Introduction
Hepatocellular carcinoma (HCC) is one of the most
common, aggressive solid malignancies worldwide, accounting for in
excess of two-thirds of all primary liver cancer cases (1). Approximately 500,000 new cases of HCC
are reported annually, and >75% of these occur in the
Asia-Pacific region (2). In the
USA, the HCC incidence is increasing at a greater rate than the
incidence of any other cancer (3).
Furthermore, the five-year survival rate for HCC is <5%, ranking
HCC as one of the types of cancer with the worst prognosis
(4). As a result, the mechanism
underlying the tumorigenesis and the specific measures required for
the early diagnosis or effective therapy of HCC are current
research focuses (5–8).
HCC commonly arises against a background of chronic
liver disease and cirrhosis caused by hepatitis B or C virus
(9). In these patients,
surveillance strategies for the detection of early HCC are
necessary. For >40 years, the most common marker used in
clinical practice has been α-fetoprotein (AFP), which is combined
with hepatic ultrasonography. AFP is considered to be the
gold-standard serum marker for the screening of patients who are at
high risk of HCC, as well as for the monitoring of treatment
response (10); however, the
clinical value of AFP has been questioned due to its low
sensitivity and specificity (11).
As the overall survival of patients with cirrhosis has improved and
the global incidence of HCC has continued to increase, strategies
for the early detection of HCC are urgently required (12).
Golgi protein 73 (GP73, otherwise known as Golph2)
is a resident Golgi-specific membrane protein that is expressed in
the normal liver by biliary epithelial cells. The expression of
GP73 undergoes a notable increase in chronic liver diseases,
particularly in HCC cells (13). A
number of studies have described the use of GP73 as a serum marker
for HCC; however, the results have been inconsistent and shown
evident heterogeneity (14–16).
The aim of the present study, therefore, was to perform a
systematic analysis of studies evaluating the diagnostic accuracy
of serum GP73 for HCC.
Materials and methods
Inclusion and exclusion criteria
Studies were evaluated strictly for their relevance
to the selected topic. Eligible studies had to include a
representative patient spectrum. The diagnosis of HCC was
established by histopathological examination or, if histopathology
was not available, by two imaging modalities, such as ultrasound,
magnetic resonance imaging or computed tomography, showing a
vascular enhancing mass of >2 cm (17). Exclusion criteria comprised studies
that evaluated serum GP73 levels by mRNA, DNA or DNA polymorphism
analysis and those that did not provide exact values for the
sensitivity or specificity of GP73, as well as abstracts, letters,
editorials and expert opinions, reviews without original data, case
reports and studies lacking control groups.
Identification of studies
A comprehensive systematic literature review of
original investigations into the diagnostic accuracy of GP73 was
performed by searching the following electronic databases up to
September 2013: PubMed/Medline, Embase, Cochrane Database of
Systematic Reviews, Cochrane Central Register of Controlled Trials,
Science Citation Index (ISI Web of Science), Chinese Biomedical
Literature Database and Chinese National Knowledge Infrastructure
(18,18). References from the included studies
and any relevant published reports were additionally manually
searched. No restrictions were placed on language, study design,
year of publication or publisher status. The subject headings and
keywords utilized in the search strategy included i) GP73: GP73,
Golgi protein 73, Golgi phosphoprotein 2, Golgi membrane protein 1;
and ii) HCC: HCC, hepatocellular carcinoma, liver cell carcinoma,
hepatic cell carcinoma. No keywords or indexing terms for
diagnostic test accuracy were used due to the possibility of
relevant studies being missed.
Study selection
Independent reviews of the studies were performed by
two reviewers based on the titles and abstracts, prior to the full
texts of any potentially relevant studies being obtained for
further assessment. Disagreements between the reviewers were
resolved by consensus. If any further study details were required,
a request was sent to the authors. When findings from the same
patient population were reported by the same author in multiple
publications, the most recent or most complete report was
identified and used to avoid overlap between cohorts.
Data extraction
The following data were extracted independently from
the included studies by two reviewers: Authors, year of
publication, journal, study design, number of patients, type of
marker assay, cut-off values and raw data regarding the sensitivity
and specificity (number of true-positive, false-negative,
true-negative and false-positive results) for comparisons of
patients diagnosed with HCC versus controls. Disagreements were
resolved through discussion with a third reviewer.
Assessment of methodological quality
The quality of each study was evaluated according to
the Quality Assessment of studies of Diagnostic Accuracy included
in Systematic reviews (QUADAS) checklist recommended by the
Cochrane Collaboration. Each of the 14 items in the QUADAS
checklist was scored as ‘yes’, ‘no’ or ‘unclear’ (20).
Data analysis
Using Meta Disc software (version 1.4; Clinical
Biostatistics Unit, Ramón y Cajal Hospital, Madrid, Spain), the
receiver operating characteristic (ROC) plane was drawn and the
Spearman correlation coefficient was calculated to estimate if
there was a threshold effect. The overall sensitivity, specificity,
positive likelihood ratio (PLR), negative likelihood ratio (NLR)
and diagnostic odds ratio (DOR) were calculated. Data were
presented as forest plots, which showed the results of individual
studies with the corresponding 95% confidence intervals (CIs).
Summary ROC (SROC) curve analysis was used to summarize the overall
test performance. The Midas model for Stata (version 12.0;
StataCorp LP, College Station, TX, USA) was used to construct the
funnel plots and calculate the P-values. Publication bias existed
when P<0.05 was observed. Meta-regression was also performed in
an attempt to explain the observed heterogeneity.
Results
Study retrieval
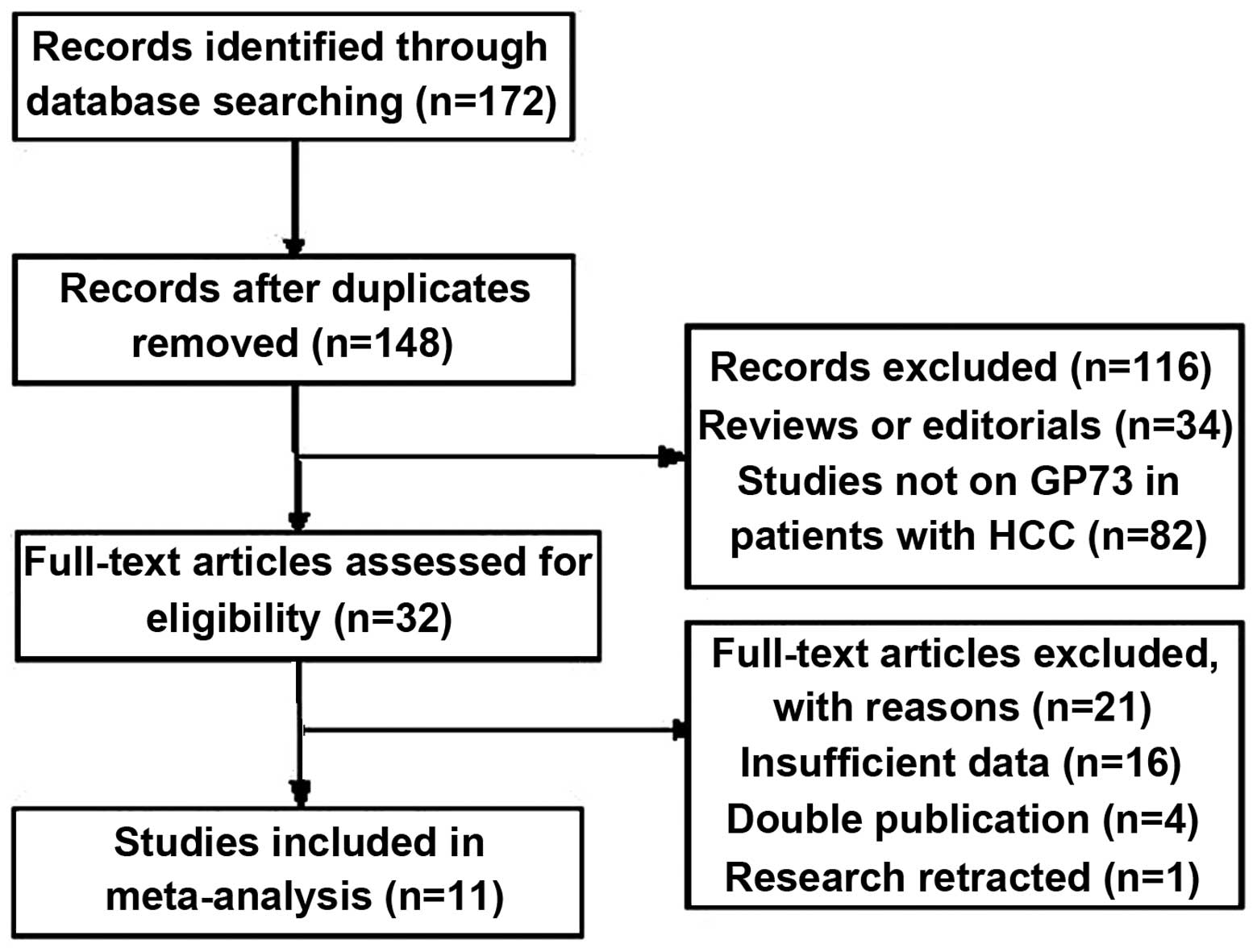
A total of 172 studies were found and 32 were
considered to be eligible for inclusion in the analysis. Following
the full-text review, 21 studies were excluded: 16 due to the
results not allowing the calculation of sensitivity or specificity;
four due to a suspected overlap in the study population or a
duplicate publication; and one due to a retraction by the author
(21). Finally, 11 studies were
available for the meta-analysis. These studies included 6,711
patients who received serum GP73 tests (22–32),
1,887 of whom were diagnosed with HCC by histopathology or two
imaging modalities. A flow diagram of the study selection process
is shown in Fig. 1.
The characteristics of each study are shown in
Table I. The number of patients in
each of the 11 studies was >100, with little difference in the
characteristics among the studies. The GP73 cutoff values differed
substantially, which may have been a source of heterogeneity. The
ethnicity in nine studies was Asian.
 | Table IMain characteristics of the studies
included. |
Table I
Main characteristics of the studies
included.
| First author, year
(ref.) | Country | TP/FP/FN/TN results
(n/n/n/n) | Assay type | Cutoff value |
HCC/cirrhosis/hepatitis/healthy/others
(n/n/n/n/n) |
|---|
| Hu, 2010 (19) | China | 24/15/7/78 | Western
blotting | 7.4 RU |
31/31/31/31/b |
| Mao, 2010 (20) | China/USA |
589/89/200/3339 | Immunoblotting | 8.5 RU |
789/512/337/1690/889 |
| Marrero, 2005
(21) | China | 99/21/45/131 | Immunoblotting | 10 RU | 144/152/b/56/b |
| Morota, 2011
(22) | USA | 62/61/8/98 | ELISA | 94.7 μg/l |
70/35/52/72/b |
| Shi, 2011 (23) | China | 50/5/23/102 | ELISA | 123.2 μg/l |
73/13/32/62/b |
| Tian, 2011
(24) | China | 115/46/38/49 | ELISA | 113.89 μg/l |
153/95/115/109/b |
| Wang, 2009
(25) | USA | 156/64/8/49 | ELISA | NK | 164/113/b/b/b |
| Xu, 2011 (26) | China | 63/38/18/208 | ELISA | NK | 81/176a/40/b |
| Zhao, 2010
(27) | China | 168/41/51/112 | ELISA | 100 ng/ml | 219/110a/43/b |
| Wang, 2013
(28) | China | 62/32/22/141 | Immunoblotting | 8.5 RU |
84/80/32/61/b |
| Hou, 2013 (29) | China | 58/16/21/58 | ELISA | 78.1 ng/l |
84/80/32/61/b |
Quality of studies
The QUADAS criteria were used to evaluate the
quality of the 11 selected studies. As shown in Table II, all the studies fulfilled
between seven and 11 of the 14 described criteria. Summary scores
were not calculated, as their interpretation can be problematic and
potentially misleading (33). All
the studies used a retrospective design. In 10 studies, healthy
individuals were recruited for the control group; the percentage of
HCC diagnoses in these studies ranged between 18.7 and 59.2%. All
the studies reported the diagnostic standard of HCC, and four
reported the tumor stage of the patients with cancer (23,24,27,28).
The serum GP73 levels were interpreted in a blinded manner in only
one out of the 11 studies.
 | Table IISummary judgments of the
methodological quality of the included studies (QUADAS
checklist). |
Table II
Summary judgments of the
methodological quality of the included studies (QUADAS
checklist).
| QUADAS item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| Representative
patient spectrum? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Selection
criteria? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Acceptable
reference standard? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Acceptable delay
between tests? | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC |
| Partial
verification avoided? | UC | Y | Y | UC | UC | Y | Y | UC | UC | Y | Y |
| Differential
verification avoided? | UC | Y | Y | UC | UC | Y | Y | UC | UC | Y | Y |
| Incorporation
avoided? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Index test
execution? | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Reference standard
execution? | N | Y | Y | N | N | Y | Y | N | N | Y | N |
| Reference standard
results blinded? | N | N | N | N | N | N | N | N | N | Y | N |
| Index test results
blinded? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Relevant clinical
information? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Uninterpretable
results reported? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Withdrawals
explained? | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC | UC |
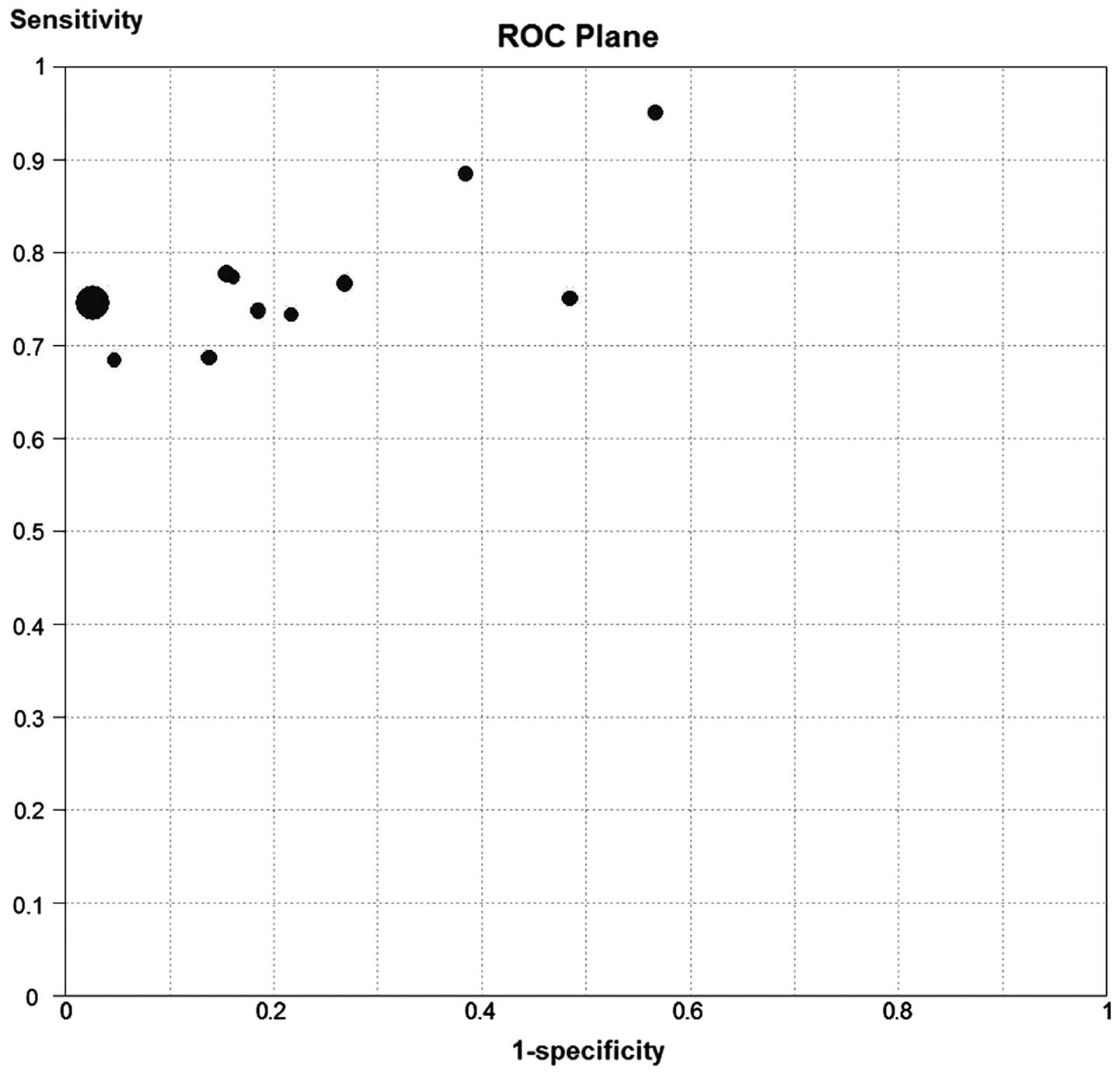
Threshold effect
When there is a threshold effect, an inverse
correlation is demonstrated between the sensitivity and
specificity, leading to a typical ‘shoulder arm’ of the ROC plane
distribution. Spearman correlation analysis also suggests a strong
positive correlation. In the present study, the ROC plane output by
the Meta Disc 1.4 software (Fig.
2) showed a nontypical shoulder arm appearance; the calculated
Spearman correlation coefficient value was 0.591 and the P-value
was 0.056, suggesting that there was no threshold effect.
Summary diagnostic accuracy of serum GP73
for HCC
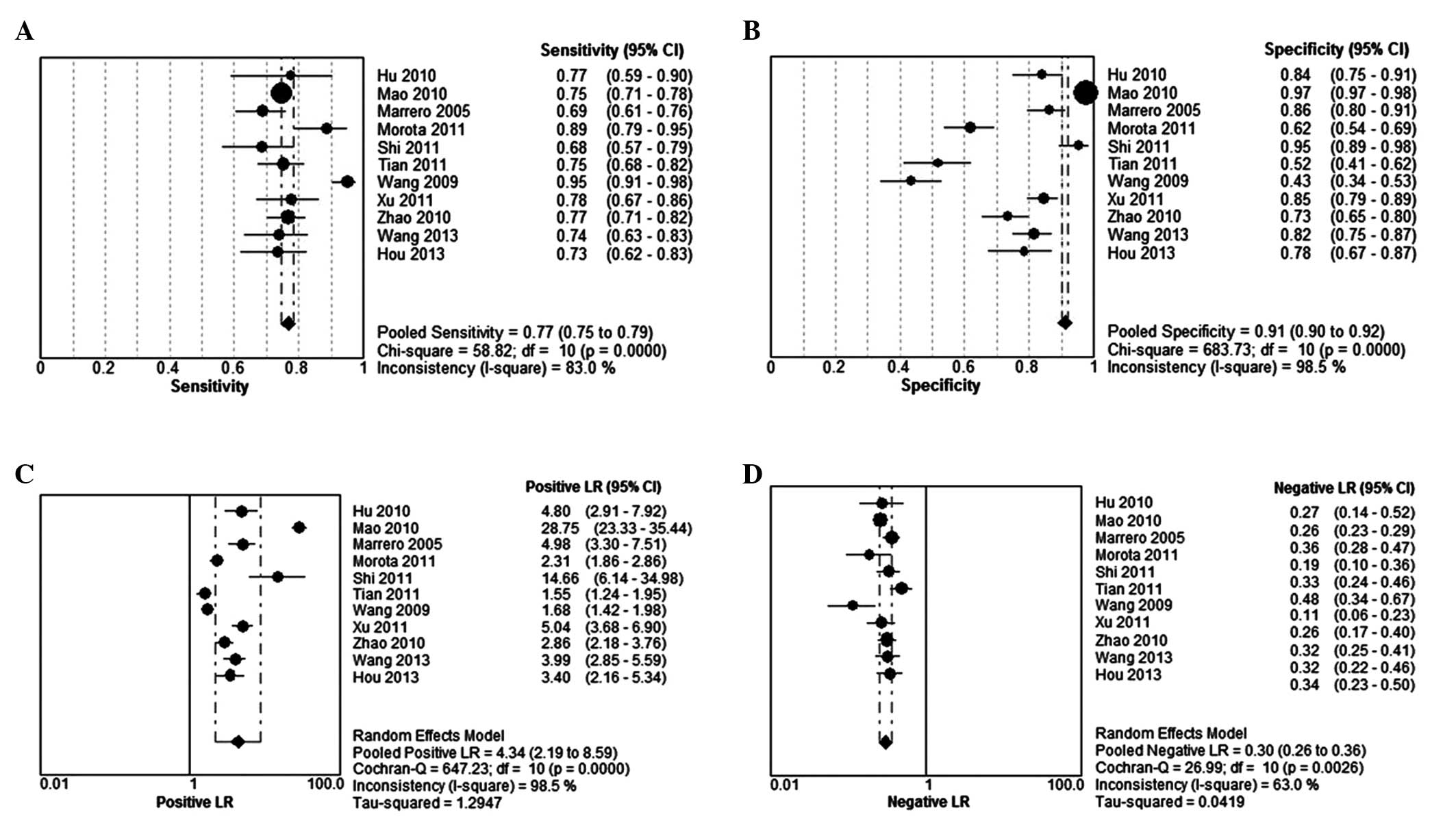
The DerSimonian-Laird (random effects) model was
used to calculate the pooled value. The sensitivity observed ranged
between 43 and 88.6% (summary, 77%; 95% CI, 75–79%) (Fig. 3A), while the specificity ranged
between 51.8 and 97.4% (summary, 91%; 95% CI, 90–92%) (Fig. 3B); the PLR was 4.34 (95% CI,
2.19–8.59) (Fig. 3C) and the NLR
was 0.30 (95% CI, 0.26–0.36) (Fig.
3D). The PLR value indicated that patients with HCC had a
4.3-fold higher chance of a positive GP73 assay compared with
patients without HCC. Similarly, the NLR indicated that, if the
GP73 assay was negative, the probability of these patients
developing HCC was ~30%. Thus, GP73-negative results may not be
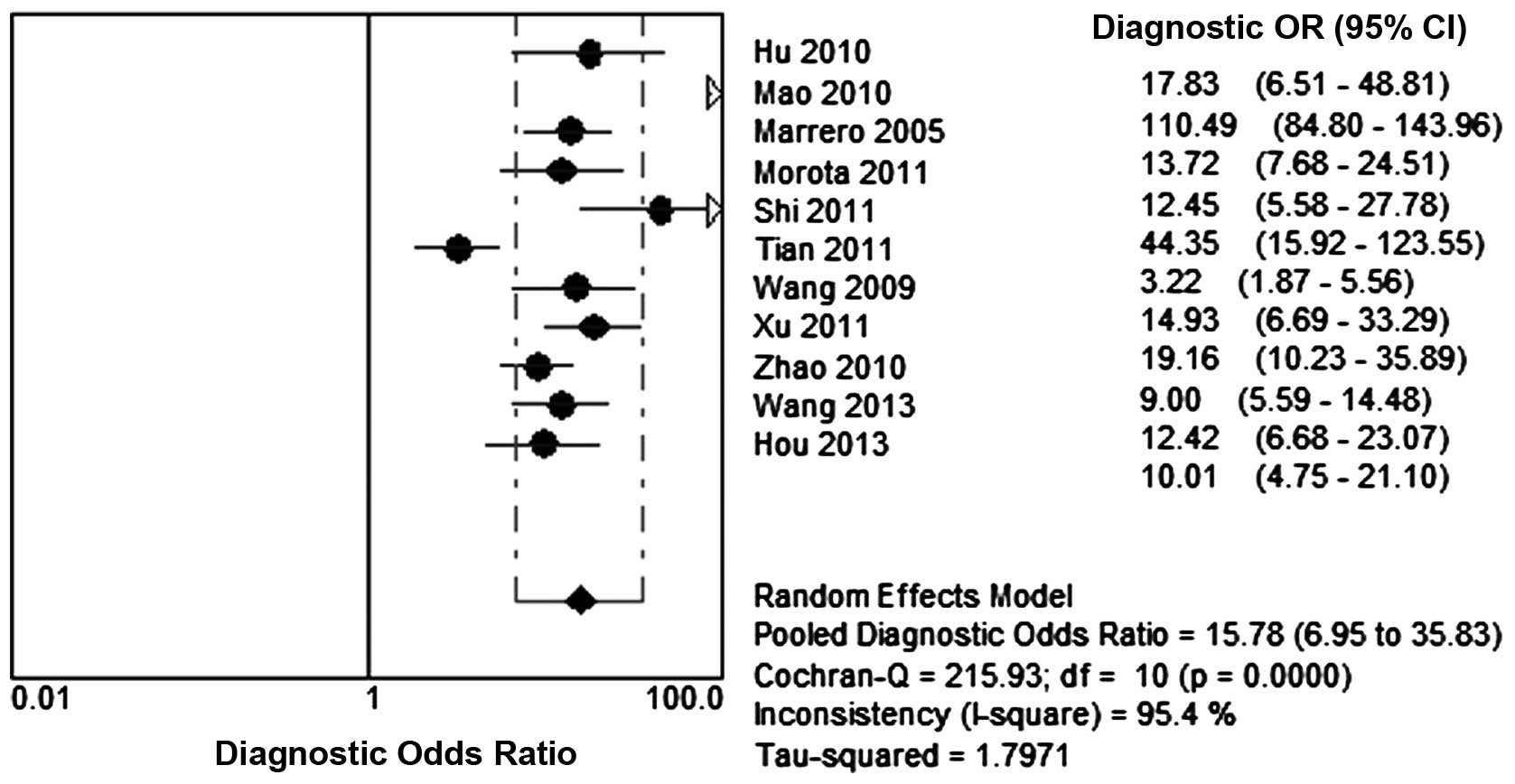
used to exclude HCC. It was also noted that the summary DOR was
15.78 (95% CI, 6.95–35.83) for GP73 (Fig. 4). The sensitivity, specificity,
PLR, NLR and DOR with the 95% CIs for each study were presented in
a forest plot, and significant heterogeneity was observed.
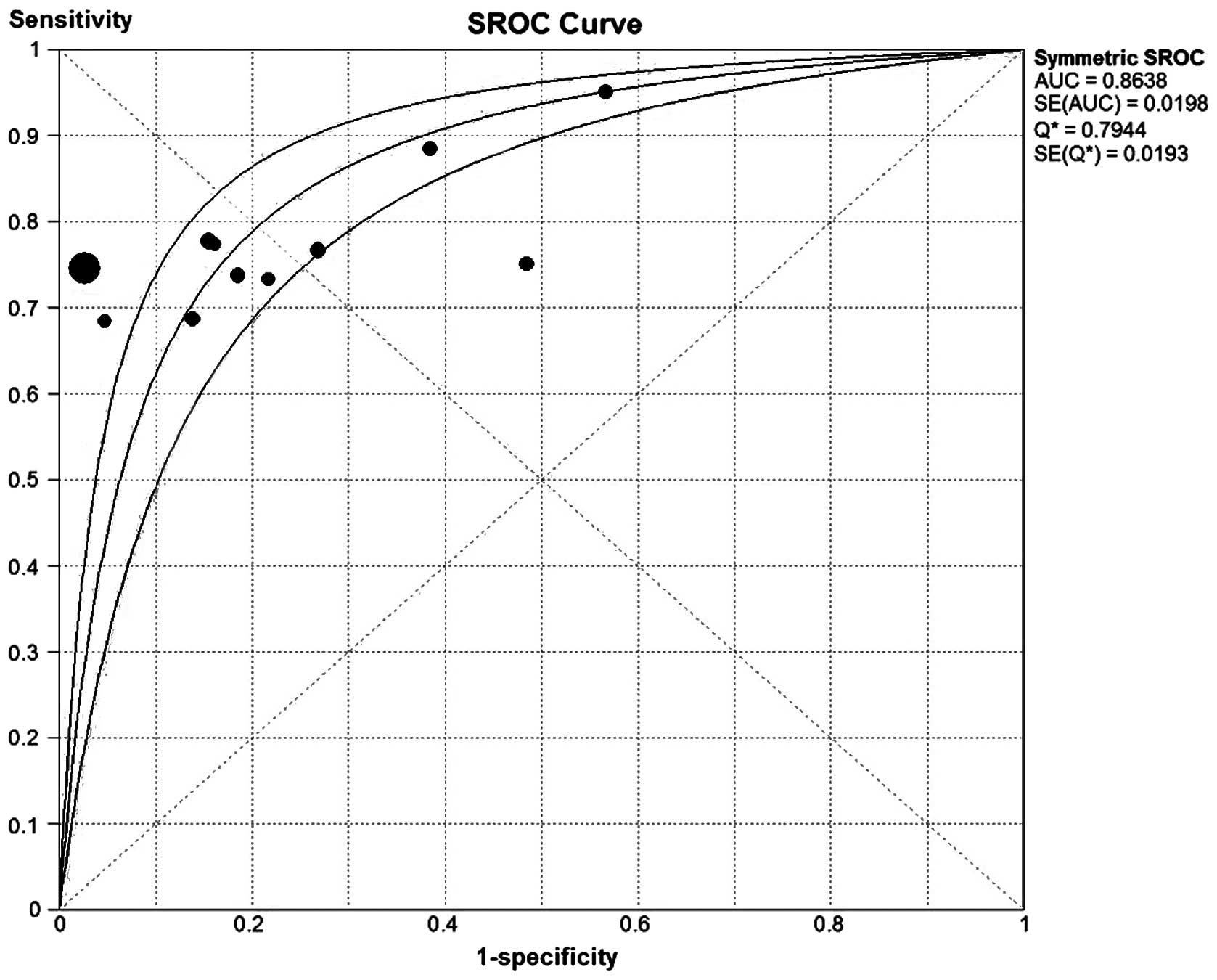
The SROC approach is the standard strategy for the
meta-analysis of the diagnostic reporting pairs of sensitivity and
specificity (34). This approach
uses DOR as the primary outcome measure, which eliminates the
effect of a possible threshold (35). As shown in Fig. 5, the area under the SROC curve was
0.8638, with a standard error of 0.0198 and Q* of
0.7944, suggesting a comparable diagnostic value of GP73 for
HCC.
Meta-regression for heterogeneity
To investigate heterogeneity, attempts were made to
explore the following study characteristics using meta-regression:
Population characteristics (gender, ethnicity, age, disease type
and stage distribution), study design (prospective or retrospective
and year of publication) and test characteristics (cutoff value,
test type and number of tests per screening round); however, due to
the unsatisfactory methodological quality of the studies or
incomplete data, year, assay type and country were the only three
features examined. The accuracy measure used was DOR, since it was
a unitary measure of diagnostic performance that encompassed
sensitivity and specificity or PLR and NLR. It was found that the
differences among the ethnicities had a significant effect on the
DOR (Table III). This may have
been due to the fact that the sample size of Western patients was
small compared with the number of Asian patients.
 | Table IIIMeta-regression of the effects of
methodological characteristics on diagnostic accuracy. |
Table III
Meta-regression of the effects of
methodological characteristics on diagnostic accuracy.
| Variable | Coefficient | Standard error | P-value | RDOR | 95% CI |
|---|
| Assay | −0.117 | 0.4307 | 0.7930 | 0.89 | 0.33–2.40 |
| Country | 2.332 | 0.5633 | 0.0033 | 10.29 | 2.81–37.73 |
| Year | −0.086 | 0.1850 | 0.6563 | 0.92 | 0.60–1.41 |
Publication bias
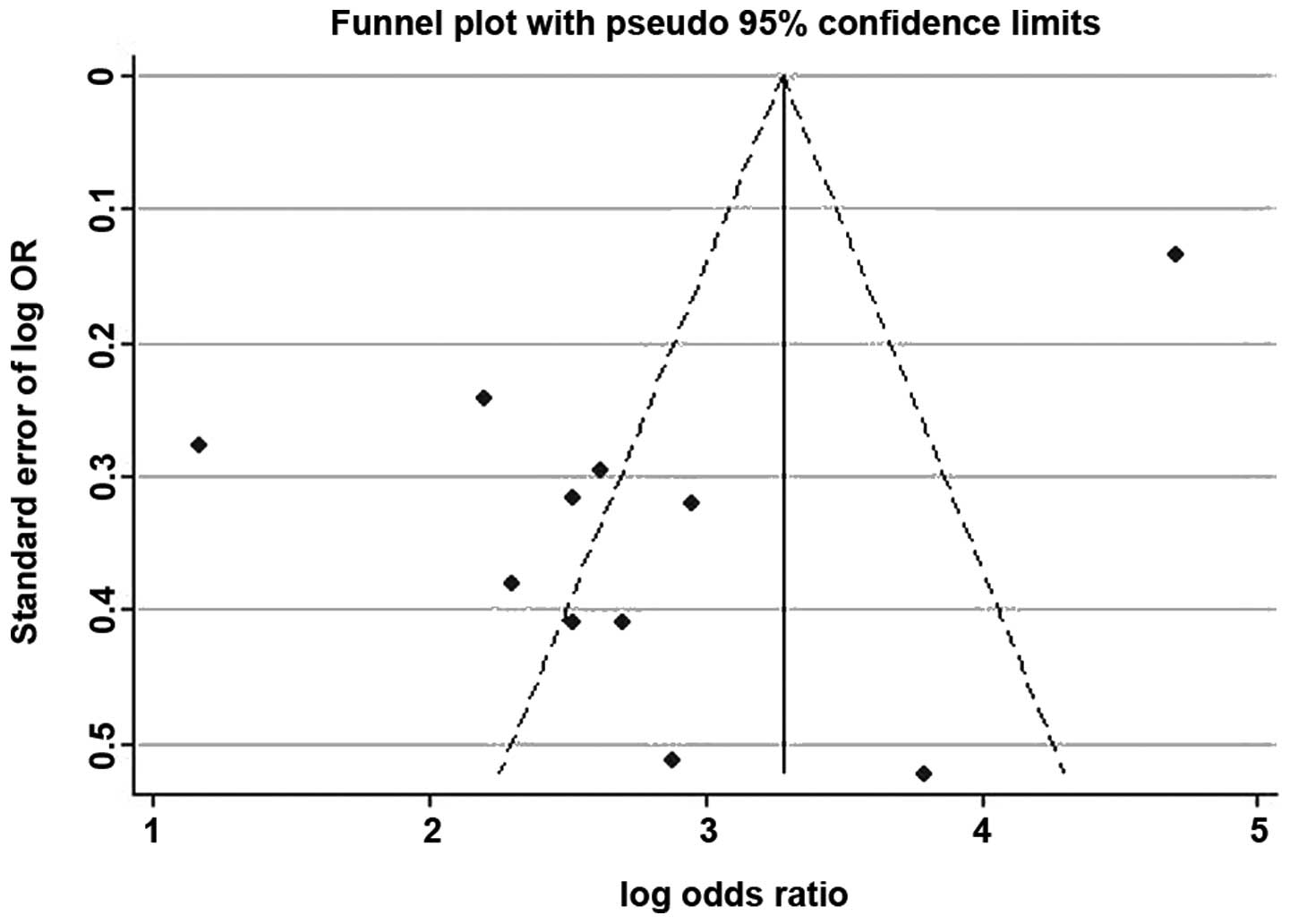
Deeks’ funnel plot was created using the
‘metafunnel’ command of Stata version 12.0. As shown in Fig. 6, the funnel plot was asymmetrical,
which meant a publication bias in our study; however, the most
recent studies have tended not to assess publication bias due to
the fact that the investigation of reporting and publication bias
in diagnostic accuracy studies has been shown to be problematic
(36,37). A possible reason is that numerous
studies are performed without study registration (36–38),
making it impossible for an exact assessment of publication and
reporting bias to be performed from registration.
Sensitivity analysis
In order to investigate the stability of the
meta-analysis, sensitivity analysis was performed from three
aspects. Firstly, one study at a time was excluded to assess the
effect of a single study on the meta-analysis. The results
suggested that the DOR was not notably affected following
sequential exclusion of each study in turn (Table IV). Secondly, the four studies
that did not use ELISA as a test method were removed; a decreased
pooled DOR (11.73; 95% CI, 6.58–20.92) was found, which suggested
that the test assay may have had an effect on the results. No
notable conclusion was drawn when the target population was limited
to Chinese patients in a similar manner to that already described
(pooled DOR, 16.29; 95% CI, 6.28–42.25). Statistical analysis was
performed using Meta Disc (version 1.4) software.
 | Table IVChanges in the DOR following
sequential exclusion of each study in turn. |
Table IV
Changes in the DOR following
sequential exclusion of each study in turn.
| First author, year
(ref.) of excluded study | Number of
studies | DOR | 95% CI |
|---|
| All studies
included (19–29) | 11 | 15.78 | 6.95–35.83 |
| Hu, 2010 (19) | 10 | 15.60 | 6.51–37.41 |
| Mao, 2010 (20) | 10 | 12.24 | 8.11–18.47 |
| Marrero, 2005
(21) | 10 | 16.01 | 6.49–39.44 |
| Morota, 2011
(22) | 10 | 16.15 | 6.70–38.92 |
| Shi, 2011 (23) | 10 | 14.34 | 5.99–34.33 |
| Tian,2011 (24) | 10 | 18.60 | 8.56–40.40 |
| Wang, 2009
(25) | 10 | 15.86 | 6.56–38.36 |
| Xu, 2011 (26) | 10 | 15.47 | 6.27–38.17 |
| Zhao, 2010
(27) | 10 | 16.73 | 6.87–40.72 |
| Wang, 2013
(28) | 10 | 16.17 | 6.61–39.53 |
| Hou, 2013 (29) | 10 | 16.51 | 6.86–39.71 |
Discussion
A total of 11 studies were analyzed to evaluate the
diagnostic accuracy of serum GP73 for HCC. The results demonstrated
that GP73 is a useful marker as an independent diagnostic tool for
HCC; however, multiple methodological limitations, a broad range of
diagnostic accuracy values and heterogeneity were found in the
included studies. Five of the studies reported that serum GP73 was
superior to AFP as a serum marker (22–24,29,30),
while the remaining six reported the opposite or had ambiguous
results.
The potential biomarker for HCC investigated in the
present study, serum GP73, is a 73-kDa transmembrane glycoprotein
composed of 400 amino acids that normally resides in the epithelial
cells of a range of human tissues (39). The presence of higher levels of
serum GP73 in patients with hepatitis-B-virus-related HCC was first
found by Block et al (13)
in 2005. The detection of GP73 in the serum was based on its
initial characterization as a resident Golgi membrane protein;
however, it has been shown that GP73 cycles to the cell membrane
for retrieval via a unique endosomal pathway (40). The results of such in vitro
studies have demonstrated that GP73 can transiently be found at the
plasma membrane, indicating a potential pathway for its release
into the circulation. The mechanism underlying the upregulation of
GP73 in HCC is yet to be elucidated, and further studies are
required to investigate whether serum GP73 levels are also altered
in patients with other types of solid tumor.
Western blotting, immunoblotting and ELISA are three
of the main methods used to assay GP73, all of which exhibit
certain disadvantages: The former two are semiquantitative and
labor-heavy, while ELISA elicits disappointing results. In seven
studies (25–30,32),
the use of ELISA was unsuccessful at finding a significant
elevation in serum GP73 levels in patients with HCC versus patients
with liver cirrhosis. It has been suggested that GP73-specific
serum autoantibodies may interfere with ELISA (10). Furthermore, several isoforms of
GP73 corresponding with different patterns or levels of
glycosylation have been found (41). Further investigation into whether
the measurement of an HCC-specific GP73 isoform would improve the
diagnostic accuracy is required.
Cancer comprises a diverse group of diseases that
exhibit considerable differences in their etiology and biology;
therefore, it is unlikely that a single biomarker would be able to
detect all the types of cancer associated with a particular organ
with sufficiently high specificity and sensitivity (11). The diagnostic value of GP73 in
combination with AFP for HCC has been reported in seven studies
(23,26–29,31,32),
and the results were improved compared with those for a single
marker.
Nine studies reported the diagnostic utility of
serum GP73 by the stage of chronic liver disease and the
conclusions were conflicting (22–24,26,28–32).
In general, the GP73 level showed an increasing trend with the
progression of liver disease. The results suggested that GP73 may
be used as a serum marker for the diagnosis of liver diseases and
for monitoring disease progression. It additionally appears that
serum levels of GP73 in patients with HCC are not consistently
affected by tumor size and differentiation, which may reflect the
potential origin of HCC from cancer stem cells. If this finding is
verified in further studies with large sample sizes, it may be
beneficial for the early detection of HCC among the at-risk
population.
The present study failed to find the reason for the
existing heterogeneity within the studies. The most important
factor contributing to this failure was that several of the studies
investigating diagnostic accuracy lacked information on key
elements of the study design and conduct. With incomplete and
inaccurate reporting, it is not possible to correctly identify
potential sources of bias and variability.
In conclusion, the present meta-analysis found that
GP73 is a valuable marker as an independent diagnostic tool for HCC
due to its high sensitivity and specificity. As such, GP73 may
improve the detection and treatment of one of the most common
global malignancies. Further studies are required to determine the
effect of the etiology of the disease on the GP73 signal strength,
the diagnostic accuracy of GP73 in detecting early HCC or cancer
recurrence and the value of a combination of GP73 and AFP.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270515).
References
|
1
|
Sherman M: Hepatocellular carcinoma:
epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirk GD, Lesi OA, Mendy M, et al: The
Gambia Liver Cancer Study: Infection with hepatitis B and C and the
risk of hepatocellular carcinoma in West Africa. Hepatology.
39:211–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benowitz S: Liver cancer biomarkers
struggling to succeed. J Natl Cancer Inst. 99:590–591. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jie L, Fan W, Weiqi D, et al: The
hippo-yes association protein pathway in liver cancer.
Gastroenterol Res Pract. 2013:1870702013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, He L, Dai W, et al: Salinomycin
inhibits proliferation and induces apoptosis of human
hepatocellular carcinoma cells in vitro and in vivo. PLoS One.
7:e506382012. View Article : Google Scholar
|
|
7
|
Dai W, Wang F, He L, et al: Genistein
inhibits hepatocellular carcinoma cell migration by reversing the
epithelial-mesenchymal transition: Partial mediation by the
transcription factor NFAT1. Mol Carcinog. Nov 14–2013.(Epub ahead
of print). View
Article : Google Scholar
|
|
8
|
Wu D, Wang F, Dai W, et al: The miR-146a
rs 2910164 G > C polymorphism increases the susceptibility to
digestive cancer in Chinese. Asian Pacific J Cancer Prev.
14:399–403. 2013. View Article : Google Scholar
|
|
9
|
Sherlock S: Viruses and hepatocellular
carcinoma. Gut. 35:828–832. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Yin X, Ying J and Zhang B: Golgi
protein 73 versus alpha-fetoprotein as a biomarker for
hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer.
12:172012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zoli M, Magalotti D, Bianchi G, et al:
Efficacy of a surveillance program for early detection of
hepatocellular carcinoma. Cancer. 78:977–985. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
el-Serag HB: Global epidemiology of
hepatocellular carcinoma. Clin Liver Dis. 5:87–107. 2001.
View Article : Google Scholar
|
|
13
|
Block TM, Comunale MA, Lowman M, et al:
Use of targeted glycoproteomics to identify serum glycoproteins
that correlate with liver cancer in woodchucks and humans. Proc
Natl Acad Sci USA. 102:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwegler EE, Cazares L, Steel LF, et al:
SELDI-TOF MS profiling of serum for detection of the progression of
chronic hepatitis C to hepatocellular carcinoma. Hepatology.
41:634–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iftikhar R, Kladney RD, Havlioglu N, et
al: Disease- and cell-specific expression of GP73 in human liver
disease. Am J Gastroenterol. 99:1087–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maitra A and Thuluvgth PJ: GP73 and liver
disease: a (Golgi)complex enigma. Am J Gastroenterol. 99:1096–1098.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruix J, Sherman M, Llovet JM, et al: EASL
Panel of Experts on HCC: Clinical management of hepatocellular
carcinoma. Conclusions of the Barcelona-2000 EASL conference
European Association for the Study of the Liver. J Hepatol.
35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu D, Wu SM, Lu J, et al: Rifaximin versus
nonabsorbable disaccharides for the treatment of hepatic
encephalopathy: a meta-analysis. Gastroenterol Res Pract.
2013:2369632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Lu J, Dai W, et al: Combination
therapy of ursodeoxycholic acid and corticosteroids for primary
biliary cirrhosis with features of autoimmune hepatitis: a
meta-analysis. Gastroenterol Res Pract. 2013:4907312013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whiting P, Rutjes AWS, Reitsma JB, et al:
The development of QUADAS: a tool for the quality assessment of
studies of diagnostic accuracy included in systematic reviews. BMC
Medical Res Methodol. 3:252003. View Article : Google Scholar
|
|
21
|
Özkan H, Erdal H, Tutkak H, et al:
Diagnostic and prognostic validity of Golgi protein 73 in
hepatocellular carcinoma. Digestion. 83:83–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu JS, Wu DW, Liang S and Miao XY: GP73, a
resident Golgi glycoprotein, is sensibility and specificity for
hepatocellular carcinoma of diagnosis in a hepatitis B-endemic
Asian population. Med Oncol. 27:339–345. 2010. View Article : Google Scholar
|
|
23
|
Mao Y, Yang H, Xu H, et al: Golgi protein
73 (GOLPH2) is a valuable serum marker for hepatocellular
carcinoma. Gut. 59:1687–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marrero JA, Romano PR, Nikolaeva O, et al:
GP73, a resident Golgi glycoprotein, is a novel serum marker for
hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morota K, Nakagawa M, Sekiya R, et al: A
comparative evaluation of Golgi protein-73, fucosylated hemopexin,
α-fetoprotein, and PIVKA-II in the serum of patients with chronic
hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab
Med. 49:711–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Y, Chen J, Li L, et al: A study of
diagnostic value of golgi protein GP73 and its genetic assay in
primary hepatic carcinoma. Technol Cancer Res Treat. 10:287–294.
2011.PubMed/NCBI
|
|
27
|
Tian L, Wang Y, Xu D, et al: Serological
AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic
diseases. Int J Cancer. 129:1923–1931. 2011. View Article : Google Scholar
|
|
28
|
Wang M, Long RE, Comunale MA, et al: Novel
fucosylated biomarkers for the early detection of hepatocellular
carcinoma. Cancer Epidemiol Biomarkers Prev. 18:1914–1921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu WF, Fei YM, Zhou JK, et al:
Significance of serum golgi protein 73 (GP73), alpha-fetoprotein
(AFP) and lectin-reactive alpha-fetoprotein (AFP-L3) expresssion in
primary hepatic carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du
Xue Za Zhi. 25:286–288. 2011.(In Chinese). PubMed/NCBI
|
|
30
|
Zhao XY, Li N, Ding HG and Jiang FF:
Detection and evaluation of serum GP73, a resident Golgi
glycoprotein, as a marker in diagnosis of hepatocellular carcinoma.
Zhonghua Zhong Liu Za Zhi. 32:943–945. 2010.(In Chinese).
|
|
31
|
Wang Y, Yang H, Xu H, et al: Golgi protein
73, not Glypican-3, may be a tumor marker complementary to
α-Fetoprotein for hepatocellular carcinoma diagnosis. J
Gastroenterol Hepatol. 29:597–602. 2014. View Article : Google Scholar
|
|
32
|
Hou SC, Xiao MB, Ni RZ, et al: Serum GP73
is complementary to AFP and GGT-II for the diagnosis of
hepatocellular carcinoma. Oncol Lett. 6:1152–1158. 2013.PubMed/NCBI
|
|
33
|
Whiting P, Harbord R and Kleijnen J: No
role for quality scores in systematic reviews of diagnostic
accuracy studies. BMC Med Res Methodol. 5:192005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deeks JJ: Systematic reviews in health
care: Systematic reviews of evaluations of diagnostic and screening
tests. BMJ. 323:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leeflang MM, Deeks JJ, Gatsonis C and
Bossuyt PM; Cochrane Diagnostic Test Accuracy Working Group.
Systematic reviews of diagnostic test accuracy. Ann Intern Med.
149:889–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song F, Khan KS, Dinnes J and Sutton AJ:
Asymmetric funnel plots and publication bias in meta-analyses of
diagnostic accuracy. Int J Epidemiol. 31:88–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kladney RD, Bulla GA, Guo L, et al: GP73,
a novel Golgi-localized protein upregulated by viral infection.
Gene. 249:53–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Puri S, Bachert C, Fimmel CJ and Linstedt
AD: Cycling of early Golgi proteins via the cell surface and
endosomes upon lumenal pH disruption. Traffic. 3:641–653. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Comunale MA, Mattu TS, Lowman MA, et al:
Comparative proteomic analysis of de-N-glycosylated serum from
hepatitis B carriers reveals polypeptides that correlate with
disease status. Proteomics. 4:826–838. 2004. View Article : Google Scholar : PubMed/NCBI
|