Introduction
Liver injury due to chemical damage or viral
infection often leads to liver fibrosis and liver failure, and this
may lead to an impairment of liver function. Liver transplantation
is the most effective treatment for liver cirrhosis, with the
ability to improve the quality of life and prognosis of patients
with liver cirrhosis. However, extensive clinical application of
the technique is limited by the lack of donor organ availability
(1). Thus, the investigation of
other treatments and therapies for cirrhosis is necessary. Bone
marrow mesenchymal stem cells (BMSCs) can differentiate into
hepatocyte-like cells, and support organ regeneration processes.
Moreover, BMSCs are safer than embryonic stem cells to use in
vivo due to their higher chromosomal stability and lower
tendency to form neoplasms in the recipient host. Therefore, BMSC
transplantation has become a novel therapeutic strategy for liver
injury (2–4). Certain clinical studies have
suggested that BMSC transplantation is an effective treatment for
patients with severe liver disease (5–7). As
a new treatment, certain issues remain to be resolved in the
application of BMSC transplantation in the treatment of liver
cirrhosis, such as the efficiencies of various transplantation
paths, the optimum cell counts of BMSC transplantation and the
timing of transplantation. In a previous study, Sun et al
(4) compared the efficiencies of
BMSC transplantation by the portal vein, abdominal cavity and
liver, and the results indicated that the best efficiency of BMSC
transplantation was obtained via the portal vein. Currently, BSMCs
are generally transplanted via the portal vein in patients with
liver diseases as the first-pass effect in the liver is much higher
than that when the transplantation is via other routes (8–10).
However, BSMC transplantation via the portal vein could transiently
increase venous pressure and create a venous embolism, which would
aggravate liver injury (11).
Moreover, puncture of the portal vein is a relatively difficult
procedure and not convenient to conduct in the clinic. Therefore,
it is important to identify effective, safe and convenient
administration methods for BMSC transplantation.
One convenient mode of administration of BMSC
transplantation is via a peripheral vein; however, the therapeutic
effect of BMSC transplantation via a peripheral vein is not clear.
Several studies have shown that the colonization and
differentiation of BMSCs in the organ are mainly induced by the
microenvironment in the injured organ, indicating that a peripheral
vein may be an alternative administration site for BMSC
transplantation (12–14). However, it is unclear whether BMSCs
transplanted via a peripheral vein colonize in the liver and exert
their functions properly. In this study, BMSCs were transplanted
via the portal vein and via a peripheral vein in rats with liver
cirrhosis, and the therapeutic effect of BMSC transplantation on
liver injury and liver fibrosis in the rats was studied. The
results were analyzed to determine whether BMSC transplantation via
a peripheral vein is an effective and convenient BMSC
administration route for liver cirrhosis.
Materials and methods
Materials
Animals
A total of 58 male Sprague-Dawley (SD) rats, 6–8
weeks of age, weighing 130–150 g were purchased from the Zoology
Section of Changzhi Medical College (Changzhi, China). The rats
were bred under specific pathogen-free conditions in the Zoology
Section of Changzhi Medical College.
Cells
293T cells were purchased from Aiyan Biological
Technology Co., Ltd. (Shanghai, China). The cells were cultured in
high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS). Cells were grown at 37°C under
5% CO2.
Main reagents and antibodies
DMEM, FBS and 0.25% trypsin were purchased from
Hyclone (Shanghai, China). Assay kits to measure alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and
albumin were purchased from Zhangjiang Biotechnology Co., Ltd.
(Shanghai, China). Percoll was purchased from Amersham Pharmacia
Biotech (Piscataway, NJ, USA). LentitopoLuc-green fluorescent
protein (GFP), was obtained from the Cancer Research Institute of
Central South University (Changsha, China) (11). The ViraPower plasmid system and
liposomes were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). Hematoxylin and eosin, and Masson stains were
purchased from Yansheng Biochemical Reagents Co., Ltd. (Shanghai,
China). Polyclonal goat anti-mouse albumin antibodies were
purchased from Abcam (Cambridge, UK). Radioimmunoassay kits used to
determine the levels of the liver fibrosis markers hyaluronic acid
(HA), laminin (LN) and procollagen type III (PC-III) were purchased
from Haiyan Medical Biotechnology Co., Ltd. (Shanghai, China).
Methods
Isolation and culture of rat
BMSCs
Rats were anesthetized by an intraperitoneal
injection of 10% hydral. The femurs and tibias were removed, and
the two terminal regions of these bones were cut and flushed with
DMEM to collect cells. The cell mixture was centrifuged at 150 × g
for 10 min, to prepare a single-cell suspension, and the
supernatant was discarded. The BMSCs were isolated with Percoll and
cultured in DMEM supplemented with FBS for 48 h. After removing the
cell suspension, adherent BMSCs were obtained. The BMSCs were grown
to passage 3 and were used in experiments once they reached 80%
confluence.
BMSC labeling
For labeling, 10 μg DNA of proteolipid protein 1
(RC225379; Amsbio, Abingdon, UK), 15 μg DNA of proteolipid protein
2 (MG224890; Amsbio), 7.5 μg DNA of vesicular stomatitis Indiana
virus G protein (HT-pack; Amsbio) and 20 μg DNA of LentitopoLuc-GFP
were added to serum-free DMEM. Then, 30 μl liposomes were added,
and the mixture was placed on ice for 20 min. The mixture of DNA
and liposomes was added to 293T cells (5×106). After 12
h, the medium was replaced with DMEM and the cells were cultured
for a further 72 h. The supernatant was collected to determine the
virus titer, and then the supernatant was filtered through a
0.45-μm filter and added to BMSCs, which were cultured for 48 h.
The transfection efficiency of GFP was observed under a fluorescent
microscope (FSX100; Olympus Corporation, Tokyo, Japan) (5).
Establishment of liver cirrhosis in
rats
To establish the liver cirrhosis rat model, 50:50
(v/v) CCl4 and vegetable oil mixture was injected
subcutaneously into the 58 adult male SD rats twice a week for 10
weeks. Three rats from each group were randomly selected and the
liver was stained with hematoxylin/eosin (H&E) and Masson
stains to confirm the establishment of liver cirrhosis.
H&E and Masson staining
In the H&E staining process, paraffin-embedded
liver sections (4 μm) were deparaffinized with xylene, dehydrated
in alcohol and washed. The sections were stained in hematoxylin
solution for 5 min and then washed, followed by differentiation in
1% acid alcohol for 30 sec, and washing for 10 min. The sections
were then counterstained with eosin-phloxine solution for 3 min,
washed and dehydrated. Finally, the slides were cleared in xylene
and mounted with a xylene-based mounting medium.
For Masson staining, the slides were deparaffinized,
dehydrated, and then washed and stained in Weigert’s iron
hematoxylin for 5 min. Next, the slides were washed, stained in
Biebrich scarlet acid fuchsin solution for 5 min, washed and
differentiated in 1% phosphomolybdic-phosphotungstic acid solution
for 5 min. The sections were transferred to aniline blue solution
and stained for 5 min, and then differentiated in 1% acetic acid
solution for 1 min. Finally, the sections were washed, dehydrated,
cleared in xylene and mounted with resin mounting medium.
Treatments
Overall, 48 rats with cirrhosis (7 rats died during
model establishment) were divided into four equal groups of 12 rats
per group to receive injection of BMSCs or PBS via the portal vein
and tail vein, respectively.
Transplantation of BMSCs
In each group, the BMSCs were resuspended in 0.1
mol/l phosphate-buffered saline (PBS) at a concentration of
107 cells/ml, and each rat was injected with 500 μl
GFP-labeled BMSCs or the same volume of PBS. For the portal vein
groups, rats were anesthetized with 10% chloral hydrate and the
abdominal cavity was opened to expose the portal vein, into which
BMSCs or PBS were injected. After removing the needle, pressure
hemostasis was applied for 1 min.
Blood and tissue collection and
processing
Tail vein blood samples were collected shortly prior
to transplantation and used to measure serum ALT, AST, albumin, HA,
LN and PC-III levels. Two weeks after transplantation, three rats
in each group were sacrificed, and the liver was removed, fixed and
sectioned. Liver samples were observed under a fluorescence
microscope (Olympus FSX100) to determine the distribution of GFP in
the liver. At the same time, blood samples were taken to measure
serum ALT, AST and albumin levels. At six weeks after
transplantation, all the remaining rats in each group were
sacrificed. Cardiac puncture blood samples were obtained to measure
ALT, AST, albumin, HA, LN and PC-III levels. The liver was fixed,
sectioned and albumin expression was determined by
immunohistochemistry.
Immunohistochemistry
The rat liver tissue sections were deparaffinized,
dehydrated, washed and subjected to citrate antigen retrieval.
After incubation in hydrogen peroxide, the sections were blocked in
serum-free blocking medium, and then incubated with anti-albumin
antibody (1:1,000 dilution) overnight at 37°C. The sections were
then washed and incubated with 200 μl rabbit anti-goat
immunoglobulin G (H&L) horseradish peroxidase-conjugated
secondary antibody (1:400 dilution; cat. no. ab6741, Abcam) for 2 h
at room temperature. Following this, the sections were washed and
incubated with avidin-biotin complex [UltraSensitive™ SAP
(mouse/rabbit) IHC kit (Fuzhou Maixin Biotechnology Development
Co., Ltd., Fujian, China)] at room temperature, washed and stained
with 3,3′-diaminobenzidine (DAB) Detection (Streptavidin/Biotin)
kit (Fuzhou Maixin Biotechnology Development Co., Ltd.). Finally,
the slides were dehydrated, cleared with xylene and mounted with
permanent mounting medium.
The relative expression of albumin was determined in
terms of the degree of staining and the number of positive cells.
The degree of staining was classified as follows: 0, no staining;
1, light orange-stained cells; 2, yellow-stained cells; and 3,
brown-stained cells. The number of stained cells was quantified as
follows: 0, <5% positive cells; 1, 5–25% positive cells; 2,
26–50% positive cells; 3, 51–75% positive cells; and 4, >75%
positive cells. The sections were graded by adding together the
scores for degree and extent of staining, and the
immunohistochemical data were classified as follows: 0, negative
staining (−); 1 or 2, weakly positive (+); 3–5, moderately positive
(++); 6 or 7, strongly positive (+++).
Data analysis
The statistical software SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA) was applied to analyze the data. Data of
ALT, AST, albumin, HA, LN and PC-III are shown as the means ±
standard deviations. Groups were compared using repeated measures
analysis of variance, and the albumin immunohistochemistry data
were analyzed by nonparametric test. Values of P<0.05 are
considered statistically significant.
Results
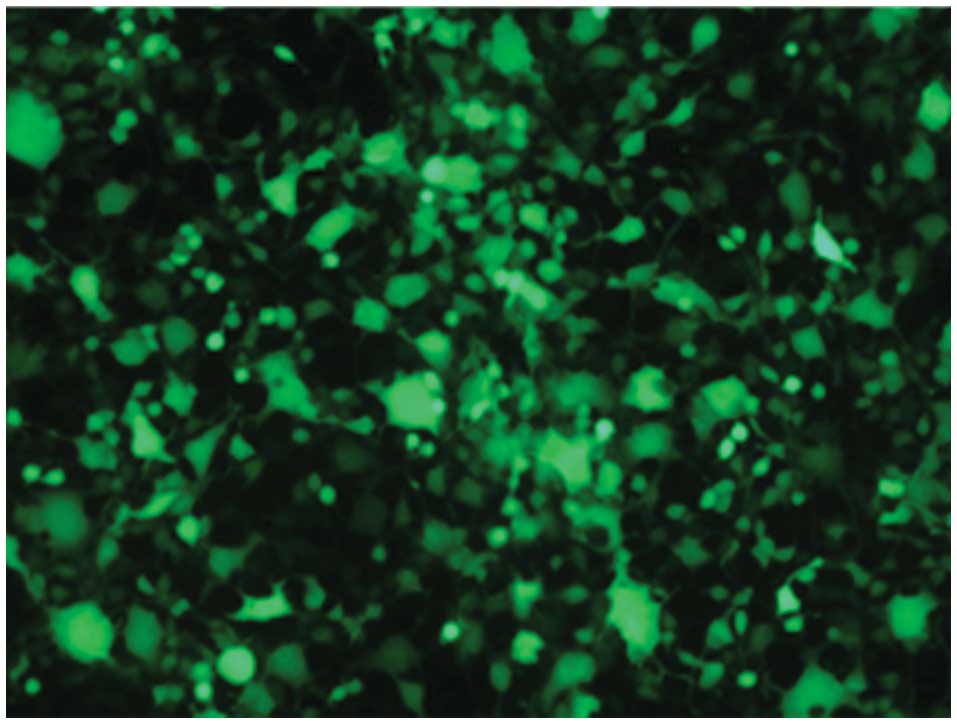
Efficiency of GFP transfection in 293T
cells
The 293T cells were co-transfected with three
plasmids and GFP. At 48 h after transfection, the efficiency of the
transfection was observed under a fluorescence microscope (Fig. 1). All the cells examined expressed
GFP, and the virus titer was 2.23×109.
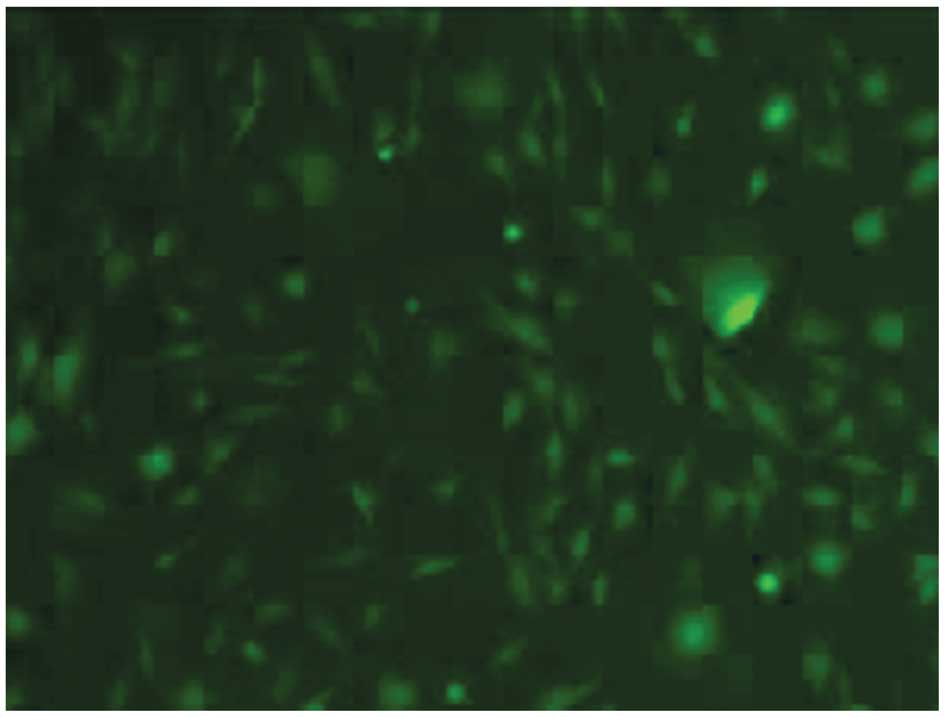
Labeling of BMSCs
BMSCs were cultured with medium collected from 293T
cells at 48 and 72 h after transfection with GFP. After 48 h of
culture, GFP-positive BMSCs were observed by fluorescence
microscopy, confirming that the BMSCs were labeled with GFP
(Fig. 2).
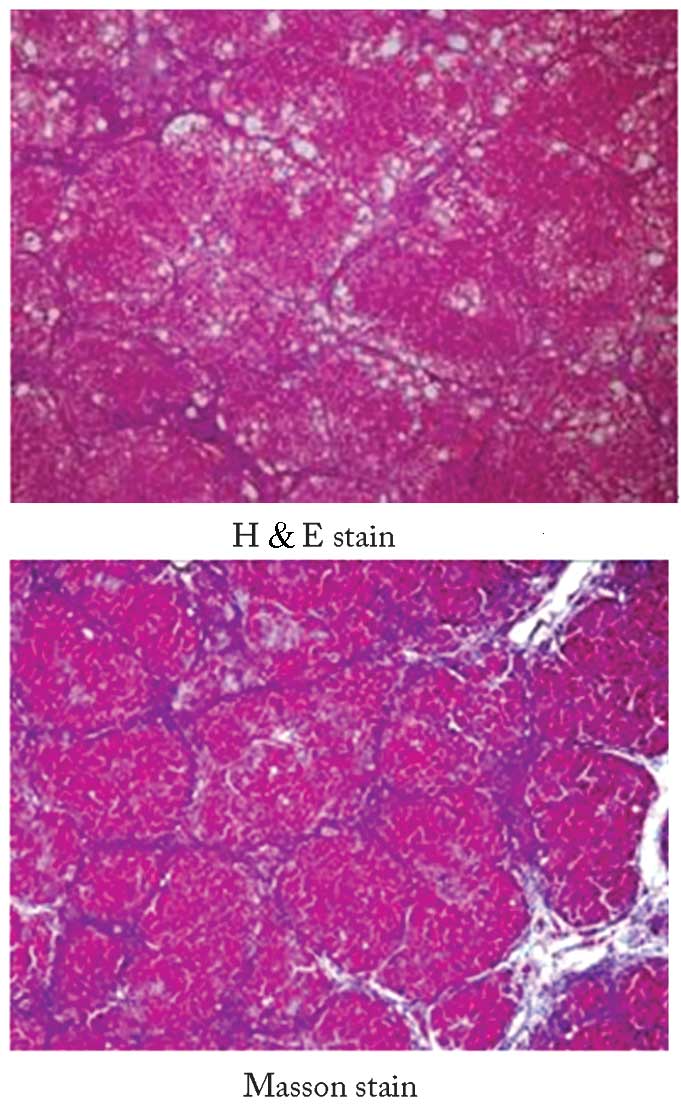
Characteristics of rats with liver
cirrhosis
Following the subcutaneous injection of 50:50 (v/v)
CCl4/vegetable oil mixture into the rats, they developed
anorexia and were emaciated. Their urine was dark yellow in color.
After 10 weeks of CCl4 administration, seven rats died.
Using a random-digits table, three rats were randomly selected for
liver staining with H&E and Masson stains. In these rats, the
formation of hepatic fibrosis and pseudolobules, enlargement of the
hepatocytes, and focal necrosis were observed, which confirmed the
successful establishment of a rat model of liver cirrhosis
(Fig. 3).
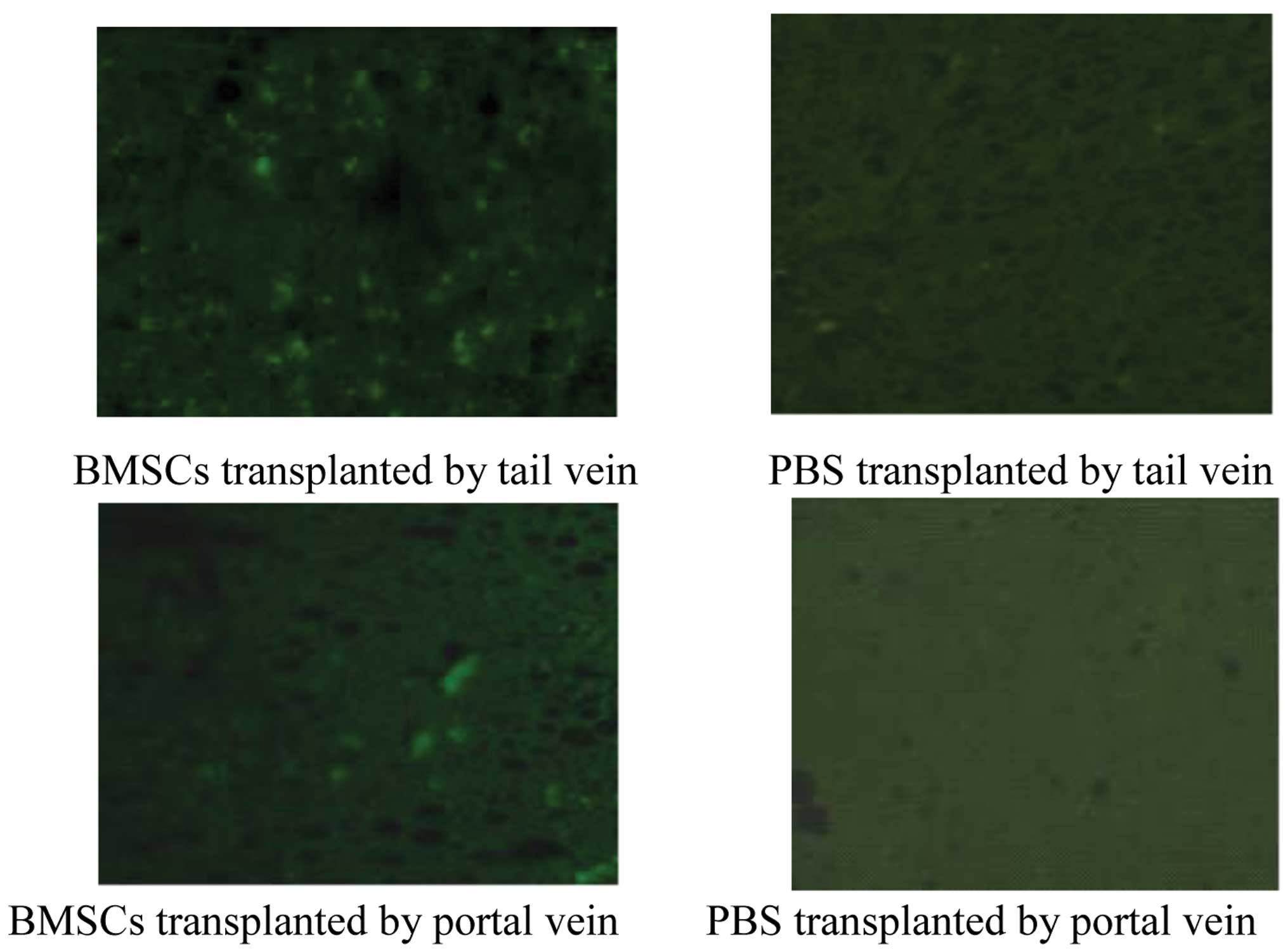
Hepatic distribution of BMSCs in rats
with liver cirrhosis
Two weeks after transplantation, three rats from
each group were sacrificed, and the hepatic distribution of BMSCs
was determined. The expression of GFP was visible in the livers of
the rats transplanted with BMSCs via the portal vein or via tail
vein; however, no GFP expression was observed in the livers of the
rats injected with PBS (Fig. 4).
These results indicate that BMSCs transplanted via the portal vein
or tail vein are able to colonize the liver.
Effects of BMSC transplantation on
markers of liver injury
The changes in ALT, AST and albumin levels were
measured prior to and at two and six weeks after BMSC
transplantation via the portal vein or tail vein. Serum ALT levels
decreased significantly at two and six weeks after BMSC
transplantation via both routes (P<0.05; Table I). AST and albumin levels measured
prior to and at two weeks after BMSC transplantation were not
significantly different in each group (P>0.05; Tables II and III). However, the serum AST level was
significantly lower and the serum albumin level was significantly
higher at six weeks after transplantation compared with the levels
measured prior to BMSC transplantation (both P<0.05).
 | Table IEffects of BMSC transplantation on
serum ALT levels (mean ± standard deviation). |
Table I
Effects of BMSC transplantation on
serum ALT levels (mean ± standard deviation).
| Serum ALT at
different time points (U/l) |
|---|
|
|
|---|
| Groups | Before
transplantation | Two weeks after
transplantation | Six weeks after
transplantation |
|---|
| BMSC transplantation
via tail vein | 148.3±15.6 | 127.3±13.6a,b | 84.7±9.9a,b |
| PBS injection via
tail vein | 142.4±14.4 | 139.3±15.3 | 128.1±13.4 |
| BMSC transplantation
via portal vein | 157.6±18.2 | 130.5±14.8a,b | 92.4±11.7a,b |
| PBS injection via
portal vein | 160.7±14.6 | 155.1±12.8 | 143.2±15.2 |
 | Table IIEffects of BMSC transplantation on
serum AST levels (mean ± standard deviation). |
Table II
Effects of BMSC transplantation on
serum AST levels (mean ± standard deviation).
| Serum AST at
different time points (U/l) |
|---|
|
|
|---|
| Groups | Before
transplantation | Two weeks after
transplantation | Six weeks after
transplantation |
|---|
| BMSC transplantation
via tail vein | 224.5±20.1 | 201.6±19.3 | 165.4±16.5a,b |
| PBS injection via
tail vein | 218.7±22.6 | 209.8±21.8 | 202.4±19.7 |
| BMSC transplantation
via portal vein | 228.4±25.2 | 206.3±21.5 | 170.6±20.5a,b |
| PBS injection via
portal vein | 222.3±22.3 | 215.2±21.8 | 211.3±20.6 |
 | Table IIIEffects of BMSC transplantation on
serum albumin levels (mean ± standard deviation). |
Table III
Effects of BMSC transplantation on
serum albumin levels (mean ± standard deviation).
| Serum albumin at
different time points (g/l) |
|---|
|
|
|---|
| Groups | Before
transplantation | Two weeks after
transplantation | Six weeks after
transplantation |
|---|
| BMSC transplantation
via tail vein | 27.2±3.4 | 28.4±2.9 | 32.5±2.5a,b |
| PBS injection via
tail vein | 26.3±3.0 | 26.9±2.2 | 27.5±2.4 |
| BMSC transplantation
via portal vein | 26.2±2.6 | 27.7±2.5 | 31.9±2.0a,b |
| PBS injection via
portal vein | 28.1±3.5 | 28.8±2.7 | 29.1±2.6 |
Serum ALT levels in rats at two and six weeks after
BMSC transplantation via the portal vein or via tail vein were
significantly lower than those in rats injected with PBS via the
portal vein or via tail vein (P<0.05). Furthermore, the serum
level of AST was significantly lower and the serum level of albumin
was significantly higher at six weeks in the rats that had
undergone BMSC transplantation compared with rats injected with PBS
(P<0.05). There were no significant differences in serum ALT,
AST or albumin levels between rats transplanted with BMSCs via the
portal vein or via tail vein either prior to or following BMSC
transplantation (all P>0.05).
The serum HA, LN and PC-III levels in the rats six
weeks after BMSC transplantation via the portal vein or tail vein
were significantly decreased compared with those measured prior to
BMSC transplantation (all P<0.05; Table IV). The levels in BMSC
transplanted rats were also significantly lower than those in rats
injected with PBS. However, there were no significant differences
between rats transplanted with BMSCs via the portal vein or tail
vein (all P>0.05). There were also no differences in the levels
of HA, LN or PC-III at six weeks after the injection of PBS
compared with those measured prior to injection.
 | Table IVEffects of BMSC transplantation on
serum HA, LN and PC-III levels of rats (mean ± standard
deviation). |
Table IV
Effects of BMSC transplantation on
serum HA, LN and PC-III levels of rats (mean ± standard
deviation).
| HA (ng/ml) | LN (ng/ml) | PC-III (ng/ml) |
|---|
|
|
|
|
|---|
| Groups | Before
transplantation | Six weeks after
transplantation | Before
transplantation | Six weeks after
transplantation | Before
transplantation | Six weeks after
transplantation |
|---|
| BMSC
transplantation via tail vein | 283.49±15.41 |
198.44±14.92a,b | 97.45±7.89 | 62.41±6.98a,b | 197.42±16.09 |
137.43±12.69a,b |
| PBS injection via
tail vein | 272.33±13.94 | 258.94±12.23 | 83.90±8.29 | 73.19±5.63 | 206.91±14.22 | 192.95±12.82 |
| BMSC
transplantation via portal vein | 265.27±12.15 |
188.73±13.05a,b | 101.57±10.56 | 70.57±6.59a,b | 189.79±12.53 |
129.17±10.54a,b |
| PBS injection via
portal vein | 280.61±14.35 | 264.48±12.27 | 89.58±9.73 | 76.38±8.73 | 190.48±13.76 | 184.18±13.79 |
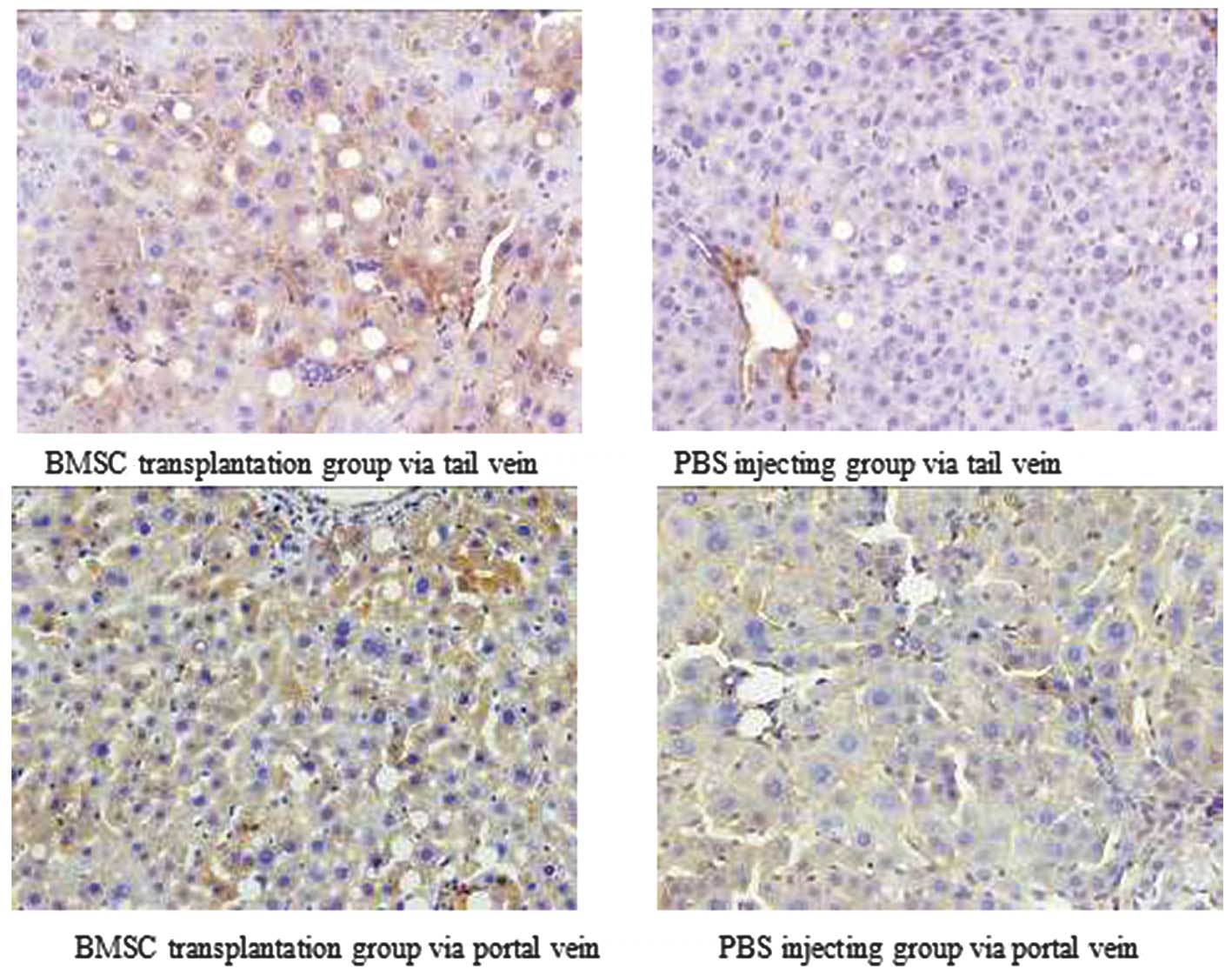
Effects of BMSC transplantation on
hepatic albumin expression
Immunohistochemistry was used to assess hepatic
albumin expression in the four experimental groups. Albumin protein
expression in the cytoplasm was represented by the accumulation of
orange or brown particles (Fig.
5). Nonparametric statistical tests revealed a significant
difference in hepatic albumin expression between the two BMSC
transplanted groups compared with their matched control groups
(P<0.05). However, there was no difference in albumin expression
between rats transplanted with BMSCs via the portal vein or tail
vein (P>0.05; Table V).
 | Table VEffects of BMSC transplantation on
albumin expression in rat liver tissue. |
Table V
Effects of BMSC transplantation on
albumin expression in rat liver tissue.
| Group | − | + | ++ | +++ |
|---|
| BMSC
transplantation via tail veina | 0 | 1 | 4 | 6 |
| PBS injection via
tail veinb | 0 | 4 | 5 | 1 |
| BMSC
transplantation via portal veina | 0 | 2 | 3 | 5 |
| PBS injection via
portal veind | 0 | 3 | 7 | 0 |
Discussion
Liver cirrhosis is a common disease in China.
Patients with aggravation of liver injury often show serious
complications, such as liver failure and hepatic encephalopathy
(15). The mortality rate
associated with this disease is extremely high and there are no
effective treatment methods for liver cirrhosis, with the exception
of liver transplantation, which is limited by the availability of
donor livers. BMSCs have the capacity of self-renewal and can
differentiate into multiple cell types. In vitro studies
have shown that BMSCs can be induced to differentiate into
hepatocyte-like cells following exposure to hepatocyte growth
factor, fibroblast growth factor-4 and epidermal growth factor
(16–20), offering a novel strategy for the
treatment for terminal liver disease. Therefore, BMSC
transplantation is increasingly being used in the treatment of
patients with severe liver disease, and much progress has been made
in the development of this therapeutic approach (21). BMSC transplantation via the portal
vein is likely to become a universal method due to the first-pass
effect of the portal circulation. However, puncture of the portal
vein is relatively difficult and may increase the risk of liver
injury. Our previous, unpublished research revealed that the
colonization of BMSCs in the liver is strongly associated with
chemotactic processes underlying organ injury. Therefore, the
identification of alternative transplantation routes is essential
to avoid these issues. Consequently, the present study sought to
investigate whether BMSCs transplanted via a peripheral vein were
capable of colonizing in the liver and were functional.
In this study, GFP-labeled BMSCs were transplanted
or PBS injected via the portal vein or tail vein into rats with
experimental liver cirrhosis. The colonization of BMSCs in the
liver and the effects of BMSC transplantation on markers of hepatic
injury and fibrosis were examined, and these parameters were
compared between the two routes of transplantation. Extensive green
fluorescence corresponding to GFP-labeled BMSCs was observed in the
livers of rats at two weeks after BMSC transplantation via the
portal vein and via the tail vein, indicating that the peripherally
transplanted BMSCs were able to colonize the injured liver, in a
similar manner to that achieved by portal vein injection. This
colonization may be at least partly associated with chemotactic
events in liver injury (22). The
effects of both routes of BMSC transplantation on markers of liver
injury (i.e., ALT, AST, albumin, HA, LN and PC-III) were then
compared. Serum ALT levels improved significantly at two and six
weeks after BMSC transplantation in both groups as compared with
levels measured prior to transplantation. Serum AST and albumin
levels measured at two weeks after BMSC transplantation were not
significantly different compared with the levels measured before
transplantation. However, at six weeks after transplantation, serum
AST and albumin levels had improved significantly in both groups;
they decreased and increased, respectively. Serum ALT, AST and
albumin levels were significantly better at six weeks after BMSC
transplantation compared with those in the PBS-injected control
groups. Serum HA, LN and PC-III levels were significantly lower at
six weeks after BMSC transplantation via the portal vein or tail
vein compared with levels measured prior to BMSC transplantation or
in the control groups. Immunohistochemical analysis of liver
sections showed that hepatic albumin expression was markedly
enhanced at six weeks after BMSC transplantation, consistent with
increases in serum albumin levels, indicating that BMSCs
transplanted via the portal vein or tail vein can improve liver
function and reduce liver fibrosis to a similar extent in rats with
liver cirrhosis. Notably, there were no differences in serum ALT,
AST, albumin, HA, LN or PC-III levels between the two groups of
rats. These effects on liver function may be achieved by the
transplanted BMSCs colonizing in the liver and then differentiating
into hepatocyte-like cells in response to the liver’s
microenvironment. The hepatocyte-like cells may suppress
inflammatory activity by secreting growth factors and cytokines,
and decrease hepatocyte apoptosis (23–27).
These results indicate that BMSCs transplanted via
the portal vein or tail vein can colonize in the liver to improve
liver function and reduce liver fibrosis. Notably, there were no
differences in these effects of BMSCs transplantation between the
two administration methods. Therefore, BMSC transplantation via a
peripheral vein is indicated to be an effective, safe and practical
method for treating liver injury.
Acknowledgements
This study was supported in part by a Hunan
Provincial Health Bureau Research grant (B2009-043).
References
|
1
|
Manns MP: Liver cirrhosis, transplantation
and organ shortage. Dtsch Arztebl Int. 110:83–84. 2013.PubMed/NCBI
|
|
2
|
Margini C, Vukotic P, Brodosi L, et al:
Bone marrow derived stem cells for the treatment of end-stage liver
disease. World J Gastroenterol. 21:9098–9105. 2014.
|
|
3
|
Yuan S, Jiang T, Zheng R, et al: Effect of
bone marrow mesenchymal stem cell transplantation on acute hepatic
failure in rats. Exp Ther Med. 8:1150–1158. 2014.PubMed/NCBI
|
|
4
|
Sun L, Fan X, Zhang L, et al: Bone
mesenchymal stem cell transplantation via four routes for the
treatment of acute liver failure in rats. Int J Mol Med.
34:987–996. 2014.PubMed/NCBI
|
|
5
|
Lau GK, Suri D, Liang R, et al: Resolution
of chronic hepatitis B and anti-HBs seroconversion in humans by
adoptive transfer of immunity to hepatitis B core antigen.
Gastroenterology. 122:614–624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Campli C, Piscaglia AC, Giuliante S, et
al: No evidence of hematopoietic stem cell mobilization in patients
submitted to hepatectomy or in patients with acute on chronic liver
failure. Transplant Proc. 97:2563–2566. 2005. View Article : Google Scholar
|
|
7
|
Gaia S, Smedile A, Omedè P, et al:
Feasibility and safety of G-CSF administration to induce bone
marrow-derived cells mobilization in patients with end stage liver
disease. J Hepatol. 45:13–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang L, Ma T, Chen W, et al: Therapeutic
potential and related signal pathway of adipose-derived stem cell
transplantation for rat liver injury. Hepatol Res. 39:822–832.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasbarrini A, Rapaccini GL, Rutella S, et
al: Rescue therapy by portal infusion of autologous stem cells in a
case of drug-induced hepatitis. Dig Liver Dis. 39:878–882. 2007.
View Article : Google Scholar
|
|
10
|
Jin SZ, Han MZ and Qu B: Advances in stem
cell transplantation for liver injury. Shi Jie Hua Ren Xiao Hua Za
Zhi. 15:3320–3323. 2007.(In Chinese).
|
|
11
|
Xiang J, Tang J, Song C, et al:
Mesenchymal stem cells as a gene therapy carrier for treatment of
fibrosarcoma. Cytotherapy. 11:516–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu N, Zhou DF, Qi JL, et al: Effect of
ligustrazine on the expression of bFGF in bone marrow stromal cells
of mice after BMT. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
14:1004–1007. 2006.(In Chinese). PubMed/NCBI
|
|
13
|
Li XH, Fu YH, Liu ZY, et al: Efficacy
comparison between transplanting microenvironmental induced and
non-induced bone marrow mesenchymal stem cells in ischemic rat
hearts. Zhonghua Xin Xue Guan Bing Za Zhi. 37:680–684. 2009.(In
Chinese). PubMed/NCBI
|
|
14
|
Li T, Zhu J, Ma K, et al: Autologous bone
marrow-derived mesenchymal stem cell transplantation promotes liver
regeneration after portal vein embolization in cirrhotic rats. J
Surg Res. 184:1161–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Longo DL: Chapter 308 Cirrhosis and Its
Complications. Harrison’s principles of internal medicine. 18th ed.
McGraw-Hill; New York: pp. 394–399. 2012
|
|
16
|
Sellamuthu S, Manikandan R, Thiagarajan R,
et al: In vitro trans-differentiation of human umbilical cord
derived hematopoietic stem cells into hepatocyte like cells using
combination of growth factors for cell based therapy.
Cytotechnolog. 63:259–268. 2011. View Article : Google Scholar
|
|
17
|
Yagi K, Kojima M, Oyagi S, et al:
Application of mesenchymal stem cells to liver regenerative
medicine. Yakugaku Zasshi. 128:3–9. 2008.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang PP, Wang JH, Yan ZP, et al:
Expression of hepatocyte-like phenotypes in bone marrow stromal
cells after HGF induction. Biochem Biophys Res Commun. 320:712–716.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin N, Lin J, Bo L, et al: Differentiation
of bone marrow-derived mesenchymal BMSCs into hepatocyte-like cells
in an alginate scaffold. Cell Prolif. 43:427–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Tao R, Wu W, et al: 3D PLGA
scaffolds improve differentiation and function of bone marrow
mesenchymal stem cell-derived hepatocytes. Stem Cells Dev.
19:1427–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan XN, Shen JK, Zhuang YP, et al:
Autologous bone marrow stem cell transplantation for treatment
terminal liver diseases. Nan Fang Yi Ke Da Xue Xue Bao.
28:1207–1209. 2008.(In Chinese). PubMed/NCBI
|
|
22
|
Zhou B, Shan H, Li D, et al: MR tracking
of magnetically labeled mesenchymal stem cell in rats with liver
fibrosis. Magn Reson Imaging. 28:394–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puglisi MA, Tesori V, Lattanzi W, et al:
Therapeutic implications of mesenchymal stem cell in liver injury.
J Biomed Biotechnol. 2011:8605782011. View Article : Google Scholar
|
|
24
|
Dalakas E, Newsome PN, Boyle S, et al:
Bone marrow stem cell contribute to alcohol liver fibrosis in
humans. Stem Cells Dev. 19:1417–1425. 2010. View Article : Google Scholar
|
|
25
|
Zheng JF and Liang LJ: Intra-portal
transplantation of bone marrow stromal cells ameliorates liver
fibrosis in mice. Hepatobiliary Pancreat Dis Int. 7:264–270.
2008.PubMed/NCBI
|
|
26
|
Shi L, Li G, Wang J, et al: Bone marrow
stromal cells control the growth of hepatic stellate cells in
vitro. Dig Dis Sci. 53:2969–2974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi LJ, Li SX, Sun B, et al: Effects of
bone marrow mesenchymal stem cells on the proliferation of
hepatocytes and cirrhotic fat-storing cells in vitro. Zhonghua Gan
Zang Bing Za Zhi. 15:681–684. 2007.(In Chinese). PubMed/NCBI
|