Introduction
Multidrug-resistant pulmonary tuberculosis (MDR-PTB)
refers to tuberculosis that is resistant to at least two major
anti-tuberculosis drugs. MDR-PTB usually cannot be treated
effectively by the use of normal drugs and procedures (1). Currently, according to the
literature, the fluoroquinolone drug levofloxacin is frequently
recommended for use in the treatment of MDR-PTB, and has been
widely investigated in clinical trials (2,3).
Moxifloxacin is a relatively new synthetic fluoroquinolone
antibacterial drug; it has several advantages including high
bioavailability, a good curative effect and minimal adverse
effects. The newly added methoxy group at position 8 of
moxifloxacin further increases its antibacterial activity, and the
antibacterial spectrum of moxifloxacin is wider than that of
levofloxacin (4). In addition,
moxifloxacin has previously been used in the treatment of MDR-PTB,
the majority of previous studies have reported only on the efficacy
of moxifloxacin in the treatment of multidrug-resistant
tuberculosis(2,4). The mechanism of moxifloxacin on
multidrug-resistant tuberculosis has received less
investigation.
In the present study, the curative effect of a
compacted treatment schedule of moxifloxacin was investigated in 92
patients with multidrug-resistant tuberculosis, randomly selected
from January 2011 and March 2013. Forty-six of the patients
received a high dose of moxifloxacin in a short-term treatment, and
were compared with the remaining 46 patients who received
moxifloxacin at the usual dosage and treatment duration. The
mechanism of multidrug-resistance in the patients was also
investigated.
Subjects and methods
Subjects
A total of 92 patients with MDR-PTB, which was
confirmed by tuberculosis culture test from January 2011 to March
2013, were randomly selected. The patients were randomly divided
into two groups, group A (n=46) and group B (n=46). The inclusion
criteria are as follows: i) received anti-tuberculosis drug
treatment for 1 year and had positive smear test results; ii)
resistant to at least two anti-tuberculosis drugs after improved
Lowenstein-Jensen medium susceptibility testing; iii) no history of
allergy to the drugs used in this study; and iv) tuberculosis
lesion detected in the lung by computed tomography or magnetic
resonance imaging examination. Patients with liver and kidney
dysfunction, psychology or other basic diseases were excluded. In
group A, there were 34 male and 12 female patients, with a disease
course of 1–24 years (mean, 11.25±7.56 years), with cavity closure
in 35 cases (48 cavities in total). In group B, there were 32 male
and 14 female patients, with a disease course of 2–23 years (mean,
13.15±8.10 years), with cavity closure in 36 cases (50 cavities in
total). There was no statistically significance between the two
groups according to age, gender or disease course (P>0.05); thus
the groups were comparable for general information. This study was
conducted in accordance with the Declaration of Helsinki and with
approval from the Ethics Committee of the First Affiliated Hospital
of Xinxiang Medical University (Weihui, China). Written informed
consent was obtained from all participants.
Treatment methods
Group A received a high dose of moxifloxacin over a
shorter than usual treatment time. The patients in group A received
moxifloxacin 0.6 g, once per day; amikacin, 0.6 g, once per day;
pasiniazid, 1 g, once each night; pyrazinamide, 0.5 g, 3 times/day;
rifabutin, 0.3 g, twice per day; pasiniazid, 0.3 g, 3 times/day and
propylthiouracil isonicotinoyl amine, 0.2 g, 3 times/day, with a
treatment course of 6 months. Patients in group B received normal
treatment with moxifloxacin (0.4 g, once per day), in addition to
the other antibtubercular drugs described above, as in group A, and
the course of treatment was 9 months. Group A and group B received
the same amount of moxifloxacin in total.
Detection methods
Gollowing one course of treatment, monocytes and
macrophages were isolated from the patients and cultured in
Dulbecco’s modified Eagle’s medium (Gibco Life Technologies,
Carlsbad, CA, USA) with 10% inactivated fetal bovine serum
(Sijiqing, Hangzhou, China). at 37°C and 5% O2 for 1
week. Following the addition of 0.5 ml washing buffer to
1×106 cells and centrifugation, another 1 ml washing
buffer was added. Fluorescent goat anti-monkey immunoglobulin
G-fluorescein isothiocyanate antibody (cat. no. FM38301; 1:16;
Sigma-Aldrich, St. Louis, MO, USA) was then added to each tube.
Following vibration of the tube, the changes in the expression of
CD80, CD40 and HLA-DR on the cell surface were monitored by flow
cytometry (EPICS® ALTRA™; Beckman Coulter, Inc., Brea,
CA, USA).
Assessment of curative effect
The curative effect was assessed according to the
guidelines approved by the Chinese Medical Association in 2005
(5). The rates of sputum negative
conversion were determined at the end of 3, 6 and 9 months using
the sputum smear test. A negative result means that no acid-fast
bacilli were found in the sputum, and a positive result means that
acid-fast bacilli were identified in the sputum. X-ray examination
of lesions was also conducted. In terms of the proportion of
lesions in the lung in the field of view: ‘evident absorption’
indicates resolution of ≥1/2 of the lesions; ‘absorption’ indicates
resolution of ≥1/3 but <1/2 of the pathological changes; and ‘no
change’ indicates <1/3 resolution of the pathological changes or
an increase in pathological changes. In the evaluation of cavity
improvement: ‘closure’ means scarless healing and the disappearance
of obstruction; ‘shrinkage’ means narrowing of the cavity diameter
by >1/2; ‘no change’ means narrowing of the cavity diameter by
<1/2 or an increase in cavity diameter. In the comprehensive
efficacy evaluation: ‘cured’ means no active tuberculosis in the
lung and cavity closure; ‘markedly effective’ means sputum negative
conversion, cavity reduction and the absorption of foci;
‘effective’ means sputum negative conversion, the absorption of or
no change in foci, and reduction or no cavity changes; ‘invalid’
means sputum positive conversion, pathological changes and no
cavity changes.
Statistical analysis
The statistical software SPSS, version 17.0 (SPSS
Inc, Chicago, IL, USA) was used to analyze the data. The data are
expressed in numbers and percentages. The χ2 test was
used to compare the differences between groups. P<0.05 was
considered to indicate a statistically significant difference
between two groups.
Results
Comparison of the curative effect between
the two groups
Following treatment, the curative rate in group A
was 82.61% and that in group B was 84.78%. There was no
statistically significant difference in curative rate between the
two groups (P>0.05; Table
I).
 | Table IComparison of curative effect between
the two groups. |
Table I
Comparison of curative effect between
the two groups.
| | Efficacy, n | |
|---|
| |
| |
|---|
| Group | No. of cases | Cured | Markedly
effective | Effective | Invalid | Curative rate
(%) |
|---|
| A | 46 | 5 | 19 | 14 | 8 | 82.61 |
| B | 46 | 4 | 17 | 18 | 7 | 84.78 |
| χ2 | | | | | | 0.526 |
| P-value | | | | | | >0.05 |
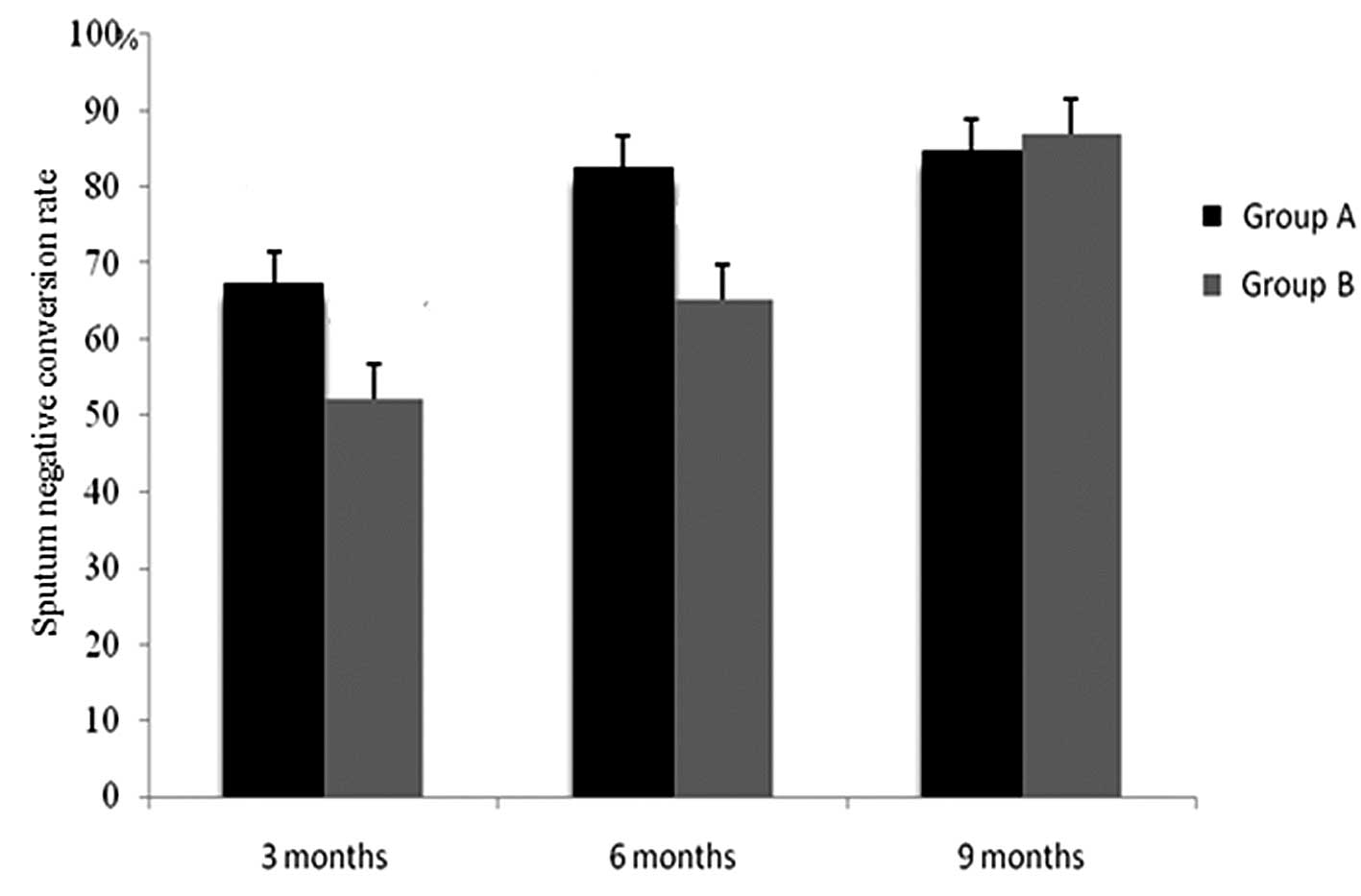
Comparison of the rates of sputum
negative conversion at 3, 6 and 9 months between the two
groups
Following 3 and 6 months of the treatment, the rates
of sputum negative conversion in group A were significantly higher
than those in group B, and there was observed to be a statistically
significant difference between the two groups
(χ32=10.956,
χ62=13.565; P<0.01). However, there was no
statistically significant difference in the rates of sputum
negative conversion between the two groups at the end of the 9
months of treatment (χ92=0.485; P>0.05;
Fig. 1).
Comparison of lesions and tuberculous
cavities between the two groups following treatment
No statistically significant difference was
identified in the improvement of lesions and cavity conditions
between the two groups at the end of the treatment period
(P>0.05; Table II).
 | Table IIComparison of lesions and tuberculous
cavities between the two groups after treatment. |
Table II
Comparison of lesions and tuberculous
cavities between the two groups after treatment.
| Lesions, n (%) | Cavities, n (%) |
|---|
|
|
|
|---|
| Group | No. of cases | Evident
absorption | Absorption | No change | Total no. | Closure | Shrinkage | No change |
|---|
| A | 46 | 14 (30.44) | 26 (56.52) | 6 (13.04) | 48 | 31 (64.58) | 7 (14.58) | 10 (20.83) |
| B | 46 | 12 (26.09) | 27 (58.70) | 7 (15.22) | 50 | 31 (62.00) | 8 (16.00) | 11 (22.00) |
| χ2 | | 2.145 | 1.236 | 1.321 | | 1.452 | 1.756 | 1.358 |
| P-value | | >0.05 | >0.05 | >0.05 | | >0.05 | >0.05 | >0.05 |
Comparison of adverse effects between the
two groups
The main adverse effects observed in the two groups
were reductions of peripheral white blood cell counts, liver
function damage, gastrointestinal symptoms and neurological
symptoms. The incidence of each of these adverse effects in group A
was significantly lower than its incidence in group B (P<0.05;
Table III).
 | Table IIIComparison of adverse effects between
the two groups [n (%)]. |
Table III
Comparison of adverse effects between
the two groups [n (%)].
| Group | No. of cases | Reduction of white
blood cell count | Liver function
damage | Gastrointestinal
symptoms | Neurological
symptoms |
|---|
| A | 46 | 1 (2.17) | 2 (4.35) | 5 (10.87) | 6 (13.04) |
| B | 46 | 5 (10.87) | 5 (10.87) | 14 (30.45) | 12 (26.09) |
| χ2 | | 4.125 | 3.892 | 16.125 | 8.751 |
| P-value | | <0.05 | <0.05 | <0.01 | <0.01 |
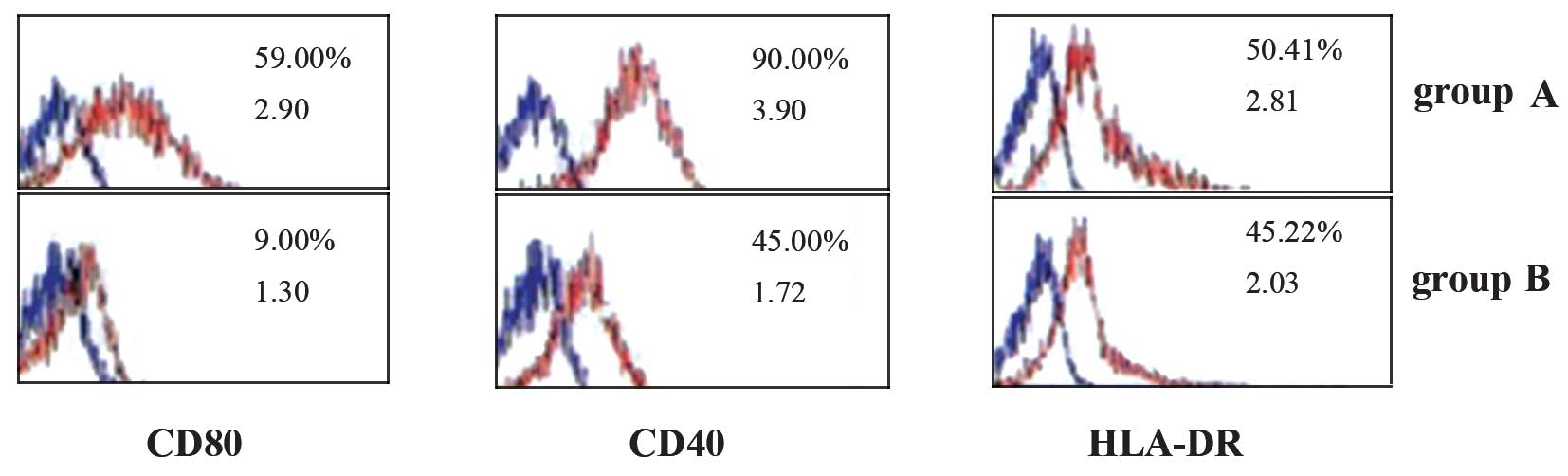
Effect of moxifloxacin on the expression
of antigen-presenting functional molecules on the surfaces of
mononuclear cells in the two groups
In group A, on the surfaces of the mononuclear cells
(monocytes and macrophages), the expression of antigen-presenting
functional molecules CD80 and CD40 was higher than that in group B
(P<0.05); however, the expression of HLA-DR was not
significantly different between groups A and B (P>0.05; Fig. 2).
Discussion
Fluoroquinolone antibiotics are critical in MDR-PTB
treatment, and are known as second-line anti-tuberculosis drugs in
the medical field. The mechanism of fluoroquinolone antibiotics is
mainly the inhibition of the activity of DNA gyrase, and thus
destruction of the replication and transcription of DNA in
Mycobacterium tuberculosis, which further destroys the
genetic material in the cells, leading to the death of
Mycobacterium tuberculosis (6). However, as antibacterial drugs are
widely used, the degree of drug-resistance of tuberculosis also
increases. Therefore, the appropriate medication is vital for
preventing an increase in the amount of MDR-PTB (7). Moxifloxacin is a relatively new
8-methoxyquinolone derivative, which has antibacterial activity
against Mycobacterium tuberculosis and non-tuberculous
mycobacteria (8). During the
treatment of MDR-PTB, the duration of treatment with
anti-Mycobacterium tuberculosis drugs is largely associated
with sputum drug resistance; a longer duration of treatment usually
leads to increasing drug-resistance and a worse curative effect
(9). Therefore, the present study
considered the condition of drug-resistance in patients with
MDR-PTB. This study, with reference to tuberculosis prevention and
control conducted in institutions in multiple regions, moderately
increased the dose of moxifloxacin and decreased the length of the
cycle of treatment, with the aim of decreasing the incidence of
tolerance to moxifloxacin among patients.
The results indicated that there was no
statistically difference in curative effect, sputum negative
conversion rate, the resolution of lesions and cavity improvement
between patients with MDR-PTB who received super-compact treatment
with a high dose of moxifloxacin and those who received
moxifloxacin treatment at the normal dosage and duration. However,
the incidence of adverse effects in the patients who received
super-compact treatment with a high dose of moxifloxacin was
significantly reduced compared with that in the normal moxifloxacin
treatment group. Abbate et al (10) observed that the antibacterial
activity of moxifloxacin is 4–8-fold stronger than that of the
antituberculosis drug levofloxacin and that patients with MDR-PTB
are generally sensitive to it. Isoniazid may be used in combination
with amantadine hydrochloride and p-aminosalicylic acid.
Isoniazid mainly inhibits the proliferation and growth of
mycobacteria. However, p-aminosalicylic acid slows down the
acetylation of isoniazid in the organism, and provides a stable
concentration of amantadine hydrochloride in the blood, which
decreases the toxicity of isoniazid in the liver (11). Rifabutin is spiro derivative of
piperidine and rifamycin; its activity against Mycobacterium
tuberculosis activity is 4-fold stronger than that of the
commonly clinically used drug rifampicin, and it is active against
rifampicin-resistant tuberculosis (12,13).
Amikacin, pasiniazid, pyrazinamide and propylthiouracil
isonicotinoyl amine are classical anti-tuberculosis drugs. In the
present study, the advantages of the new moxifloxacin
administration strategy were demonstrated to be high activity
against Mycobacterium tuberculosis, with evident
bactericidal activity and applicability for patients with
tuberculosis resistant to multiple drugs, including rifampin,
ofloxacin and rifampicin. In addition, this new strategy was found
to be safe, with high tolerance, which supports the proposition of
super-compact treatment with a high dose of moxifloxacin for
MDR-PTB (14). In addition, Liang
et al (15) found that
long-term anti-tuberculosis treatment is highly likely to be
associated with new infections, and the pulmonary lesions were
mainly exudative. This explains to some extent why fewer adverse
effects occur when anti-tuberculosis treatment is conducted for a
shorter time period. The limitation of the present study is that
the cost of treatment with moxifloxacin and rifabutin is high.
Thus, it would be difficult to clinically apply this new treatment
strategy; this safer strategy is only likely to be available to
patients who have good economic conditions.
Mononuclear macrophages are antigen-presenting
cells, with large quantities of antigen-presenting functional
molecules on the surface. Mononuclear macrophages may present
tuberculosis antigen to T cells (CD4+ and
CD8+), activate the immune response to differing
degrees, and induce the accumulation of T cells. Therefore,
antigen-presenting functional molecules on mononuclear cell
surfaces have important immunological functions against
tuberculosis. In the present study, in group A, the expression of
the antigen-presenting functional molecules CD80 and CD40 on the
mononuclear cell surface was greater than that in group B, but no
significant difference in HLA-DR expression was observed between
the two groups. This indicates that moxifloxacin treatment over a
shorter time period may induce the release of many cytokines,
facilitate activation of the immune response and increase the
immune function in patients with tuberculosis. However, these
effects decreased gradually during the long-term treatment with
moxifloxacin. The study only compared moxifloxacin treatments of
different concentrations and durations, without an empty control,
and only focused on the mechanism by which moxifloxacin affects T
cells; an investigation of the overall mechanism is lacking.
Therefore, further research and investigation are needed.
In summary, the short-duration treatment with a high
dose of moxifloxacin is effective for MDR-PTB, and its advantages
are a reduction in the incidence of adverse reactions and a lack of
drug resistance. The treatment strategy used in the present study
is worthy of further study and testing in clinical trials.
References
|
1
|
Chen JL, Shi JW, Gu DL and Chen XL: The
value of moxifloxacin and rifabutin strategy on super compact
treatment of initial smear positive pulmonary tuberculosis.
Chongqing Shi Yi. 41:2966–2968. 2012.(In Chinese).
|
|
2
|
Koh WJ, Lee SH, Kang YA, et al: Comparison
of levofloxacin versus moxifloxacin for multidrug-resistant
tuberculosis. Am J Respir Crit Care Med. 188:858–864. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WH, Xu B, Tang WQ, Zong XN, Luo PF,
Cao S and Wei PM: Meta-analysis of refractory, recurrent and
multi-drug resistant tuberculosis treatment by moxifloxacin.
Nanjing Yi Ke Da Xue Xue Bao. 30:1166–1171. 2010.(In Chinese).
|
|
4
|
Jiang RH, Xu HB and Li L: Comparative
roles of moxifloxacin and levofloxacin in the treatment of
pulmonary multidrug-resistant tuberculosis: a retrospective study.
Int J Antimicrob Agents. 42:36–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chinese Medical Association. Guidance for
Clinical Detection and Treatment (Tuberculosis). Beijing People’s
Medical Publishing House; pp. 87–91. 2005
|
|
6
|
Manika K, Chatzika K, Zarogoulidis K and
Kioumis I: Moxifloxacin in multidrug-resistant tuberculosis: is
there any indication for therapeutic drug monitoring? Eur Respir J.
40:1051–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nosova EY, Bukatina AA, Isaeva YD,
Makarova MV, Galkina KY and Moroz AM: Analysis of mutations in the
gyrA and gyrB genes and their association with the resistance of
Mycobacterium tuberculosis to levofloxacin, moxifloxacin and
gatifloxacin. J Med Microbiol. 62:108–113. 2013. View Article : Google Scholar
|
|
8
|
Zvada SP, Denti P, Geldenhuys H, et al:
Moxifloxacin population pharmacokinetics in patients with pulmonary
tuberculosis and the effect of intermittent high-dose rifapentine.
Antimicrob Agents Chemother. 56:4471–4473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berdot S, Papy E, Rioux C, et al: Use of
moxifloxacin in tuberculosis regimen in a French teaching hospital.
Med Mal Infect. 40:568–573. 2010.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbate E, Vescovo M, Natiello M, et al:
Successful alternative treatment of extensively drug-resistant
tuberculosis in Argentina with a combination of linezolid,
moxifloxacin and thioridazine. J Antimicrob Chemother. 67:473–477.
2012. View Article : Google Scholar
|
|
11
|
Liu XY, Lin JX, Chen R and Peng DD:
Clinical observation of super compact treatment of moxifloxacin in
the initial treatment sputum positive pulmonary tuberculosis.
Guangdong Yi Xue. 32:1749–1751. 2011.(In Chinese).
|
|
12
|
Fouad M and Gallagher JC: Moxifloxacin as
an alternative or additive therapy for treatment of pulmonary
tuberculosis. Ann Pharmacother. 45:1439–1444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pranger AD, van Altena R, Aarnoutse RE, et
al: Evaluation of moxifloxacin for the treatment of tuberculosis: 3
years of experience. Eur Respir J. 38:888–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balasubramanian V, Solapure S, Gaonkar S,
et al: Effect of coadministration of moxifloxacin and rifampin on
Mycobacterium tuberculosis in a murine aerosol infection model.
Antimicrob Agents Chemother. 56:3054–3057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang LL, Liu X and Ma Y: Effects of
anti-tuberculosis containing with moxifloxacin or levofloxacin on
multidrug resistant pulmonary tuberculosis. Zhongguo Yi Yuan Xie
Hui. 14:1451–1453. 2011.(In Chinese).
|