61 | ±0.32 | ±0.79 | ±0.41 | ±1.64 | ±0.47 | ±2.67 | ±0.25 |
| HGB (g/dl) | 15.13 | 12.58 | 14.28 | 13.90 | 13.50 | 15.03 | 15.20 | 14.63 | 12.83 | 13.93 | 16.18 | 15.30 | 16.00 | 14.90 | 12.25 | 15.73 |
| ±0.87 | ±5.25 | ±0.53 | ±2.95 | ±0.90 | ±0.52 | ±0.64 | ±0.80 | ±3.24 | ±0.47 | ±0.95 | ±0.66 | ±2.94 | ±0.36 | ±4.97 | ±0.55 |
| HCT (%) | 41.41 | 35.77 | 40.68 | 38.75 | 38.08 | 41.99 | 42.02 | 40.96 | 37.17 | 37.93 | 43.95 | 41.55 | 44.38 | 40.35 | 33.77 | 43.65 |
| ±1.78 | ±12.90 | ±1.49 | ±7.17 | ±2.24 | ±0.94 | ±1.55 | ±1.62 | ±7.74 | ±1.98 | ±3.54 | ±1.86 | ±7.63a | ±1.55 | ±12.84a | ±0.96 |
| MCV (fl) | 47.50 | 46.75 | 47.25 | 46.75 | 47.25 | 46.00 | 45.75 | 46.50 | 46.50 | 46.67 | 47.00 | 46.00 | 47.00 | 46.67 | 47.50 | 47.00 |
| ±1.29 | ±0.96 | ±0.96 | ±0.96 | ±0.96 | ±1.15 | ±0.50a | ±1.29 | ±0.58 | ±0.58 | ±0.82 | ±0.82 | ±0.97 | ±1.15 | ±0.58 | ±1.00 |
| MCH (pg) | 17.35 | 16.00 | 16.55 | 16.75 | 16.75 | 16.48 | 16.63 | 16.68 | 15.93 | 17.13 | 17.35 | 16.88 | 17.03 | 17.23 | 17.05 | 16.93 |
| ±0.47 | ±1.90a | ±0.25a | ±0.83 | ±0.31 | ±0.21 | ±0.31 | ±0.30 | ±1.07a | ±0.06 | ±0.86 | ±0.19 | ±0.81 | ±0.80 | ±1.05 | ±0.71 |
| MCHC (g/dl) | 36.45 | 34.13 | 35.13 | 35.78 | 35.40 | 35.75 | 36.15 | 35.73 | 34.20 | 36.77 | 36.83 | 36.85 | 36.07 | 36.90 | 35.80 | 36.07 |
| ±0.66 | ±3.76a | ±0.62 | ±1.45 | ±0.50 | ±0.53 | ±0.37 | ±0.60 | ±2.12a | ±0.67 | ±1.11 | ±0.21 | ±1.54 | ±1.15 | ±2.15 | ±1.06 |
| PLT
(K/μgl) | 465.00 | 574.75 | 579.00 | 382.00 | 456.25 | 490.75 | 583.00 | 710.00 | 409.67 | 400.50 | 533.75 | 571.25 | 650.33 | 596.33 | 380.75 | 667.33 |
| ±53.20 | ±128.90 | ±180.30 | ±239.50 | ±174.70 | ±96.60 | ±25.20 | ±230.10 | ±172.30 | ±99.70 | ±82.90 | ±91.50 | ±177.30 | ±67.30 | ±216.60 | ±218.30 |
The results of the blood biochemical tests are shown
in Table III. Compared with the
control group, minor changes were observed in the low-dose
isotretinoin, misoprostol, methotrexate, mifepristone and
levonorgestrel groups. However, various changes related to drug
treatment were observed in the medium- and high-dose groups.
Isotretinoin caused significant increases in alkaline phosphatase
and total bilirubin and decreases in glucose and uric acid at doses
of 10 and 100 μg/kg. Misoprostol caused a significant increase in
alkaline phosphatase and a reduction in uric acid at a dose of 670
μg/kg. Methotrexate caused a significant increase in lipase and a
decrease in uric acid at the high dose of 8,300 μg/kg. Treatment
with mifepristone led to a significant increase in total
cholesterol and calcium as well as a decrease in glucose at doses
of 33 and 330 μg/kg. Levonorgestrel caused a significant increase
in calcium and a decrease in glucose at the high dose of 2,500
μg/kg.
 | Table IIIClinical chemistry parameters
following 3 days of oral treatment of the drugs in female mice
(mean ± SD, n=3–5 mice). |
Table III
Clinical chemistry parameters
following 3 days of oral treatment of the drugs in female mice
(mean ± SD, n=3–5 mice).
| | Isotretinoin
(μg/kg/day) | Misoprostol
(μg/kg/day) | Methotrexate
(μg/kg/day) | Mifepristone
(μg/kg/day) | Levonorgestrel
(μg/kg/day) |
|---|
| |
|
|
|
|
|
|---|
| Control | 1 | 10 | 100 | 6.7 | 67 | 670 | 83 | 830 | 8300 | 3.3 | 33 | 330 | 25 | 250 | 2500 |
|---|
| BUN (μg/Dl) | 24.75 | 29.00 | 25.67 | 18.00 | 30.00 | 19.75 | 13.75 | 20.50 | 20.00 | 22.33 | 23.67 | 26.75 | 24.75 | 20.50 | 29.00 | 26.00 |
| ±10.24 | ±8.72 | ±7.09 | ±4.55 | ±14.00 | ±3.50 | ±2.50 | ±4.20 | ±7.07 | ±5.69 | ±7.23 | ±3.77 | ±6.75 | ±9.19 | ±12.46 | ±4.00 |
| CRSC (μg/Dl) | 0.20 | 0.20 | 0.23 | 0.08 | 0.27 | 0.15 | 0.13 | 0.15 | 0.15 | 0.17 | 0.20 | 0.13 | 0.18 | 0.15 | 0.20 | 0.13 |
| ±0.08 | ±0.08 | ±0.12a | ±0.05 | ±0.12 | ±0.06 | ±0.05 | ±0.06 | ±0.07 | ±0.06 | ±0.00 | ±0.05 | ±0.10 | ±0.07 | ±0.00 | ±0.05 |
| ALT (IU/l) | 37.40 | 32.00 | 78.00 | 51.50 | 90.50 | 57.25 | 36.25 | 61.50 | 58.50 | 30.00 | 40.00 | 38.50 | 40.50 | 38.33 | 42.50 | 38.25 |
| ±4.62 | ±6.38 | ±51.81 | ±32.35 | ±89.35a | ±31.16 | ±13.40 | ±36.74 | ±40.25 | ±3.00 | ±4.32 | ±10.41 | ±15.07 | ±14.50 | ±23.90 | ±9.22 |
| AST (IU/l) | 98.25 | 98.25 | 230.75 | 178.50 | 159.00 | 139.25 | 102.25 | 143.50 | 258.50 | 96.67 | 116.25 | 106.00 | 156.00 | 162.67 | 134.25 | 112.75 |
| ±18.63 | ±10.40 | ±185.91 | ±83.01 | ±79.28 | ±65.61 | ±20.01 | ±83.05 | ±300.88a | ±10.50 | ±14.13 | ±22.05 | ±161.36 | ±105.08 | ±68.81 | ±31.13 |
| ALP (IU/l) | 76.80 | 91.25 | 144.00 | 116.00 | 97.00 | 123.50 | 144.00 | 113.00 | 97.75 | 89.00 | 107.00 | 118.00 | 81.50 | 76.33 | 124.50 | 93.00 |
| ±15.34 | ±11.32 | ±54.36b | ±15.38a | ±6.83 | ±27.81a | ±14.51b | ±17.51 | ±48.94 | ±31.11 | ±26.99 | ±29.86a | 9.19 | 54.88 | 23.81a | 16.55 |
| CK (IU/l) | 101.25 | 92.33 | 208.00 | 272.00 | 176.00 | 178.25 | 87.50 | 69.50 | 100.00 | 432.00 | 420.00 | 136.50 | 508.75 | 541.00 | 477.75 | 202.00 |
| ±87.52 | ±32.59 | ±158.04 | ±202.03 | ±128.31 | ±186.91 | ±36.13 | ±32.30 | ±45.21 | ±611.42 | ±660.05 | ±123.85 | ±819.60 | ±802.81 | ±462.31 | ±162.57 |
| LDH (IU/l) | 536.25 | 705.33 | 609.00 | 536.00 | 391.67 | 539.50 | 384.75 | 369.33 | 666.00 | 465.33 | 609.33 | 369.75 | 448.50 | 486.00 | 452.00 | 462.75 |
| ±310.51 | ±158.86 | ±341.74 | ±175.92 | ±161.13 | ±122.24 | ±105.39 | ±203.64 | ±260.92 | ±131.35 | ±243.94a | ±49.65 | ±161.60 | ±285.67 | ±137.25 | ±89.02 |
| TBIL (μg/Dl) | 0.09 | 0.10 | 0.11 | 0.06 | 0.06 | 0.09 | 0.06 | 0.08 | 0.10 | 0.05 | 0.07 | 0.05 | -nd | 0.05 | 0.05 | 0.04 |
| ±0.02 | ±0.01 | ±0.06 | ±0.02a | ±0.03 | ±0.02 | ±0.01 | ±0.01 | ±0.02 | ±0.00 | ±0.04 | ±0.02 | - | ±0.03a | ±0.02a | ±0.02 |
| Tchol (μg/Dl) | 94.60 | 93.25 | 102.50 | 95.25 | 78.25 | 88.50 | 93.25 | 97.75 | 109.25 | 90.33 | 90.25 | 127.00 | 158.33 | 93.00 | 105.00 | 109.25 |
| ±22.61 | ±14.93 | ±28.62 | ±19.52 | ±7.23 | ±12.23 | ±21.41 | ±10.78 | ±8.92 | ±21.13 | ±8.18 | ±30.08a | ±22.03c | ±29.21 | ±38.22 | ±23.64 |
| LIPA (IU/l) | 26.00 | 23.00 | 23.00 | 24.00 | 43.00 | 23.25 | 22.00 | 28.33 | 28.75 | 36.67 | 32.00 | 24.25 | 29.25 | 32.67 | 27.75 | 23.50 |
| ±7.48 | ±0.00 | ±5.00 | ±3.65 | ±15.12c | ±2.75 | ±2.83 | ±6.66 | ±2.99 | ±8.33a | ±11.34 | ±3.50 | ±6.18 | ±3.21 | ±0.96 | ±4.73 |
| GLU (μg/Dl) | 164.20 | 158.25 | 169.50 | 131.25 | 214.50 | 157.00 | 124.75 | 185.25 | 203.25 | 182.33 | 181.50 | 229.75 | 115.50 | 197.33 | 184.75 | 128.00 |
| ±22.53 | ±30.21 | ±21.05 | ±6.02a | ±32.42 | ±8.12 | ±23.19 | ±48.67 | ±40.73 | ±15.31 | ±21.52 | ±80.16 | ±7.59a | ±11.72 | ±23.95 | ±27.78a |
| TP (g/Dl) | 5.55 | 6.00 | 6.53 | 6.15 | 5.43 | 6.13 | 6.38 | 5.48 | 6.20 | 5.60 | 6.00 | 6.10 | - | 4.20 | 5.95 | 6.03 |
| ±0.47 | ±0.22 | ±2.11 | ±0.19 | ±0.25 | ±0.29 | ±0.28 | ±0.40 | ±0.40 | ±0.85 | ±0.37 | ±0.34 | - | ±3.38 | ±0.44 | ±0.60 |
| ALB (g/Dl) | 3.85 | 3.95 | 3.40 | 3.73 | 3.67 | 3.63 | 3.90 | 3.63 | 3.70 | 3.50 | 3.88 | 3.60 | 3.75 | 4.05 | 3.65 | 3.97 |
| ±0.57 | ±0.13 | ±0.72 | ±0.10 | ±0.21 | ±0.13 | ±0.16 | ±0.62 | ±0.28 | ±0.52 | ±0.43 | ±0.20 | ±0.26 | ±0.35 | ±0.17 | 0.21 |
| Ca (μg/Dl) | 9.75 | 11.00 | 11.85 | 11.15 | 11.38 | 11.05 | 11.18 | 11.73 | 11.28 | 11.30 | 10.95 | 10.98 | 14.07 | 11.60 | 11.45 | 12.63 |
| ±3.00 | ±0.00 | ±2.22 | ±0.37 | ±0.82 | ±0.25 | ±0.42 | ±0.57 | ±0.91 | ±1.27 | ±1.04 | ±0.15 | ±3.02c | ±0.70 | ±0.66 | ±1.47c |
| P (μg/Dl) | 8.93 | 8.93 | 9.63 | 7.90 | 10.93 | 8.95 | 7.80 | 10.20 | 9.43 | 8.05 | 10.30 | 7.60 | 14.80 | 7.10 | 9.33 | 9.37 |
| ±1.45 | ±1.11 | ±1.35 | ±0.65 | ±0.67 | ±0.89 | ±0.91 | ±1.28 | ±2.23 | ±0.35 | ±0.68 | ±0.24 | ±0.00 | ±6.09 | ±1.17 | ±2.63 |
| UA (μg/Dl) | 2.80 | 2.10 | 1.43 | 1.70 | 1.80 | 2.15 | 1.73 | 1.73 | 2.15 | 1.37 | 2.08 | 1.85 | 1.98 | 2.33 | 1.73 | 2.05 |
| ±0.53 | ±0.00 | ±0.42a | ±0.18a | ±0.52 | ±0.74 | ±0.21a | ±0.49 | ±1.18 | ±0.32a | ±1.16 | ±0.47 | ±0.86 | ±1.10 | ±0.22a | ±0.77 |
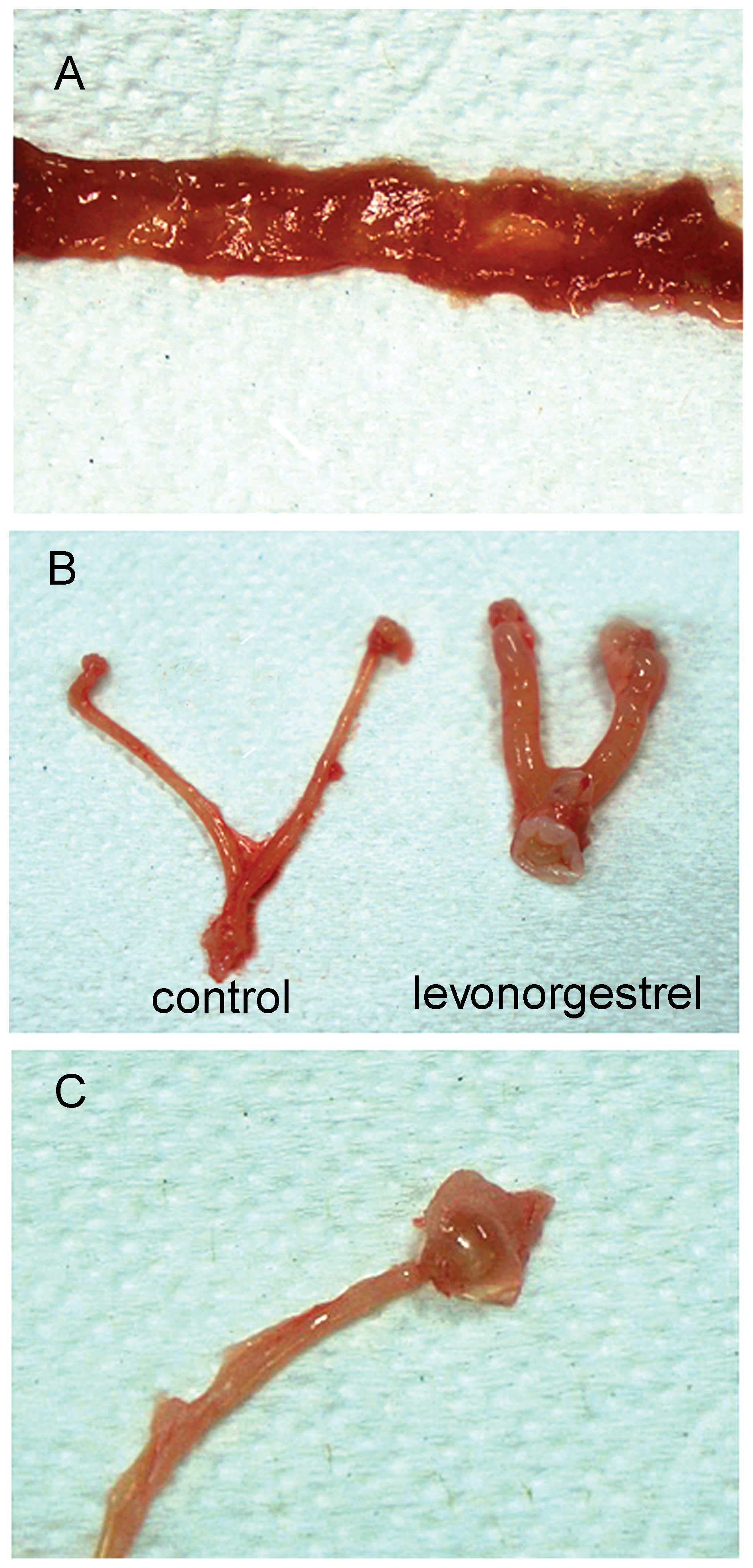
At the scheduled necropsy, treatment-related gross
findings were examined and compared with those of control animals.
The single dead animal observed in the high-dose methotrexate
(8,300 μg/kg) group exhibited severe gastrointestinal and lung
bleeding. As shown in Table IV
and Fig. 1, gross changes
including intestinal redness, uterine enlargement and ovarian
dropsy were detected in the drug treatment groups. Intestinal
redness was frequently observed in animals treated with
methotrexate at higher doses. Uterine enlargement and ovarian
dropsy were repeatedly observed in the mifepristone and
levonorgestrel groups.
 | Table IVGross necropsy findings following 3
days of oral treatment of the drugs in female mice. |
Table IV
Gross necropsy findings following 3
days of oral treatment of the drugs in female mice.
| | Isotretinoin
(mg/kg/day) | Misoprostol
(μg/kg/day) | Methotrexate
(μg/kg/day) | Mifepristone
(mg/kg/day) | Levonorgestrel
(μg/kg/day) |
|---|
| |
|
|
|
|
|
|---|
| Finding | Control | 1 | 10 | 100 | 6.7 | 67 | 670 | 83 | 830 | 8300 | 3.3 | 33 | 330 | 25 | 250 | 2500 |
|---|
| Number/group | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Uterus
enlargement | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 1 |
| Ovarian dropsy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 |
| Intestine
congestion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 1 |
Discussion
The present study was conducted to assess the oral
toxicity of pregnancy category X drugs, namely isotretinoin,
misoprostol, methotrexate, mifepristone and levonorgestrel, in ICR
mice. The results of the study revealed that daily oral
administration of the drugs for 3 days caused certain changes in
the hematological and serum biochemical parameters as well as the
gross parameters of organs (based on necropsy findings).
No abnormal clinical signs were observed in any of
the animals with the exception of those in the high-dose
methotrexate (8,300 μg/kg) group, which demonstrated decreased
spontaneous activity. Additionally, there was one dead animal in
this group. Methotrexate is a potent antiproliferative and
immunosuppressive agent that is widely used against a broad
spectrum of cancer-related diseases and arthritis. However, the
drug has demonstrated significant toxicity in a number of organs,
including the liver, lung, intestine, bone, heart and blood as well
as the reproductive and nervous system (10). The LD50 of methotrexate is known to
be approximately 50–200 μg/kg in mice.
The hematological system is one of the most
sensitive targets for toxic chemicals and is a significant index of
physiological and pathological status in humans and animals. In our
study, significant changes in the WBC count were observed in the
hematological analysis following treatment with category X drugs.
All drugs demonstrated similar dose-response correlations, and
marked reductions in the numbers of granulocytes and granulocyte
differentiation were observed, as well as an increase in the
lymphocyte differentiation. These findings indicate that the drugs
exhibit hematological toxicity related to WBCs, which are the cells
of the immune system that are involved in defending the body
against infectious disease and foreign materials. It was reported
that isotretinoin treatment induces oxidative toxicity in the blood
of patients (11), and several
studies have indicated that methotrexate exhibits blood toxicity.
Murakami et al (12)
observed a decreased number of blood cells in rats that were
administered low-dose methotrexate. Kose et al (13) also reported the severe
hematological toxicity of methotrexate. Mifepristone causes severe
blood loss due to vaginal bleeding (14–16).
Significant reductions in the mean platelet count have been
observed due to long-term treatment with levonorgestrel (17).
Based on the serum biochemical analysis, higher
doses of the drugs were observed to cause various changes in
biochemical parameters including uric acid, glucose, alkaline
phosphatase, total bilirubin, lipase, total cholesterol and
calcium. Alkaline phosphatase was significantly increased by
treatment with isotretinoin and misoprostol, and calcium was
increased by mifepristone and levonorgestrel. Total bilirubin,
lipase and total cholesterol were also considerably elevated in
serum following treatment with isotretinoin, methotrexate and
mifepristone. However, the serum glucose level was significantly
decreased following treatment with isotretinoin, mifepristone and
levonorgestrel. Uric acid was also decreased following treatment
with isotretinoin, misoprostol and methotrexate. This result may
indicate that the drugs have side effects including hypoglycemic
and hypouricemic activity. It has been documented that these drugs
exert various effects on serum biochemical parameters. For example,
it has been reported that isotretinoin significantly increases the
serum levels of aspartate aminotransferase, total cholesterol and
triglycerides (18). Another study
indicated that oral isotretinoin therapy inhibits bone turnover and
calcium homeostasis (19).
Elevation of the alkaline phosphatase level in serum was observed
in misoprostol-treated rats (20).
Ettinger (21) reported a marked
increase in uric acid caused by methotrexate treatment. The level
of glutamic-oxaloacetic transaminase in serum was also altered
following treatment with high-dose methotrexate (22). It was also reported that
mifepristone induced severe hypokalemia in cancer patients
(23). Other studies have
indicated that mifepristone and levonorgestrel are closely
associated with calcium and bone metabolism (24,25).
At necropsy, intestinal redness was observed in
animals receiving high-dose methotrexate. Indeed,
methotrexate-induced intestinal damage in mice has been well
documented in earlier studies (26–30).
Uterus enlargement and ovary dropsy were frequently detected in the
groups receiving mifepristone and levonorgestrel. Although it is
not known whether these findings are normal biological variations,
Tamura et al demonstrated ovarian toxicity in female rats
following repeated doses of mifepristone (31).
In conclusion, 3-day repeated oral administration of
pregnancy category X drugs to mice resulted in notable changes in
WBCs, including a marked reduction of granulocytes and an increase
of lymphocytes. Based on the serum analysis, the drugs also caused
changes in various biochemical parameters. Therefore, the present
study suggests that these drugs may induce blood toxicity in mice
despite the short-term exposure. Thus, it is essential to protect
mothers from illegally abusing the drugs for the purpose of early
pregnancy termination.
Acknowledgements
This study was supported by Konkuk University in
2014.
References
|
1
|
Rademaker M: Isotretinoin: dose, duration
and relapse. What does 30 years of usage tell us? Australas J
Dermatol. 54:157–162. 2013. View Article : Google Scholar
|
|
2
|
Ward A, Brogden RN, Heel RC, Speight TM
and Avery GS: Isotretinoin. A review of its pharmacological
properties and therapeutic efficacy in acne and other skin
disorders. Drugs. 28:6–37. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watkinson G and Akbar FA: Misoprostol in
peptic ulcer disease. Prostaglandins. 33:78–92. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watkinson G, Hopkins A and Akbar FA: The
therapeutic efficacy of misoprostol in peptic ulcer disease.
Postgrad Med J. 64(Suppl 1): 60–77. 1988.PubMed/NCBI
|
|
5
|
Bleyer WA: The clinical pharmacology of
methotrexate: new applications of an old drug. Cancer. 41:36–51.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonelli RM: Mifepristone (RU 486).
Diskussionsforum Med Ethik. XXXVIII–XLIII. 1992.(In German).
|
|
7
|
Grimes DA: Mifepristone (RU 486) for
induced abortion. Womens Health Issues. 3:171–175. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldzieher JW: Advances in oral
contraception. An international review of levonorgestrel and
ethinyl estradiol. J Reprod Med. 28(Suppl 1): 53–56.
1983.PubMed/NCBI
|
|
9
|
Fotherby K: Levonorgestrel. Clinical
pharmacokinetics. Clin Pharmacokinet. 28:203–215. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodman TA and Polisson RP: Methotrexate:
adverse reactions and major toxicities. Rheum Dis Clin North Am.
20:513–528. 1994.PubMed/NCBI
|
|
11
|
Erturan İ, Naziroǧlu M and Akkaya VB:
Isotretinoin treatment induces oxidative toxicity in blood of
patients with acne vulgaris: a clinical pilot study. Cell Biochem
Funct. 30:552–557. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami Y, Sakauchi N, Ogasawara H,
Yamashita N, Masuda T and Tauchi K: A one-month repeated oral dose
toxicity study of methotrexate in unilaterally nephrectomized rats.
J Toxicol Sci. 23(Suppl 5): 681–699. 1998. View Article : Google Scholar
|
|
13
|
Kose F, Abali H, Sezer A, Mertsoylu H,
Disel U and Ozyilkan O: Little dose, huge toxicity: profound
hematological toxicity of intrathecal methotrexate. Leuk Lymphoma.
50:282–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang OS, Xu J, Cheng L, Lee SW and Ho PC:
The effect of contraceptive pills on the measured blood loss in
medical termination of pregnancy by mifepristone and misoprostol: a
randomized placebo controlled trial. Hum Reprod. 17:99–102. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harper C, Winikoff B, Ellertson C and
Coyaji K: Blood loss with mifepristone - misoprostol abortion:
measures from a trial in China, Cuba and India. Int J Gynaecol
Obstet. 63:39–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prasad RN, Choolani M, Roy A and Ratnam
SS: Blood loss in termination of early pregnancy with mifepristone
and gemeprost. Aust N Z J Obstet Gynaecol. 35:329–331. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aisien AO and Enosolease ME: Haemostatic
function in Norplant (levonorgestrel) users: a 3-year prospective
experience in Benin-City, Nigeria. Niger Postgrad Med J.
16:126–131. 2009.PubMed/NCBI
|
|
18
|
Karadag AS, Ertugrul DT, Tutal E and Akin
KO: Short-term isotretinoin treatment decreases insulin-like growth
factor-1 and insulin-like growth factor binding protein-3 levels:
does isotretinoin affect growth hormone physiology? Br J Dermatol.
162:798–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kindmark A, Rollman O, Mallmin H,
Petrén-Mallmin M, Ljunghall S and Melhus H: Oral isotretinoin
therapy in severe acne induces transient suppression of biochemical
markers of bone turnover and calcium hom eostasis. Acta Derm
Venereol. 78:266–269. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milcan A, Colak M and Eskandari G:
Misoprostol enhances early fracture healing: a preliminary
biochemical study on rats. Bone. 41:611–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ettinger LJ: Pharmacokinetics and
biochemical effects of a fatal intrathecal methotrexate overdose.
Cancer. 50:444–450. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perez C, Sutow WW, Wang YM and Herson J:
Evaluation of overall toxicity of high-dosage methotrexate
regimens. Med Pediatr Oncol. 6:219–228. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castinetti F, Fassnacht M, Johanssen S,
Terzolo M, Bouchard P, Chanson P, Do Cao C, Morange I, Picó A,
Ouzounian S, et al: Merits and pitfalls of mifepristone in
Cushing’s syndrome. Eur J Endocrinol. 160:1003–1010. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang J, Lu J and Wu R: Mifepristone
following conservative surgery in the treatment of endometriosis.
Zhonghua Fu Chan Ke Za Zhi. 36:717–720. 2001.(In Chinese).
|
|
25
|
Purdie DW, Hay AW and Everett M: Short
term effects of SHD 386L and levonorgestrel on bone and mineral
metabolism in the postmenopause: a double-blind randomised
placebo-controlled trial. Maturitas. 14:189–199. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen C, Tian L, Zhang M, Sun Q, Zhang X,
Li X, Cao X, Liu Q, Li X and Hao L: Protective effect of amifostine
on high-dose methotrexate-induced small intestinal mucositis in
mice. Dig Dis Sci. 58:3134–3143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Koning BA, Sluis M,
Lindenbergh-Kortleve DJ, Velcich A, Pieters R, Büller HA, Einerhand
AW and Renes IB: Methotrexate-induced mucositis in mucin
2-deficient mice. J Cell Physiol. 210:144–152. 2007. View Article : Google Scholar
|
|
28
|
Nakamaru M, Masubuchi Y, Narimatsu S,
Awazu S and Horie T: Evaluation of damaged small intestine of mouse
following methotrexate administration. Cancer Chemother Pharmacol.
41:98–102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramadan AA, Badr WY and Ali AM: The effect
of methotrexate (MTX) on the small intestine of the mouse. I A
macroscopic study. Folia Morphol (Praha). 36:68–78. 1988.
|
|
30
|
Baskerville A and Batter-Hatton D:
Intestinal lesions induced experimentally by methotrexate. Br J Exp
Pathol. 58:663–669. 1977.PubMed/NCBI
|
|
31
|
Tamura T, Yokoi R, Okuhara Y, Harada C,
Terashima Y, Hayashi M, Nagasawa T, Onozato T, Kobayashi K, Kuroda
J and Kusama H: Collaborative work on evaluation of ovarian
toxicity. 2) Two- or four-week repeated dose studies and fertility
study of mifepristone in female rats. J Toxicol Sci. 34 Suppl
1:SP31–SP42. 2009. View Article : Google Scholar : PubMed/NCBI
|