Introduction
With the improvement of perinatal medicine and
neonatal intensive care in recent years, the survival rate of
low-birth weight infants, premature infants and infants with severe
asphyxia has been significantly improved. Consequently, the
incidence of neonatal brain injuries has increased; in particular,
periventricular leukomalacia (PVL) has attracted increasing
attention (1).
It has been proposed that PVL occurs due to
perinatal inflammation and/or hypoxia-ischaemia induced by various
factors during the perinatal period (2). Anatomical pathology has shown that
the periventricular white matter of the watershed areas of the
lateral ventricles exhibits oedema and coagulative necrosis with a
macrophage response to form cysts following hypoxia and ischaemia
of the brain. Areas of cystic necrosis can collapse to form scars
resulting in gliosis. The periventricular white matter is
extensively replaced with numerous cysts due to white matter
deficiency in periventricular areas and the centrum semiovale. The
ependyma between the softened area of the cyst and the ventricle is
destroyed; the integrity of the cysts with the ventricle is also
disrupted. The ventricle locally or passively expands to form
jagged edges, exhibiting an irregular shape. Commonly observed in
preterm infants and full-term children with suffocation, PVL is a
significant manifestation of infant brain injuries. PVL is the main
cause of infant mortality; patients who survive suffer from nervous
system disorders and mental retardation later in life. The
pathogenesis of this condition has been linked to perinatal
inflammation and/or hypoxia-ischaemia. Ischaemia/reperfusion
results in the formation of reactive oxygen species and reactive
nitrogen species, causing cell death (3).
In addition to early mortality due to the poor
prognosis of PVL, 50% of the sequelae from cerebral palsy (CP) are
related to the manifestation of PVL in survivors (4). Studies have shown that PVL is an
independent risk factor of CP (5,6). CP
is characterised by a group of developmental processes involving
movement and posture; as a result, activity is limited and this
finding is attributed to non-progressive disturbances that occur in
the developing foetal or infant brain (7). CP is a serious disorder in children
and influences the survival and quality of life of infants.
However, at present, no specific treatment is available to
alleviate PVL; hence, the prevention of various risk factors
associated with PVL is necessary.
Therefore, in the present study, the clinical data
of 408 children with CP were retrospectively analysed by examining
images of PVL in the Rehabilitation Centre of the Children’s
Hospital of Zhengzhou (Zhengzhou, China). The correlations of the
PVL degree, gestational age, low birth weight, asphyxia and other
high-risk factors, as well as the formation, type and comorbidities
of CP were analysed to provide an objective basis for the
prevention of illness, clinical analysis and prognosis of children
with CP. This study provides guidelines for perinatal prevention,
the early clinical diagnosis of PVL, early intervention and the
reduction of sequelae.
Subjects and methods
Subjects
Among the 2,700 children with CP that were subjected
to rehabilitation therapy in the Rehabilitation Centre from August
2008 to August 2012, the following cases were observed: 43.57%
spastic diplegia; 42.53% spastic quadriplegia; 5.39% spastic
hemiplegia; 3.73% muscle hypotonia; and 2.49% involuntary
movements. Approximately 89% of these patients exhibited
abnormalities in the computed tomography (CT) or magnetic resonance
imaging (MRI) of the head relevant to the ages of the children
examined in the hospital. The common results of the CT or MRI were
listed as follows: widened subarachnoid cavity; enlarged
ventricles; mild brain atrophy; PVL; and low density. The CT/MRI
results of the heads of 2,700 patients indicated that 408 children
(15.11%) suffered from PVL including 301 males and 107 females. The
ages of these 408 patients ranged between five months and 10 years
with an average of 18.67±17.38 months. Among the 408 cases
included, 330 were aged below two years, 57 were aged between two
and four years, 13 were aged between four and six years, and 8 were
aged more than six years (the symptoms of these children were mild
and inappropriate for surgery). The gestational age was between 24
and 41.6 weeks, with an average of 34.67±4.62 weeks. The birth
weight ranged between 900 and 5,500 g, with an average of
2,480.47±750.60 g, including 226 cases with a birth weight of
<2,500 g. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of the Children’s Hospital of Zhengzhou. Written informed consent
was obtained from the parents or guardians of the participants.
PVL diagnosis and analysis
MRI has been demonstrated to be a valuable tool for
monitoring the development and pathology of the preterm brain. In
this retrospective study, some of the CT results were obtained from
other hospitals prior to the children being admitted to the
Children’s Hospital of Zhengzhou. In addition, the MRI examination
exhibits high noise, and some children with CP exhibited sleep
disorders. Although these children were anaesthetised, the MRI
examination could not be completed. As a result, some children were
subjected to CT scanning.
A 64 row spiral head CT scanner (GE speed-A1-type
spiral CT; GE Healthcare, Pewaukee, WI, USA) was used to study the
children. The scan parameters were as follows: tube voltage, 120
kVp; tube current, 100 mA; thickness, 5–10 mm; pitch, l;
reconstruction matrix, 512×512; and scan mode, helical scan.
Certain children were subjected to brain MRI
(Magnetom Aera 1.5T MRI; Siemens AG, Munich, Germany). The MRI
examination was conducted approximately at the time of the
patients’ hospitalisation. All of the MRI procedures used a 1.5T
system. A conventional MRI sequence protocol was applied in all of
the participants: spin-echo (SE) T1-weighted (T1-W) sagittal; SE
dp/T2-W; fluid attenuation inversion recovery (FLAIR) axial; and
turbo spin echo (TSE) T2-W and inversion-recovery (IR) coronal
scans.
According to the imaging diagnostic standards of the
brain and spinal cord injury, the congenital brain and skull
deformities and PVL in children and infants were categorised into
three degrees based on the damage in the periventricular region
(8,9): i) for a mild degree, the white matter
reduction was limited to the peritrigonal, anterior or posterior
white matter; the central circle of the white matter appeared
normal or slightly reduced, and the lateral ventricles were of
normal size; the cerebral sulci were widened adjacent to the
trigonal area of the lateral ventricle, and the lateral area
exhibited a small visible area of softening, with long T1 and long
T2 signals; ii) for a moderate degree, the white matter of the
centrum semiovale was reduced, in addition to the previously
described abnormalities; the optic radiation was involved;
periventricular white matter was decreased significantly; deep
lateral fissures were widened extensively with enlarged occipital
horns located close to the lateral fissure with grey matter in
close proximity to the walls of the lateral ventricles; the
ependyma was separated from gray matter only by a small amount of
white matter; the trigonal area was abnormally enlarged; and a
small amount of malacia of the periventricular white matter was
present. iii) for a severe degree, the cerebral white matter had
almost disappeared; periventricular white matter was replaced with
cystic areas and connected to the lateral ventricle; the lateral
ventricle appeared irregular; the centre measured only half of the
total residual amount of the white matter, grey matter and parietal
grey matter, in which the lateral fissures were deep and close to
the lateral ventricle. The group of patients with MRI was very
small and not used for indexing purposes.
The reading, analysis and diagnosis of the imaging
were conducted by two chief physicians of radiology and two chief
physicians of children’s neurology and were blinded to other
patient data. All radiology scans were read independently by two
radiologists who were unaware of the clinical information. If these
two radiologists disagreed in their analysis of the lesions, the
films were sent to a third radiologist who was unaware of the
opinions of the first two radiologists. Details regarding the
procedure and efforts to minimise observer variability are
presented in a previous study (10).
Clinical diagnosis and evaluation
The diagnosis and evaluation involved the following.
i) High risk factors of perinatal brain injury were obtained from
information contained in a questionnaire that was completed by the
mother of each child and provided to the doctors during the medical
consultation. ii) CP was diagnosed, typed and classified according
to the classification presented at an international symposium of
cerebral palsy in 2006 (11). In
this study, the following categories were considered for type
according to clinical symptoms: spastic (diplegia, spastic
quadriplegia, spastic hemiplegia, spastic monoplegia); flaccid;
dyskinesia; ataxic; and mixed. iii) The children’s intelligence was
evaluated according to the Gesell Developmental Scale and Wechsler
Intelligence Scale. Children with a developmental quotient (DQ) of
<75 or intelligence quotient (IQ) of <70 were diagnosed with
mental retardation. iv) The brainstem auditory-evoked potential
(MEB-9100 type-evoked potential) was used to assess the hearing
threshold. Children with a hearing threshold >35 dB were
diagnosed with hearing impairment. v) The sign-significant
relations (S-S) (12) evaluation
method developed by the China Rehabilitation Research Centre was
used to diagnose significant language development delay. Children
with a language assessment, language comprehension or expression
delay of half a year or more compared with normal children of the
same age were diagnosed with delayed language development. vi)
Children with seizures were included as epilepsy cases and treated
with antiepileptic drugs to control their symptoms. Epilepsy was
diagnosed with reference to the diagnosis and classification
criteria developed by the International League Against Epilepsy
(13,14), and a 24-h dynamic abnormal EEG
monitoring report was obtained for all children with epilepsy. vii)
The children’s eyes were examined by the professional chief
ophthalmologists in the hospital. Visually impaired patients were
diagnosed to determine whether or not visual abnormalities,
strabismus, amblyopia, eye involuntary movement, cortical blindness
or nystagmus were present. The visual evoked potential and fundus
examination were performed for non-responsive children with the
application of strong light stimulation to the eyes.
Statistical analysis
Data were stored on a computerised database and
analysed using SPSS version 13.0 for Windows (Statistical Package
for Scientific Studies for Windows; SPSS Inc., Chicago, IL, USA).
The conditional logistic regression analysis method was used, in
which PVL was considered as an independent variable, and the other
factors were considered as dependent variables. Measurements are
presented as the mean ± standard deviation and Wald tests were used
in the statistical analysis. P<0.05 was considered to indicate a
statistically significant result. Logistic regression analysis was
performed using relevant factors.
Results
High risk factors
Among the 408 patients, 251 were born prematurely,
154 were born at full-term, and three were adopted and had an
unknown gestational age. In addition, 80 cases had a gestational
age of ≤28 weeks, with the lowest gestational age being 24 weeks. A
total of 154 cases had a gestational age of ≥37 weeks. A total of
35 cases (8.58%) were born with a weight of ≤1,500 g, with the
lowest weight being 900 g, and 181 cases (44.36%) were born with a
weight of ≥2,500 g (Table I).
 | Table IGestational age and birth weight in
children with PVL. |
Table I
Gestational age and birth weight in
children with PVL.
| Variable | No. of cases (%) |
|---|
| Gestational age
(weeks) |
| 24–28 | 80 (19.61) |
| 28–30 | 12 (2.94) |
| 30–32 | 58 (14.22) |
| 32–34 | 29 (7.11) |
| 34–37 | 72 (17.65) |
| 37–41.6 | 154 (37.75) |
| Unknowna | 3 (0.74) |
| Birth weight (g) |
| 900–1500 | 35 (8.58) |
| 1500–2000 | 111 (27.21) |
| 2000–2500 | 80 (19.61) |
| 2500–5500 | 181 (44.36) |
| Unknown | 1 (0.24) |
A total of 390 cases (95.59%; Table II) had one or more high risk
factors, including 29 cases with simple prenatal risk factors, 94
cases with simple neonatal period risk factors and 263 cases with
concomitant prenatal and neonatal risk factors. The three adopted
children were born with an unknown history of birth or the mother’s
pregnancy. Furthermore, 62 cases (15.20%) were twins or multiple
births, including 7 cases with stillbirth, 9 cases with mortality
after birth and 12 cases with CP. Among the 408 children, 181 cases
were born with a birth weight of ≥2,500 g, considered a normal
birth weight (44.36%), and these included 77 cases with neonatal
asphyxia as one of the PVL risk factors (42.54%); only 12 cases had
no risk factors (Tables III and
IV).
 | Table IIHigh risk factors in children with
PVL. |
Table II
High risk factors in children with
PVL.
| Factors | No. of cases (%) |
|---|
| Prenatal |
| Abnormal amniotic
fluid | 54 (13.24) |
| Umbilical cord
abnormalities | 16 (3.92) |
| Placental
abnormalities | 29 (7.11) |
| Intrauterine
distress | 27 (6.62) |
| Threatened
abortion | 57 (13.97) |
| Pregnancy-induced
hypertension | 26 (6.37) |
| Pre-eclampsia | 1 (0.25) |
| Infection during
pregnancy | 86 (21.08) |
| TORCH
infection | 175 (42.89) |
| Habitual
abortion | 7 (1.72) |
| Gestational
diabetes | 9 (2.21) |
| Intrapartum and
postnatal |
| Caesarean
section | 208 (50.98) |
| Twins/multiple
births | 62 (15.20) |
| Neonatal
asphyxia | 181 (44.36) |
| Neonatal
encephalopathy | 123 (30.15) |
| Neonatal
sepsis | 5 (1.23) |
| Neonatal
respiratory distress | 11 (2.69) |
| Neonatal
seizures | 18 (4.41) |
| Neonatal
mechanical ventilation | 9 (2.21) |
| Neonatal
intracranial haemorrhage | 37 (9.07) |
| Neonatal
jaundice | 169 (41.42) |
| Neonatal
pneumonia | 25 (6.13) |
 | Table IIILogistic regression analysis of high
risk factors for PVL. |
Table III
Logistic regression analysis of high
risk factors for PVL.
| | | | | | | 95% CI for EXP
(B) |
|---|
| | | | | | |
|
|---|
| Variablesa | B | S.E. | Wald | df. | Sig | Exp (B) | Lower | Upper |
|---|
| Gestational
age | 0.384 | 0.089 | 18.763 | 1 | <0.001 | 1.468 | 1.234 | 1.747 |
| Birth weight | −0.181 | 0.133 | 1.870 | 1 | 0.172 | 0.834 | 0.643 | 1.082 |
| Single/multiple
births | 0.843 | 0.282 | 8.927 | 1 | 0.003 | 2.324 | 1.337 | 4.040 |
| Mode of
delivery | 0.893 | 0.168 | 28.255 | 1 | <0.001 | 2.442 | 1.757 | 3.393 |
| Amniotic fluid
abnormalities | 1.459 | 0.355 | 16.927 | 1 | <0.001 | 4.304 | 2.147 | 8.625 |
| Asphyxia | 1.381 | 0.210 | 43.209 | 1 | <0.001 | 3.980 | 2.636 | 6.008 |
| Complications
during pregnancy | 1.613 | 0.205 | 62.078 | 1 | <0.001 | 5.015 | 3.358 | 7.490 |
| Encephalopathy | 0.045 | 0.226 | 0.040 | 1 | 0.841 | 1.046 | 0.671 | 1.631 |
| Intracranial
hemorrhage | 2.338 | 0.412 | 32.199 | 1 | <0.001 | 10.358 | 4.620 | 23.226 |
| Constant | −3.561 | 0.393 | 82.071 | 1 | <0.001 | 0.028 | | |
 | Table IVHigh risk factors in 181 children
with normal birth weight and PVL. |
Table IV
High risk factors in 181 children
with normal birth weight and PVL.
| Factors | Cases (%) |
|---|
| Prenatal |
| Abnormal amniotic
fluid | 30 (16.57) |
| Umbilical cord
abnormalities | 15 (8.29) |
| Placental
abnormalities | 11 (6.08) |
| Intrauterine
distress | 12 (6.63) |
| Threatened
abortion | 22 (12.15) |
| Pregnancy-induced
hypertension | 6 (3.31) |
| Pre-eclampsia | 1 (0.55) |
| Infection during
pregnancy | 49 (27.07) |
| TORCH
infection | 82 (45.30) |
| Neonatal
pneumonia | 11 (6.07) |
| Intrapartum and
postnatal |
| Cesarean
section | 91 (50.27) |
| Twins/multiple
births | 15 (8.28) |
| Neonatal
asphyxia | 77 (42.54) |
| Neonatal
encephalopathy | 52 (28.73) |
| Neonatal
sepsis | 4 (2.21) |
| Neonatal
respiratory distress | 3 (1.66) |
| Neonatal
seizures | 12 (6.63) |
| Neonatal
mechanical ventilation | 4 (2.21) |
| Neonatal
intracranial hemorrhage | 11 (6.08) |
| Neonatal
jaundice | 76 (41.99) |
Logistic regression analysis
The analysis undertaken for gestational age, birth
weight, single/multiple births, mode of delivery, amniotic fluid
abnormalities, asphyxia, complications during pregnancy,
encephalopathy and intracranial hemorrhage found a P-value<0.05
that indicated a statistically significant difference. Thus, these
factors were associated with the occurence of PVL.
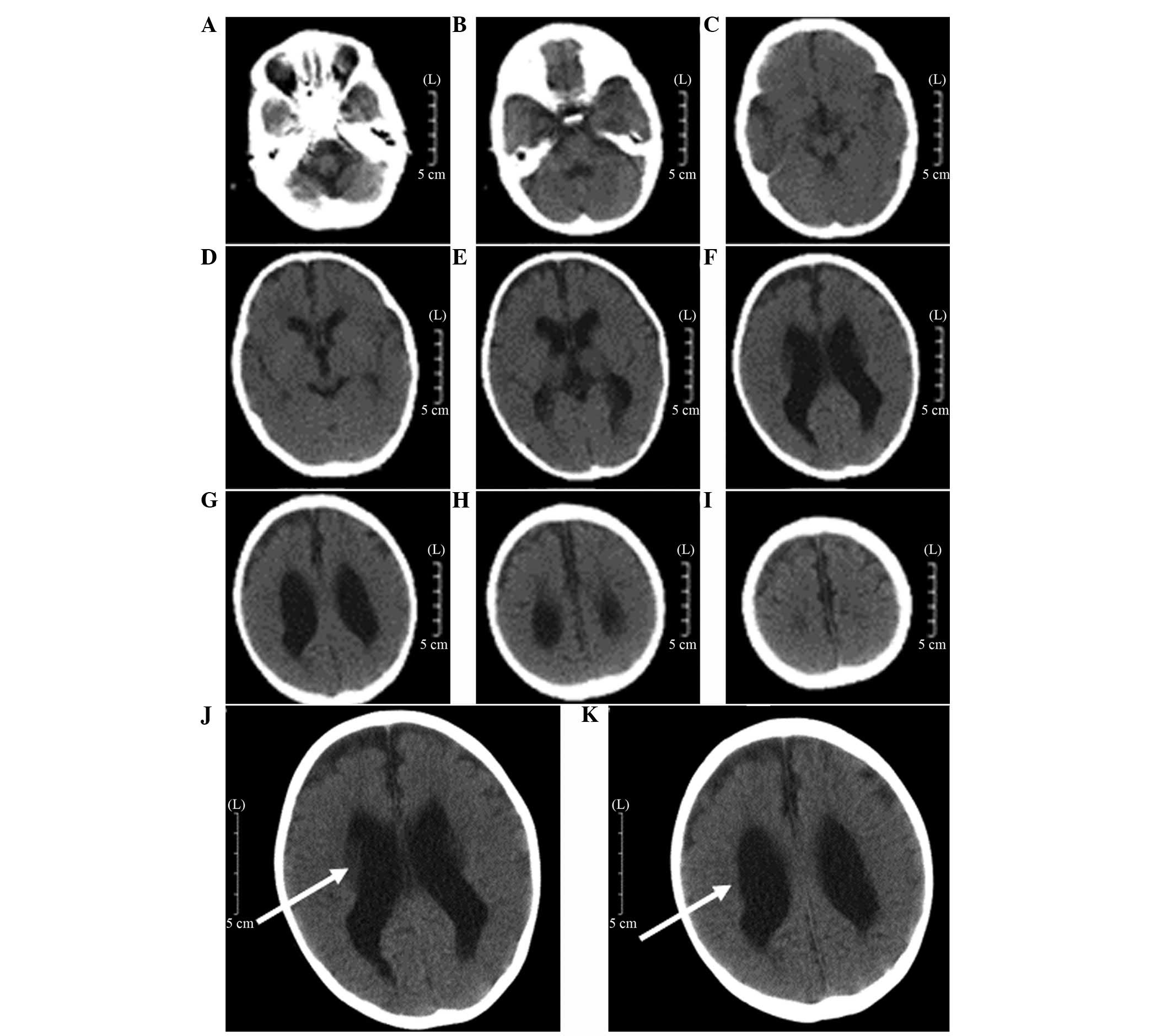
CT findings and results
The characteristics of PVL are generally consistent
with the MRI findings (15,16).
CT examination is not able to detect early damage in the
periventricular white matter until cystic lesions are formed. The
end-stage appearance of the PVL was observed by CT as follows
(15): the lateral ventricle was
enlarged, expanding from the rear of the lateral ventricles to the
full lateral ventricle and the edges of the lateral ventricle were
irregular and uneven. The quantity of the periventricular white
matter was reduced mainly around the trigonal zone of the lateral
ventricle and along the centrum semiovale in severe cases. The CT
scan exhibited a low-density (Fig.
1). Analysis of the results indicated that 172 cases were
classified as mild, 195 cases were moderate and 41 cases were
severe (Table V).
 | Table VComparison of gestational age with
the degree of PVL [n (%)]. |
Table V
Comparison of gestational age with
the degree of PVL [n (%)].
| Degree of PVL |
|---|
|
|
|---|
| Gestational age
(weeks) | Mild | Moderate | Severe |
|---|
| 24–28 | 14 (3.43) | 40 (9.80) | 26 (6.37) |
| 28–30 | 1 (0.25) | 2 (0.49) | 9 (2.21) |
| 30–32 | 16 (3.92) | 39 (9.56) | 3 (0.74) |
| 32–34 | 10 (2.45) | 18 (4.41) | 1 (0.25) |
| 34–37 | 41 (10.05) | 30 (7.35) | 1 (0.25) |
| 37–41.6 | 89 (21.81) | 64 (15.69) | 1 (0.25) |
| Unknowna | 1 (0.25) | 2 (0.49) | 0 |
| Total | 172 | 195 | 41 |
Association between PVL severity and
gestational age, birth weight and type of CP
In this study, among the cases of severe PVL, 63.41%
of the children had a gestational age of 24–28 weeks, 21.95% had a
gestational age of 28–30 weeks and 97.56% with a gestational age of
<37 weeks. Also, among the severe cases of PVL, 63.41% of
children had a birth weight of 900–1,500 g, 26.83% had a birth
weight of 1,500–2,000 g and 97.56% had a birth weight of <2,500
g. Spastic quadriplegia was present in 43.90% of the severe PVL
cases. The results indicate that severe PVL was more common in
infants born prematurely or with a low-birth weight. Hence, a young
gestational age and low birth weight are associated with severe
PVL. This result also indicated a high possibility of developing
spastic quadriplegia in such cases (Tables V and VI).
 | Table VIComparison of birth weight and the
degree of PVL [n(%)]. |
Table VI
Comparison of birth weight and the
degree of PVL [n(%)].
| Degree of PVL |
|---|
|
|
|---|
| Birth weight
(g) | Mild | Moderate | Severe |
|---|
| 900–1500 | 3 (0.74) | 6 (1.47) | 26 (6.37) |
| 1500–2000 | 30 (7.35) | 70 (17.16) | 11 (2.70) |
| 2000–2500 | 36 (8.82) | 41 (10.05) | 3 (0.74) |
| 2500–5500 | 103 (25.25) | 77 (18.87) | 1 (0.25) |
| Unclear | 0 | 1 (0.25) | 0 |
| Total | 172 | 195 | 41 |
The types of CP in the 408 children with PVL
included the following: spastic CP in 390 cases (diplegia in 213
cases, quadriplegia in 114 cases, hemiplegia in 56 cases and
monoplegia in 7 cases); 16 cases with hypotonia and 2 cases with
dyskinetic CP. The incidences of other comorbidities were
distinctly different among the different types of CP. The highest
was hearing impairment in spastic diplegia. The total incidences of
various comorbid disabilities with were higher in quadriplegia than
in the other types (Tables VII
and VIII).
 | Table VIIComparison of cerebral palsy type and
the degree of PVL (no. of cases). |
Table VII
Comparison of cerebral palsy type and
the degree of PVL (no. of cases).
| Degree of PVL |
|---|
|
|
|---|
| Type of cerebral
palsy | Mild | Moderate | Severe |
|---|
| Diplegia | 97 | 103 | 13 |
| Spastic
quadriplegia | 45 | 51 | 18 |
| Spastic
hemiplegia | 21 | 30 | 5 |
| Spastic
monoplegia | 6 | 1 | 0 |
| Hypotonia | 3 | 9 | 4 |
| Dyskinesia | 0 | 1 | 1 |
| Total | 172 | 195 | 41 |
 | Table VIIIComparison of the type of cerebral
palsy and comorbidities in children with PVL (no. of cases). |
Table VIII
Comparison of the type of cerebral
palsy and comorbidities in children with PVL (no. of cases).
| Type of cerebral
palsy | Cases | Visual
impairment | Hearing
impairment | Language
barriers | Mental
retardation | Epilepsy |
|---|
| Diplegia | 213 | 7 | 47 | 8 | 4 | 3 |
| Spastic
quadriplegia | 114 | 10 | 30 | 4 | 10 | 26 |
| Spastic
hemiplegia | 56 | 2 | 4 | 2 | 2 | 1 |
| Spastic
monoplegia | 7 | 1 | 2 | 0 | 0 | 0 |
| Hypotonia | 16 | 1 | 6 | 0 | 1 | 0 |
| Dyskinesia | 2 | 0 | 0 | 2 | 0 | 0 |
Discussion
CP is one of the most common pediatric neuromotor
developmental disabilities with a prevalence rate of 2.37%
(17). With the improvement of the
conditions and technical advancements in perinatal medicine and
neonatal emergency medicine, the survival rates of children born
prematurely and other high-risk children have increased. The
incidence of CP significantly increased by 66% to 100% (2). PVL has been defined as cyst formation
with necrosis of myelinated fibres of the white matter in the
trigonal region, situated at the posterior side and lateral to the
external angles of the lateral ventricles in survivors of the
perinatal asphyxia (18), with an
incidence of 25–75% (19). PVL has
a poor prognosis because of its unclear etiology and pathogenesis.
As a major cause of early mortality, mental retardation and damage
to the nervous system (20), PVL
seriously affects the survival rates and the quality of life of
preterm infants. Therefore, PVL has been the focus of numerous
studies of the perinatal period. For instance, Drougia et al
(4) demonstrated that PVL is the
main cause of CP (particularly in children born prematurely), with
~50% of cases having CP and PVL. Considering that different onset
types and comorbidities for CP affect the degree of disability and
quality of life, the present study retrospectively analysed the
images of 408 children with CP treated in the rehabilitation centre
at a single hospital. The correlation between high risk factors
(degree of PVL, gestational age, low birth weight and asphyxia) and
CP, as well as clinical types and comorbidities, were investigated
to evaluate the cause and clarify the underlying mechanism. The
focus of the study was on perinatal period care, risk and early
prevention, early diagnosis, early treatment and morbidity
reduction.
Recent studies have indicated that PVL is associated
with immature vascular development of the periventricular region,
the imperfect autoregulation of cerebral blood flow and specific
damage to oligodendrocyte precursors (21). Children born prematurely with
immature brain development are very sensitive to hypoxia and
ischaemia, and the periventricular area is the zone with the
highest incidence of hypoxic-ischaemic damage in preterm infants.
Therefore, damage of the periventricular white matter is more
likely to occur in children born prematurely than in those born at
full-term, and the degree is more severe. Perlman et al
(22) observed that PVL mainly
occurs in children born prematurely with a gestational age of
<32 weeks and a birth weight of <1,500 g; the lower the birth
weight, the greater the severity of the PVL. Vollmer et al
(23) also found that the
mortality rate of children born prematurely with a gestational age
of <28 weeks was greater than that of those with a gestational
age of 28–32 weeks after the PVL, and that such children usually
succumb within 2 weeks after birth. Analysis of the 408 children
with PVL in the present study indicates that 251 cases (61.51%)
were born prematurely and 154 cases (37.74%) were born at
full-term. A total of 35 cases (8.58%) had a weight at birth of
≤1,500 g, among which 26 cases had severe PVL, accounting for
63.41% of the total cases of severe PVL. A total of 226 cases
(55.39%) were born with a weight ≤2500 g, of which 40 cases were
severe PVL, accounting for 97.56% of the total cases of severe PVL.
A total of 181 cases (44.36%) were born with a weight ≥2500 g, of
which 1 case was severe PVL (0.24%). A total of 80 cases (19.61%)
were born at a gestational age of ≤28 weeks, of which 26 cases were
severe PVL (63.41% of all cases of severe PVL). A total of 150
cases (36.76%) were born at a gestational age ≤32 weeks, of which
38 cases were severe PVL (92.68%). Furthermore, 251 cases (61.52%)
were born at a gestational age ≤37 weeks, of which 40 cases were
severe PVL (accounting for 97.56% of all severe cases; Tables I, V and VI). The results indicate that the lower
the gestational age and the lower the birth weight, the greater the
severity of the PVL. This finding is roughly in accordance with the
results of the studies previously mentioned. However, the
proportion of the total cases was not very large, which is related
to the objectives of the study, that the survival rate of children
with more severe PVL is low, and that the lower the gestational age
and the birth weight, the higher the incidence of the PVL.
In investigations of the etiology of PVL and the
sequela CP, intrauterine infection has become topic of particular
interest. According to epidemiological and animal studies,
intrauterine infection/inflammatory response has an important role
in the pathogenesis of PVL, as an alternative to hypoxia and
ischaemia (24,25). Intrauterine infection can cause
premature rupture of the membranes and preterm delivery (a high
risk factor of PVL), and also directly causes fetal periventricular
white matter damage (26). The
incidence of neonatal PVL may be as high as 60–70% when
intrauterine infection occurs in mothers, and more than half of the
survivors have long-term neurobehavioral defects (27). A total of 154 children with CP and
PVL in the present study had a normal gestational age, and mostly
had PVL to a mild or moderate extent. Analysis of the high risk
factors showed that infection, intrauterine hypoxia and other high
risk factors were present in children with CP, suggesting that
infection during pregnancy and intrauterine hypoxia are the main
reasons for PVL in full-term infants.
Clinical studies have found that hypoxia and
ischaemia in children and the degrees of asphyxia are closely
associated with brain damage; wherein, the more severe the degree
of the asphyxia caused by hypoxia, the more severe the brain damage
(28). Other high risk factors,
such as the mother suffering from pre-eclampsia, antepartum
haemorrhage, premature rupture of membranes, fetal distress,
intrauterine hypoxia and ischaemia and intrauterine infection
(29), and the newborn baby
suffering from seizures, mechanical ventilation, double fetal
asphyxia, apnea, hypoxemia, hypocapnia and sepsis (27), easily cause changes in cerebral
vascular and cerebral hemodynamics and damage to the autoregulation
cerebral blood flow (15),
resulting in hypoxic-ischaemic conditions in the brain (30), thereby leading to the occurrence of
PVL. The results of the logistic regression analysis (Table III) showed that the high risk
factors associated with PVL included gestational age, hypoxia,
various complications during pregnancy, amniotic fluid
abnormalities, intracranial hemorrhage, mode of delivery and
single/multiple births. The correlation between birth weight and
PVL was low, which was inconsistent with previous studies (31,32).
The reason for this is hypothesised to be that the children in the
present study were survivors of severe PVL, but the survival rate
of very low birth weight infants with severe PVL is low.
Encephalopathy was found to have a low correlation with PVL,
suggesting that the occurrence of PVL is closely associated with
the high risk factors of prenatal pregnancy; encephalopathy may be
a secondary illness in these children. Reducing the birth rate of
babies with a very low birth weight would decrease the occurrence
of severe PVL and improve the neonatal survival rate.
The major clinical manifestations in children with
PVL and CP were changes such as various degrees of movement
disorders and mental retardation. Melhem et al found that
the severity of motor impairment and cognitive impairment was
closely associated with the lateral ventricular volumes, which is
similar to the results of the present study (33). The movement disorders are
considered to be associated with lesioning of the periventricular
white matter affecting the pyramidal tract (34). The pyramidal tract starts from the
cerebral cortex down to the anterior horn motor neurons of the
spinal cord along the lateral ventricles. The order of distribution
of nerve fibers from the pyramidal tract is lower limbs, trunk and
upper extremities from the inside out. Therefore, based on the
severity of PVL, lower limb disorders occur first, with clinical
manifestations including spastic diplegia, and expansion of the
lesions results in spastic quadriplegia (35). In the present study, 213 cases
(52.21%) had spastic diplegia, and 114 cases (27.94%) had spastic
quadriplegia. Spastic hemiplegia, spastic hemoplagia, hypotonia and
dyskinesia accounted for 13.73, 1.72, 3.92 and 0.49% of cases,
respectively. Imaging studies demonstrate the anatomical basis for
the abnormal functioning of the brain (36). The clinical manifestations are
associated with the severity of PVL in children. The neuromotor
development of children with PVL can be predicted based on imaging
findings. In the present study, 18 (43.90%) of the 41 children with
severe PVL had spastic quadriplegia, whereas 13 cases (31.71%) had
diplegia, and these were commonly found in children born
prematurely or with a low birth weight. This finding suggests that
the lower the gestational age and the lower the birth weight, the
more severe the PVL, and the greater the scope of involvement in
the corticospinal tract, the more likelihood the development of
spastic quadriplegia and diplegia. The imaging of one of the
children with dyskinesia showed severe PVL; however, the specific
mechanisms remain unclear, but may be caused by concurrent
pyramidal and extrapyramidal involvement (2).
In addition to movement disorders, the
manifestations of PVL are often associated with epilepsy, visual
and auditory damage, mental retardation and delayed language
development. Optic radiation and auditory tract involvement in the
wide ranging reduction of the periventricular white matter is the
main cause of dysfunctions and damage of vision and hearing in
children with PVL and CP. Epilepsy, hearing impairment and visual
impairment were the three most common disorders. In the present
study, the prevalence rate of vision and hearing impairment was
26.96%, in accordance with the literature. The prevalence of
epilepsy was 7.35% (Table
VIII), lower than the rate reported in the literature (5), which may be related to the fact that
partial frequent seizures in children with CP were not
rehabilitated and were missed. It has been suggested that the
degree of lateral expansion and periventricular reduction of the
white matter, thinning of the corpus callosum and optic radiation
involvement are associated with IQ (37). In the present study, 17 children
had various degrees of mental retardation. It has previously been
shown that intellectual impairment is severe when the degree of
lateral expansion and cortical damage increase with concurrent
reduction of the periventricular white matter, indicating that PVL
is an important clinical cause of mental retardation (38). In the present study, 16 children
had different levels of language retardation. The language centres
of Broca’s area, Wernicke’s area, the supramarginal gyrus and
angular gyrus are all located in the periventricular area. When the
range of the PVL incorporated the speech centre, the children would
be likely to have various degrees of language retardation. The
degree of audio-visual impairment, levels of intelligence, seizures
and degree of language development can affect the therapeutic
effect of CP, as well as the prognosis. Thus, vision and hearing
examinations, intellectual measurement, language evaluation and EEG
examination are recommended to be performed routinely in children
with CP for early diagnosis and intervention, contributing to the
improvement of the rehabilitation effect.
As one of the significant causes of CP in children,
PVL is an ischaemic necrosis of the brain cells commonly found in
infants born prematurely or with a low birth weight. The degree of
PVL was negatively correlated to the gestational age and birth
weight of the children. The degree of the PVL in full-term infants
was associated with infection during pregnancy and intrauterine
hypoxia. Quadriplegia and diplegia were most common among the
subtypes of CP, and hearing impairment was the most common
comorbidity. The type of CP most commonly associated with
comorbidity was quadriplegia.
A retrospective clinical analysis with certain
limitations was conducted in this study. However, this analysis was
useful for evaluating the cause of the disease and understanding
the correlation between the type of CP, comorbidities and the
degree of PVL, and is a reminder that attention to perinatal care
is important to avoid the birth of children with severe CP. The
study suggested that improvement of the prognosis for children born
prematurely or with a low birth weight with severe PVL may be
achieved by observation and early intervention, and should be the
direction of future research. The various pre-natal and post-natal
risk factors affecting children with CP were strictly observed with
the establishment of a collaborative network among the maternity,
paediatrics and rehabilitation services. This was considered to be
key for the prevention, diagnosis and treatment of children with
CP, contributing to enhancement of the quality of the population,
reduced morbidity rates and improved life quality of children with
CP.
References
|
1
|
Xu XY, Shu H, lu XM, et al: The
relationship between MRI manifestations and clinical of the White
matter around children. [J]. Journal of pediatric clinical
practical magazine. 26:1347–1348. 2011.
|
|
2
|
Kinney HC: Human myelination and perinatal
white matter disorders. J Neurol Sci. 228:190–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma BX and Chen JY: The pathogenesis
research status of Ventricle white matter around the softening
disease. Chinese Pediatrics of IntegratedTraditional and Western
Medicine. 3:506–508. 2011.
|
|
4
|
Drougia A, Giapros V, Krallis N, et al:
Incidence and risk factors for cerebral palsy in infants with
perinatal problems: a 15-year review. Early Hum Dev. 83:541–547.
2007. View Article : Google Scholar
|
|
5
|
Deng W, Pleasure J and Pleasure D:
Progress in periventricular leukomalacia. Arch Neurol.
65:1291–1295. 2008.PubMed/NCBI
|
|
6
|
Deguchi K, Oguchi k, Matsuura N, et al:
Periventricular Leukomalacia: relation to gestational age and
axonal injury. Pediatr Neuro. 20:370–374. 1999. View Article : Google Scholar
|
|
7
|
Bax M, Goldstein M, Rosenbaum P, et al:
Proposed definition and classification of cerebral palsy, April
2005. Dev Med Child Neuro. 47:571–576. 2005. View Article : Google Scholar
|
|
8
|
Rosenbaum P, Paneth N, Leviton A, et al: A
report: the definition and classification of cerebral palsy April
2006. Dev Meal Child Neurol Suppl. 109:8–14. 2007.
|
|
9
|
Venkateswaran S and Shevell MI:
Comorbidities and clinical determinants of outcome in children with
spastic quadriplegic cerebral palsy. Dev Med Child Neurol.
50:216–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie S, Guo XM, Cui AG, et al: The study of
MR diffusion tensor imaging on white matter around ventricle of
children. Zhonghua Fangshexue Zazhi. 39:325–327. 2005.(In
Chinese).
|
|
11
|
Kuban KC, Allred EN, O’Shea M, Paneth N,
Pagano M and Leviton A: An algorithm for identifying and
classifying cerebral palsy in young children. J Pediatr.
153:466–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong T: Applied language therapy. Language
retardation of children. Wu HS: People’s Military Medical
Publishing House; Beijing: pp. 140–158. 1995
|
|
13
|
Berg AT, Berkovic SF, Brodie MJ, et al:
Revised terminology and concepts for organization of seizures and
epilepsies: Report of the ILAE Commission on Classification and
Terminology, 2005–2009. Epilepsia. 51:676–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banerjee PN, Filippi D and Allen Hauser W:
The descriptive epidemiology of epilepsy - a review. Epilepsy Res.
85:31–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho HK, Jang SH, Lee E, et al: Diffusion
tensor imaging-demonstrated differences between hemiplegic and
diplegic cerebral palsy with symmetric periventricular
leukomalacia. AJNR Am J Neuroradiol. 34:650–654. 2013. View Article : Google Scholar
|
|
16
|
Kobayashi S, Fujimoto S, Fukuda S, et al:
Periventricular leukomalacia with late-onset circulatory
dysfunction of premature infants: correlation with severity of
magnetic resonance imaging findings and neurological outcomes.
Tohoku J Exp Med. 210:333–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Zhang ZH, Zhu DN, et al: Epidemic
characteristics and current prevention of cerebral palsy in
children of Henan Province. Maternal and Child Health Care of
China. 28:2109–2112. 2013.(In Chinese).
|
|
18
|
Mallard C, Welin AK and Peebles D: White
matter injury following systemic endotoxemia or asphyxia in the
fetal sheep. Neurochemical. 28:215–223. 2003. View Article : Google Scholar
|
|
19
|
Zhu LH and Jiang l: Research progress of
white matter around the premature ventricular softening. Chinese
Journal of Pediatrics. 44:192–196. 2006.
|
|
20
|
Zach T and Brown JC: Periventricular
leukomalacia. eMedicine; 2003, (http://www.emedicine.com/ped/topic1773.htmuri).
|
|
21
|
Kinney HC, Haynes RL, Xu G, Folkerth RD,
Sleeper LA and Volpe JJ: Neuron deficit in the white matter and
subplate in periventricular leukomalacia. Ann Neurol. 71:397–406.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perlman JM, Risser R and Broyles RS:
Bilateral cystic periventricular leukomalacia in the premature
infant: associated risk factors. Pediatrics. 97:822–827.
1996.PubMed/NCBI
|
|
23
|
Vollmer B, Roth S, Baudin J, Stewart AL,
Neville BG and Wyatt JS: Predictors of long-term outcome in very
preterm infants: gestational age versus neonatal cranial
ultrasound. Pediatrics. 112:1108–1114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon BH, Park CW and Chaiworapongsa T:
Intrauterine infection and the development of cerebral palsy. BJOG.
110(Suppl 20): 124–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li DY, Li JH and Mu DZ: Study Progress in
Pathology and Pathogenesis of Neonatal Preventricular White Matter
Injury. Appl Clin Pediatr. 22:943–945. 2007.
|
|
26
|
Sameshima H and Ikenoue T: Developmental
effects on neonatalmortality and subsequent cerebralpalsy in
infants exposed to intrauter-ine infection. Early Hum Dev.
83:517–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murata Y, Itakura A, Matsuzawa K, Okumura
A, Wakai K and Mizutani S: Possible antenatal and perinatal related
factors indevelopment of cystic periventricular leukomalacia. Brain
Dev. 27:17–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuji M, Saul JP, Du Plessis A, et al:
Cerebral intravascular oxygenation correlates with mean arterial
pressure in critically illpremature infants. Pediatrics.
106:625–632. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wharton KN, Pinar H, Stonestreet BS, et
al: Severe umbilical coad inflammation - a predictor of
periventricular leukomalacia in very low birth weight infants.
Early Hum Dev. 77:77–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volpe JJ: Neurobiology of periventricular
leukomalacia in the premature infant. Pediatr Res. 50:553–562.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inage YW, Ltoh M and Takashina S:
Correlation between cerebrovscular matury and periventricular
leukomalacia. Pediatr Neurol. 22:204–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao JH and De J: To explore the high risk
factors of premature infants leukomalacia intraventricular
hemorrhage and periventricular. Hai Nan Yi Xue. 22:106–108.
2011.(In Chinese).
|
|
33
|
Melhem ER, Hoon AH Jr, Ferrucci JT Jr,
Quinn CB, Reinhardt EM, et al: Periventricular leukomalacia:
relationship between lateral ventricular volume on brain MR images
and severity of cognitive and motor impairment. J Radiology.
214:199–204. 2000. View Article : Google Scholar
|
|
34
|
Staudt M, Pavlova M, Böhm S, Grodd W and
Krägeloh-Mann I: Pyramidal tract damage correlates with motor
dysfunction in bilateral periventricular leukomalacia (PVL).
Neuropediatrics. 34:182–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Haastert IC, De Vries LS, et al: Gross
motor functional abilities in preterm-born children with cerebral
palsy due to periventricular leukomalacia. Dev Med Child Neurol.
50:684–689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Argyropoulou MI: Brain lesions in preterm
infants: Initial diagnosis and follow-up. Pediatr Radiol.
40:811–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fedrizzi E and Inverno M: MRI features of
cerebral lesions and cognitive functions in preterm spastic
diplegic children. Pediatric Neurology. 15:207–212. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Andiman SE, Haynes RL, Trachtenberg FL, et
al: The cerebral cortex overlying periventricular leukomalacia:
Analysis of pyramidal neurons. Brain Patho. 20:803–814. 2010.
View Article : Google Scholar
|