Introduction
Alzheimer’s disease (AD) is a neurodegenerative
brain disorder and is the most common cause of dementia. AD is
characterized by Aβ plaque accumulation, intracellular tangles,
neuronal loss in selective brain regions and a wide range of
challenging behavioral disturbances, which may ultimately result in
fatality (1).
Previous studies have indicated that iron is
involved in the progression of AD (2), and that altered iron homeostasis may
be an important factor in the pathogenesis of the disease (3). However, the underlying mechanisms
resulting in the abnormal iron load in the AD brain remain unclear.
It has been hypothesized that the brain iron load may be affected
by the altered expression of certain brain iron
metabolism-associated proteins, including divalent metal
transporter 1 (DMT1) and ferroportin 1 (FPN1) (4).
DMT1, also known as natural resistance-associated
macrophage protein 2 (Nramp2) or divalent cation transporter 1
(DCT1), is a proton-coupled metal ion transport protein, and was
the first transmembrane iron transporter to be identified in
mammals (5). DMT1 is a widely
expressed protein, with 12 putative transmembrane-spanning domains,
and is responsible for the uptake of a broad range of divalent
metal ions (6), including
Fe2+, Zn2+, Mn2+, Co2+,
Cd2+, Cu2+, Ni2+ and
Pb2+ (7–9). The four isoforms of DMT1 are
distinguished through their mRNA transcripts, which vary at their
5′-UTR and 3′-UTR. Two of these four transcripts contain an IRE at
the 3′-end (10,11). Thus, the carboxy-terminal DMT1
protein isoforms are designated -IRE and -nonIRE. FPN1 is the sole
exporter of iron and is responsible for iron absorption in the
intestines, recycling of erythrocyte iron by macrophages and
maternal delivery of iron to the fetus (12,13).
Deferoxamine (DFO) is an iron chelator that
significantly alleviates the symptoms of patients with AD,
resulting in a notable neuroprotective effect (14,15).
However, chemical drugs may exhibit adverse effects, including oral
side effects, and are costly, suggesting that research into
alternative therapies is required. The use of the active components
of Epimedium, Astragalus and Radix Puerariae
may circumvent these shortcomings. Furthermore, these compounds may
scavenge free radicals, reduce inflammation, adjust multiple
viscera functions (16) and reduce
brain iron overload (17,18).
Therefore, the present study aimed to investigate
the potential role of the active components of Epimedium,
Astragalus and Radix Puerariae on brain iron load in AD.
An APP/PS1 transgenic mouse model of AD was established and treated
with a mixture of the active component compounds. The Morris water
maze test was used to evaluate whether the active component
treatment was able to attenuate the cognitive deficits of AD in the
mouse model. Following behavioral testing, the Aβ plaque
accumulation and brain iron load in the mouse hippocampus were
determined. Furthermore, the expression levels of DMT1 and FPN1
were examined to clarify the molecular mechanisms underlying the
abnormal brain iron load in AD.
Materials and methods
Animals and treatments
The present study was approved by the Ethics
Committee of Chengde Medical University (Chengde, China). In total,
30 male APPswe/PS1ΔE9 (APP/PS1) mice and ten
C57BL/6J (C57) mice (both obtained from Beijing HFK Bioscience Co.,
Ltd., Beijing, China) were used in the present study. The APP/PS1
transgenic mouse model of AD overexpresses the Swedish
(K594M/N595L) mutation of APP, with presenilin 1 (PS1) deleted in
exon 9 and a C57 genetic background. The APP/PS1 mice were
genotyped using polymerase chain reaction (PCR). The mice were
housed under 12-h light/dark cycle conditions and were fed and
watered regularly. Six-month-old APP/PS1 transgenic mice were
divided at random into three groups. The AD model group contained
APP/PS1 mice that received no treatment. The active component group
consisted of APP/PS1 transgenic mice that received the active
components of Astragalus, Radix Puerariae and
Epimedium (120, 80 and 80 mg/kg, respectively), which was
administered via oral gavage. The DFO group received 30 mg/kg DFO
and was used as a positive control. In addition to the these three
groups, a fourth (C57) group contained ten male C57 mice and was
included as a normal control.
Preparation of the active component
treatment
The treatment comprised of the active components of
three Chinese medicinal herbs, Epimedium, Astragalus
and Radix Puerariae, including icariin, astragaloside IV and
puerarin, respectively (Fig. 1).
These substances were purchased from Nanjing Zelang Medical
Technology Co., Ltd (Nanjing, China) and were >98% pure.
Icariin, astragaloside IV and puerarin were dissolved or suspended
in distilled water at a ratio of 3:2:2.
Morris water maze
Behavioral examinations were conducted in a Morris
water maze at week 8 of drug treatment, as described in a previous
study (19). Briefly, a circular
black pool (diameter, 1.2 m; depth, 55 cm) was filled with water to
a depth of 30 cm at 22°C. A clear circular platform (diameter, 10
cm) was submerged 2 cm underwater in the northeast quadrant of the
pool. Each mouse underwent four trials per day for six consecutive
days. During the place navigation trial, mice were placed randomly
into the pool facing the wall individually from four preset
starting points, and were allowed to swim for a maximum of 120 sec
or until they located the platform. On the sixth day, the spatial
probe trial was conducted, in which the platform was removed from
the pool and the mice were allowed to swim for 120 sec. The total
swim time (escape latency; sec); the number of times the animal
crossed the previous location of the platform (platform-crossing);
the time that the animal spent in the quadrant where the platform
in (time spent in the target quadrant; sec); and the average
swimming velocity (m/s) were recorded using a video tracking system
(SLY-WMS Morris Water Maze System; Beijing Sunny Instruments Co.,
Ltd., Beijing, China). The scores of the animal behavior when
searching for the platform (search strategies score) were recorded
by a researcher blind to the treatment of the mouse. The search
strategy categories used were direct swim, tendency search, random
search and circle search, which received a score of 1, 2, 3 and 4,
respectively.
Tissue preparation
Following the Morris water maze experiments, the
mice were anesthetized with 50 mg/kg sodium pentobarbital (Tianjin
Fu Chen Chemical Reagents Factory, Tianjin, China) administered
intraperitoneally, then euthanized by decapitation. The brains were
removed rapidly and divided into hemispheres on an ice-cooled
board. The hippocampus and cerebral cortex were dissected from one
hemisphere and stored at −80°C for western blot analysis. The
remaining hemisphere was fixed in 4% paraformaldehyde (Tianjin Fu
Chen Chemical Reagents Factory) in phosphate-buffered saline (PBS;
Tianjin Fu Chen Chemical Reagents Factory) at 4°C overnight. The
hemisphere was then embedded in paraffin (Tianjin Fu Chen Chemical
Reagents Factory), cut into 5-μm sections and stored at room
temperature until required for morphological analysis.
Immunohistochemistry (IHC) and Aβ load
measurement
Standard avidin-biotin complex IHC staining was
performed to analyze the distribution of Aβ plaques in the APP/PS1
mouse brain. Briefly, paraffin sections were dewaxed, rehydrated
and treated in 0.1 M Tris-hydrogen chloride (HCl) buffer (pH 7.4)
containing 3% hydrogen peroxide (H2O2;
Tianjin Fu Chen Chemical Reagents Factory) for 10 min to reduce
endogenous peroxidase activity. Following washing with
Tris-buffered saline, the sections were boiled in citric acid
buffer (Tianjin Fu Chen Chemical Reagents Factory) for 3 min at 800
W in a microwave oven. The sections were then rinsed in running
water, treated with 5% bovine serum albumin (Biotin-Streptavidin
HRP Detection system, ZSGB-BIO, Beijing, China) for 30 min, and
subsequently incubated overnight with monoclonal mouse anti-human
Aβ antibody (1:500, #A5213; Sigma-Aldrich, St. Louis, MO, USA) at
4°C. The sections were rinsed in running water and subsequently
incubated with biotinylated goat anti-mouse immunoglobulin G (IgG)
(1:200; Biotin-Streptavidin HRP Detection system) for 1 h and with
streptavidin peroxidase (Biotin-Streptavidin HRP Detection system)
for a further 1 h at room temperature. Following rinsing, the
sections were stained with 0.025% diaminobenzidine (DAB;
Sigma-Aldrich) for 1 min. The stained sections were dehydrated,
cleared, covered with neutral balsam and examined under a light
microscope equipped with a digital camera (BH-2; Olympus
Corporation, Tokyo, Japan). Control group mice were treated with
identical solutions but without primary antibody, followed by all
subsequent incubations as described above.
Quantitative image analysis was performed
for Aβ IHC with micrographs of five sections per brain
The number of Aβ-positive plaques in the cortex and
hippocampus was counted, and comparisons between the control and
treatment groups were made. Aβ burden was assessed as the
percentage of the total area of the cortex and hippocampus that
contained regions of Aβ deposits. The data were analyzed using
Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Perls’ reaction
Perls’ reagent, potassium ferrocyanide, reacts with
Fe3+ in the presence of HCl to form an insoluble pigment
known as Prussian blue (20).
Following fixation with 4% paraformaldehyde, the brains were
stained with Perls’ solution. Paraffin sections were deparaffinized
and rehydrated. For the enhancement of iron staining signals, the
sections were incubated with 0.75 mg/ml 3,3′-DAB and 0.07%
H2O2 in 1 M Tris-HCl (pH 7.5) for 5 min,
followed by rinsing with PBS. The sections were immersed for 2 h in
Perls’ blue staining solution, which was prepared immediately prior
to use by mixing equal parts of 20% HCl and 20% potassium
ferrocyanide (Tianjin Fu Chen Chemical Reagents Factory). Light
blue spots were detected using the Perls’ reaction without DAB;
however, without DAB enhancement, Perls’ reaction is not very
sensitive. Therefore the concentration of the Perls’ liquid was
increased and the contrast of the image was altered. The stained
sections were dehydrated, cleared, covered with neutral balsam and
examined under a light microscope equipped with a digital
camera.
Western blot analysis
The cerebral cortex tissue fragments were minced
into small pieces and homogenized in chilled lysis buffer (Tianjin
Fu Chen Chemical Reagents Factory) overnight at 4°C. The lysis
buffer contained 50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40,
0.25% sodium deoxycholate, 1 mM phenylmethanesulfonylfluoride, 10
mg/ml leupeptin, 1 mM Na3VO4, 0.1% SDS and 1
mM NaF. Cell pellets were lysed directly on the culture dishes
using the lysis buffer. The lysates were collected, centrifuged at
80,000 × g for 30 min and total proteins were quantified using a
bicinchoninic acid kit (Multisciences Biotech Co., Ltd., Hangzhou,
China). The supernatant was removed, portioned into aliquots and
stored at −80°C. A 50-μg sample of each of the total proteins was
subjected to SDS-PAGE using 10% gradient Tris/glycine gels (Tianjin
Fu Chen Chemical Reagents Factory) and the separated proteins were
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Temecula, CA, USA). Subsequent to blocking in 5% nonfat
milk for 1 h, the membranes were incubated overnight with the
following primary antibodies at 4°C: Polyclonal rabbit anti-rat
DMT1-IRE (#NRAMP21-A; 1:3,000) and DMT1-without IRE (#NRAMP23-A;
1:2,000) antibodies from Alpha Diagnostics International Inc.,
Owings Mills, MD, USA; polyclonal rabbit anti-mouse FPN1 (1:13,000;
#MTP11-A; Alpha Diagnostics International Inc., San Antonio, TX,
USA) and monoclonal rabbit anti-rat anti-β-actin antibody (1:5,000;
Hua Han Biopharmaceutical Holdings Ltd., Shijiazhuang, China). The
membranes were washed, then incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:10,000; Kirkegaard & Perry Lab Inc., Gaithersburg, MD, USA)
for 2 h. Protein levels were quantified from western blot analyses
using a densitometer (DU800; MJ Research, Inc., St. Bruno, QC,
Canada), and normalized against β-actin.
Immunoreactive bands were visualized using
SuperSignal West Pico Chemiluminescent Substrate (Pierce
Biotechnology Inc., Rockford, IL, USA) using ChemiDoc XRS system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with Image-Pro Plus
software, version 6.0.
Statistical analysis
Data from the water maze escape latency were
analyzed using repeated-measures analysis of variance (ANOVA) with
the group as the between-subjects independent variable and day of
trial as the within-subjects independent variable. Univariate
ANOVAs were conducted for single dependent variables in the water
maze probe trial and neurochemical assays, with the group as the
between-subjects independent variable. Following a significant
result in an omnibus ANOVA, Bonferroni post-hoc comparisons were
conducted. The comparisons of most interest were the AD model group
vs. C57 control group and the drug-treated groups vs. the AD group.
All analyses were performed using SPSS software for Windows,
version 17.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
Morris water maze
The Morris water maze experiment was conducted to
investigate whether the active component treatment was able to
reduce cognitive deficits in APP/PS1 mice. The ability of the mice
to learn and process spatial information was tested using a Morris
water maze. The analysis of the place navigation trial demonstrated
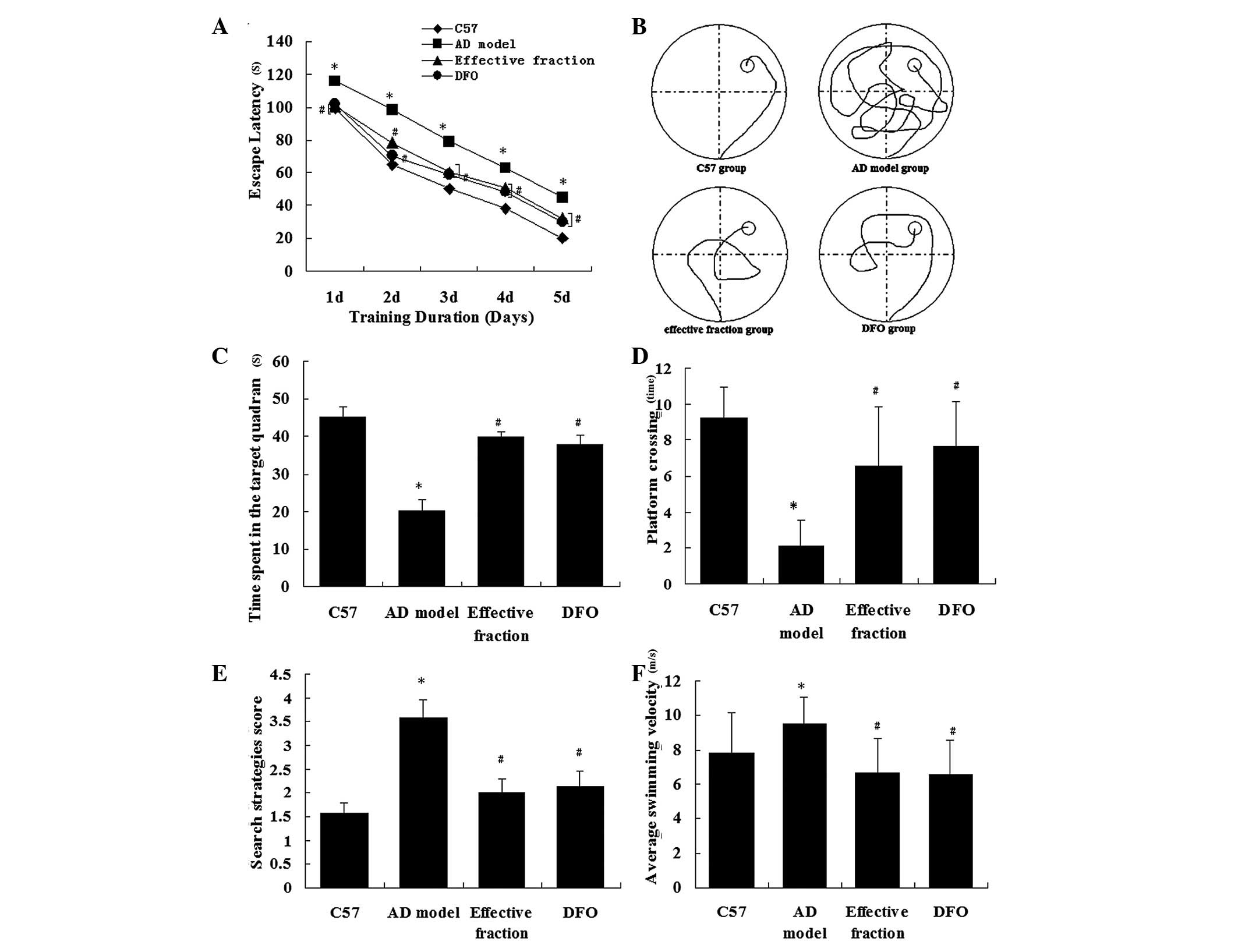
that the escape latencies reduced between days 1–5 in all groups
(Fig. 2A). The AD model mice
exhibited longer escape latencies than the C57 control mice
(P<0.01). Mice in the active component and DFO groups displayed
significantly reduced escape latencies compared with the AD model
mice (P<0.01). There was no significant difference in escape
latency between the active component and DFO groups (P>0.79).
These results indicate that the active component treatment
mitigated the impairment of spatial learning and memory in the AD
model mice. Fig. 2B displays the
representative swimming paths of mice in the four groups. The
analysis of the spatial probe trial indicated that the percentage
time spent in the target quadrant (Fig. 2C), the search strategies score
(Fig. 2D), the platform-crossing
times (Fig. 2E) and the average
swimming velocity (Fig. 2F) varied
significantly between the groups. The AD model mice spent
significantly less time in the quadrant containing the platform
than the C57 group mice (P<0.01; Fig. 2C). The search strategies score was
reduced in the AD model group compared with the C57 group
(P<0.01; Fig. 2D). Furthermore,
the number of crossings to the previous location of the platform
was reduced in the AD model group compared with the C57 control
group (P<0.01; Fig. 2E). The
mice in the active component group spent more time in the target
quadrant, scored higher in search strategies and exhibited more
platform-crossing times than those in the AD model group. These
results indicate that the ability of the mice to use spatial cues
for the localization of the platform was impaired in the AD model
group (P<0.01) and was improved by treatment with the active
components of Astragalus, Radix Puerariae and
Epimedium. The average swimming velocity was increased in AD
model group compared with the C57 group (P<0.01). Mice in the
active component and DFO groups exhibited slower average swimming
velocities (P<0.01), indicating that the AD model mice did more
unnecessary swimming. Collectively, these results suggest that
treatment with the active components of Astragalus, Radix
Puerariae and Epimedium attenuated the cognitive
deficits on learning and memory performance in AD model mice.
Treatment with the active components of
Astragalus, Radix Puerariae and Epimedium inhibits Aβ plaque
accumulation and reverses Aβ burden in the brains of APP/PS1
mice
The effects of the active components of
Astragalus, Radix Puerariae and Epimedium on
Aβ deposition in the APP/PS1 mouse brain were evaluated using IHC
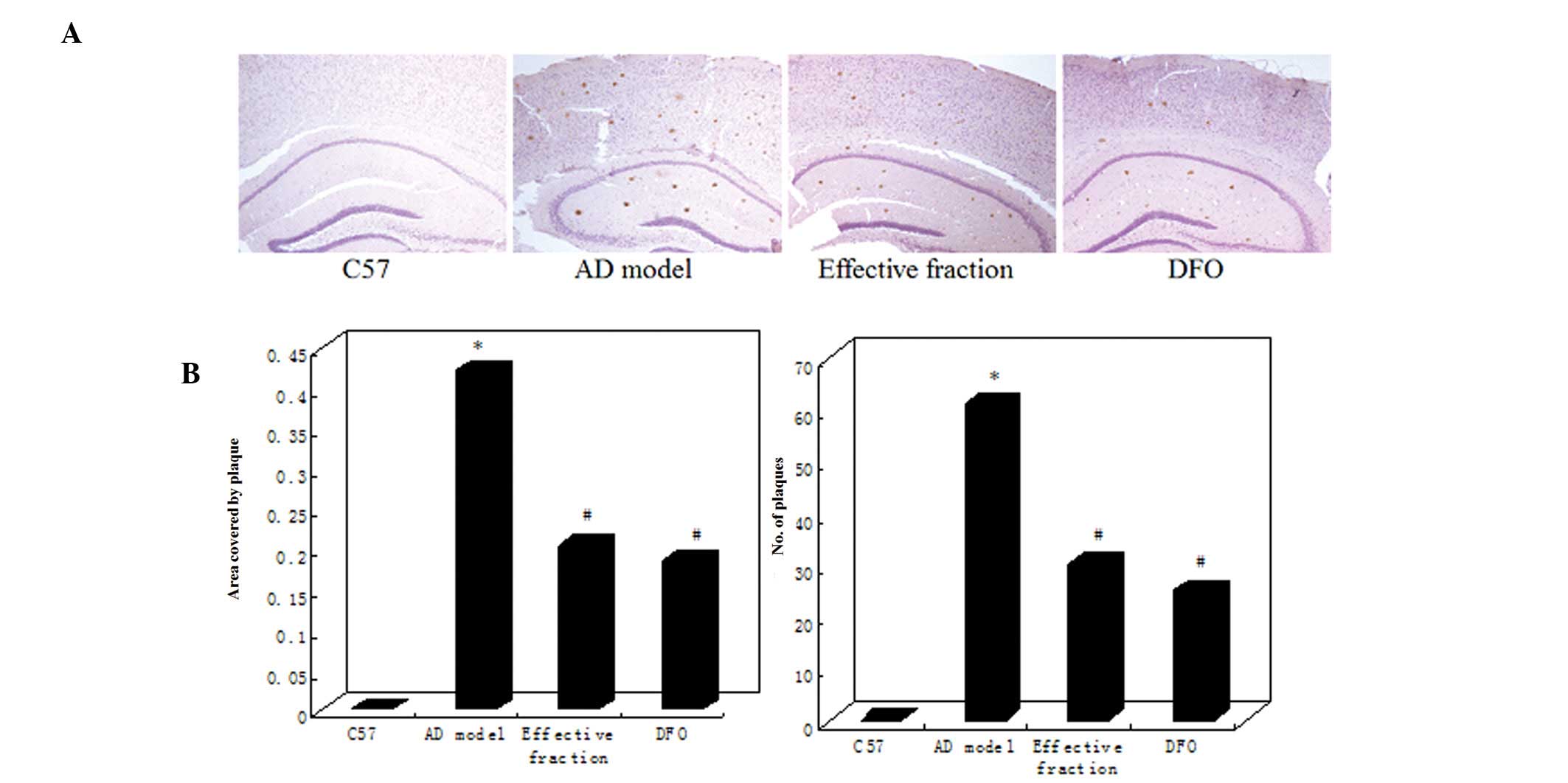
(Fig. 3). The APP/PS1 model mice
exhibited a marked increase in the number and size of
Aβ-immunoreactive senile plaques in the cortex and hippocampus.
However, the active component and DFO group mice displayed a clear
reduction in plaque number and Aβ burden compared with the AD model
mice (Fig. 3A). Quantitative
analysis indicated that the plaque numbers in the brains of the AD
model group significantly increased to 62.13% compared with the C57
group (P<0.01; Fig. 3B). The Aβ
burden was determined by measuring the areas of Aβ-positive
neuritic plaques in the brain. The Aβ plaque area was significantly
increased in the AD model group compared with the C57 group
(Fig. 3B). However, the brains of
active component and DFO group mice exhibited a significant
reduction in plaque count and Aβ burden compared with AD model
mice. No differences in plaque number or Aβ burden were identified
between the active component and DFO groups (Fig. 3B).
Brain iron deposition was assessed using
Perls’ staining
Light blue spots were detected using the Perls’
reaction without DAB; however, without DAB enhancement, Perls’
reaction is not very sensitive. Therefore the concentration of the
Perls’ liquid was increased and the contrast of the image was
altered. The influence of the active components of
Astragalus, Radix Puerariae and Epimedium on
brain iron load in the APP/PS1 mouse brain was evaluated using
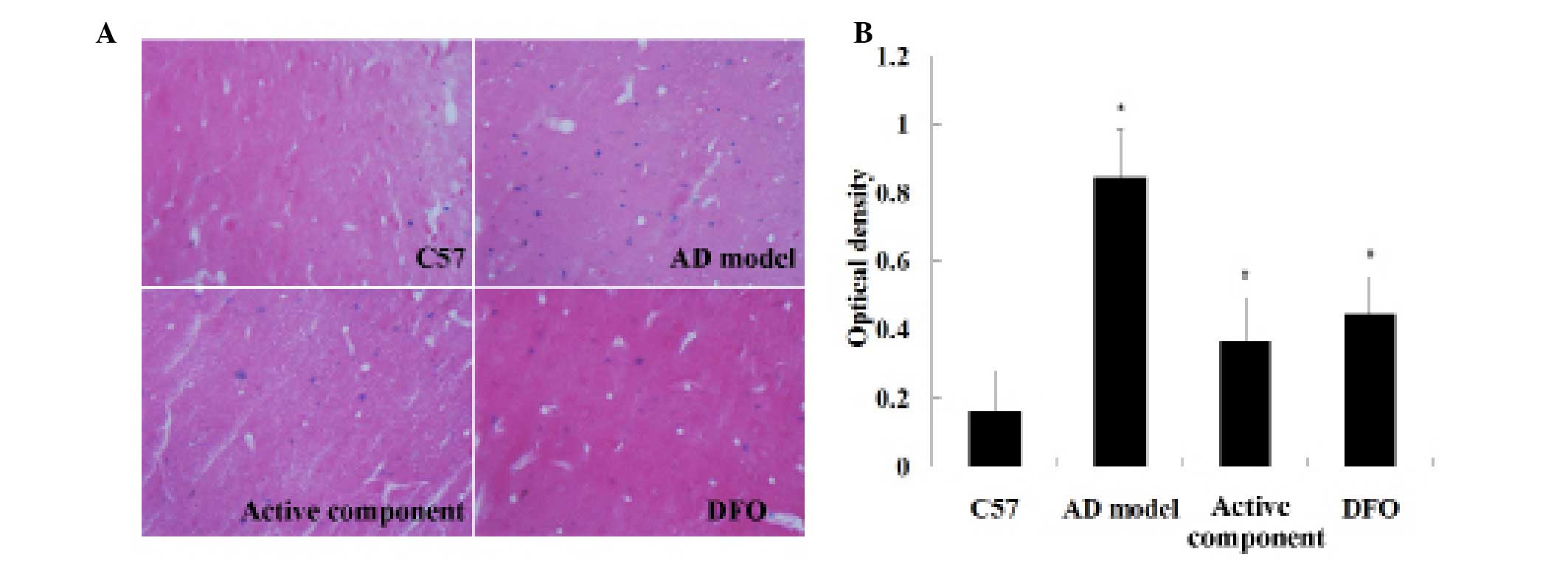
Perls’ staining (Fig. 4A).
Quantitative analysis indicated that the blue spot number in the
brains of the AD model group increased significantly compared with
the C57 group (P<0.01; Fig.
4B). The brains of the active component and DFO groups
displayed a significant reduction in blue spots compared with the
AD model mice. No difference in the number of blue spots was
observed between the active component and DFO groups (Fig. 4B). Thus, the AD model group
exhibited a marked increase in brain iron load and the active
component treatment appeared to reduce this iron load in the AD
model mouse brain.
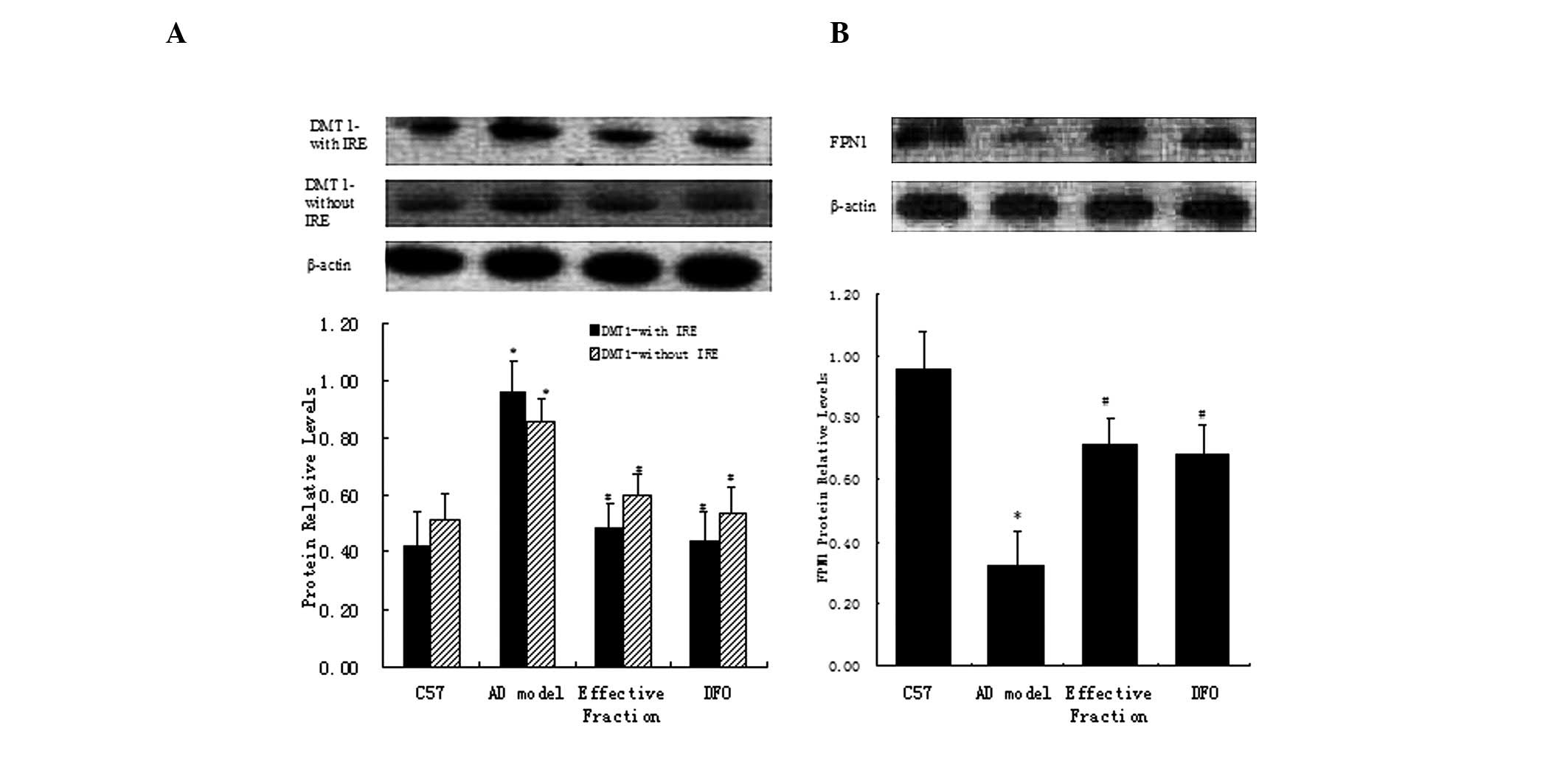
Western blot analysis
DMT1 and FPN1 proteins were extracted from the mouse
hippocampus in order to quantify the differences in their
expression levels between the groups. Western blot analyses for
DMT1-IRE and DMT1-nonIRE revealed a clear band at 64 kDa, matching
the predicted molecular mass of these proteins. Quantification of
the expression levels of DMT1-IRE and DMT1-nonIRE indicated that
the levels of the two proteins were significantly higher in the
hippocampus of the AD model group than the C57 group, whilst the
active component and DFO group mice presented a significant
reduction compared with the AD model mice (Fig. 5A). Western blot analysis of FPN1
displayed a major band at 62 kDa, which matched the predicted
molecular mass of these proteins. Quantification of the expression
levels of FPN1 indicated a significant reduction in the hippocampus
of AD model mice compared with the C57 group mice (Fig. 5B), which was partly reversed in the
active component and DFO groups.
Discussion
AD is a neurodegenerative disorder, characterized
clinically by progressive memory loss and neuropathologically by
extracellular amyloid plaques (21). AD is a typical age-dependent
neurodegenerative disease that affects 5% of individuals >65
years, 20% of those >85 years and >33% of those >90 years;
>20 million people are affected worldwide (22) and this figure is predicted to rise
in the future, due to an increase in the elderly population.
Marked progress has been made in the last 20 years
in understanding the pathophysiology of AD (23). However, effective preventative and
therapeutic treatments remain to be produced. In the absence of
preventative and therapeutic methods, the prevalence of AD will
continue to increase as life expectancy increases (24). Therefore, the development of novel
treatments for AD is an urgent requirement, in view of the
increasingly aged populations (25).
Previous evidence has indicated that a disturbance
of normal iron homeostasis may contribute to the pathology of AD,
which suggests that iron chelation may be an effective therapeutic
intervention (26). The use of an
iron-chelating agent, such as DFO, may significantly alleviate
symptoms in patients with AD and produce a notable neuroprotective
effect. However, these chemical drugs present a number of problems,
including adverse effects, such as oral side effects.
Traditional Chinese medicine (TCM) is a complete
medical system that has been practiced for >3,000 years. The
active component treatment used in the present study was composed
of the active components of the TCM herbs Epimedium,
Astragalus and Radix Puerariae, including icariin,
astragaloside IV and puerarin, respectively. According to TCM
theories, these active components may be useful in the treatment of
patients with neuropsychiatric disorders. The active components of
Epimedium, Astragalus and Radix Puerariae may
circumvent the shortcomings of chemical iron-chelators.
Furthermore, these compounds may scavenge free radicals, reduce
inflammation, adjust the functions of multiple viscera(16) and reduce iron overload of recession
in the central nervous system (17,18).
The present study aimed to demonstrate these effects and to explain
the underlying mechanisms using contemporary methodology and
transgenic animal models of AD.
Multiple genetically modified transgenic animal
models include APP/PS1/tau (27,28)
and Cdk5/P35/tau. APP/PS1/tau mice express human APP695(Swe),
PS1(M146V) and Tau (P301L) under the control of the mouse Thy-1
promoter.
In the present study, APP/PS1 mice were used as the
AD model. The mice express human APP with the Swedish mutations
(K670N/M671L) at the β-secretase cleavage site and PS1 (PS1dE9)
(29); with a C57BL/6J background.
In addition, C57BL/6J mice were used in the normal control group.
APP/PS1 mice have been reported to demonstrate impaired learning
and memory in the Morris water maze test (30) and the results of the present study
were consistent with this. The neuroprotective effects of the
active components of Epimedium, Astragalus and
Radix Puerariae were investigated in this mouse model in the
current study.
A number of previous studies have demonstrated the
neuroprotective effect of metal chelators, which may be clinically
applicable in the treatment of AD (31,32).
One previous study reported that the iron chelator DFO slowed the
clinical progression of cognitive decline associated with AD
(3); therefore, the present study
utilized DFO as a positive control.
The clinical manifestations of cognitive deficits in
the early stages of AD include impaired learning and memory
ability. Behavioral data obtained using the Morris water maze in
the present study confirmed a significant impairment in learning
and memory performance in APP/PS1 mice, which was consistent with
previous studies (33,34). Collectively, the escape latency,
platform-crossing, time spent in the target quadrant and average
swimming velocity results suggested that the active component
treatment improved the learning and memory deficits of the AD model
mice. These results substantiated the presence of neurotoxicity in
the model mouse brain and indicated that the active component
treatment was able to attenuate the resulting cognitive deficits
associated with AD.
The deposition of Aβ in the brain induces a series
of neurotoxic effects and is considered to be the primary factor in
the emergence and progression of AD (35,36).
The most common form of Aβ is Aβ1–42, located primarily in discrete
Aβ deposits. The more soluble Aβ1–40 form is associated with blood
vessels (37) and may develop
later in the course of the disease (38). The reduction of Aβ levels,
particularly the more toxic Aβ1–42 form, is considered to be one of
the most important therapeutic targets for the treatment of AD.
Prior studies indicate that increased Aβ production in the
hippocampus and cortex leads to synaptic impairment, neuronal loss
and memory deficits (39,40). Furthermore, synaptic dysfunction
has been identified in the associated cortices and hippocampus of
the AD brain (41,42). These observations suggest that the
excessive production of Aβ peptides in the brain is a pivotal
factor in AD pathology. In the present study, Aβ neuropathology was
quantified using immunohistochemistry. Treatment with the active
components of Astragalus, Radix Puerariae and
Epimedium inhibited Aβ plaque accumulation and reversed the
Aβ burden in the APP/PS1 mouse brain.
Iron is an essential nutrient involved in numerous
vital biological processes, and iron deficiency and overdose are
harmful to the health of humans. Therefore, iron homeostasis is a
strictly regulated process in healthy individuals. The uptake, use,
storage and excretion of iron are maintained in a dynamic balance
to ensure that the iron content in the body can fulfill
physiological needs without excessive accumulation.
Iron is an intrinsic producer of reactive oxygen
species through associated processes including hydroxyl radical
formation, glutathione consumption, protein aggregation, lipid
peroxidation and nucleic acid modification (43). Factors that disturb iron
homeostasis may lead to disease; therefore, iron metabolism has
long been the focus and interest of numerous researchers.
Metal ions are implicated in the pathogenesis of AD
(44,45) and have been demonstrated to
participate in APP expression, Aβ generation and the production of
oxidative compounds (46,47). Therefore, it can be hypothesized
that metal transporters may serve important functions in the
pathogenesis of AD by altering metal homeostasis (48,49).
In the present study, the brain iron load was evaluated using
Perls’ staining and iron was observed to accumulate in the AD
brain. Treatment with the active components of Astragalus,
Radix Puerariae and Epimedium was observed to relieve
brain iron overload of the AD model mice.
In the previous ten years, considerable progress has
been achieved in the study of iron metabolism. Numerous genes and
proteins have been associated with iron metabolism, including DMT1
(5), transferrin receptor 2
(50), FPN1 (51,52),
hephaestin (53,54) and hepcidin (55,56).
These studies and others have improved the understanding of iron
metabolism and its regulation, and provide a scientific basis for
further characterizing the molecular mechanisms of iron
metabolism-associated diseases. It has been indicated that the
expression and regulation of these genes is tissue-specific and
that their expression may be directly or indirectly affected by the
iron status in the body (57).
Cellular iron homeostasis is regulated by the iron
transporters DMT1 and FPN1. The primary iron uptake transporter is
DMT1, while FPN1 (58) functions
essentially as an iron efflux transporter (59). DMT1 is involved in iron uptake,
which is widely distributed in mammalian tissues. DMT1 is known to
contribute to neurodegeneration in animal models of Parkinson’s
disease (60); however, a
comprehensive description of DMT1 in the AD pathogenesis has not
yet been established.
Notably, a previous study on rat brains demonstrated
that the expression of the IRE and non-IRE isoforms of DMT1
increase with age (60) and thus,
DMT1 has been hypothesized to be the primary risk factor in AD. In
the present study, western blot analysis was used to detect the
levels of brain iron load-associated DMT1 and FPN1. The levels of
the two isoforms of DMT1 were significantly increased in the AD
model hippocampus compared with the healthy C57 control, and the
level of FPN1 was significantly reduced. Thus, the results of the
present study are essentially consistent with the previous study
(11). However, the molecular
mechanisms responsible for these effects require further
investigation.
The underlying mechanisms that result in an abnormal
brain iron load may involve the overexpression of the brain iron
metabolism-associated proteins DMT1 and FPN1. These results suggest
that DMT1 and FPN1 serve critical functions in the iron-mediated
neuropathogenesis of AD and that pharmacological inhibition of DMT1
and FPN1 may provide novel therapeutic strategies for treating
AD.
In conclusion, treatment with the active components
of Epimedium, Astragalus and Radix Puerariae
improved the learning and memory deficits of APP/PS1 mice and
reduced the Aβ and iron load in the AD brain. Therefore, the active
components of Epimedium, Astragalus and Radix
Puerariae may be clinically applicable for the prevention or
treatment of AD.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81273983),
Natural Science Foundation of Hebei Province (no. C2010001471) and
the Science and Technology Research Youth Fund Project of Hebei
Colleges and Universities (no. Q2012036).
References
|
1
|
Zhang J, Zhen YF, Pu-Bu-Ci-Ren, et al:
Salidroside attenuates beta amyloid-induced cognitive deficits via
modulating oxidative stress and inflammatory mediators in rat
hippocampus. Behav Brain Res. 244:70–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El Tannir El Tayara N, Delatour B, Le
Cudennec C, et al: Age-related evolution of amyloid burden, iron
load, and MR relaxation times in a transgenic mouse model of
Alzheimer’s disease. Neurobiol Dis. 22:199–208. 2006. View Article : Google Scholar
|
|
3
|
Crapper McLachlan DR, Dalton AJ, Kruck TP,
et al: Intramuscular desferrioxamine in patients with Alzheimer’s
disease. Lancet. 337:1304–1308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xian-Hui D, Wei-Juan G, Tie-Mei S, et al:
Age-related changes of brain iron load changes in the frontal
cortex in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s
disease. J Trace Elem Med Biol. Dec 3–2014.(Epub ahead of
print).
|
|
5
|
Fleming MD, Trenor CC 3rd, Su MA, et al:
Microcytic anaemia mice have a mutation in Nramp2, a candidate iron
transporter gene. Nat Genet. 16:383–386. 1997.PubMed/NCBI
|
|
6
|
Li H, Li F, Sun H and Qian ZM:
Membrane-inserted conformation of transmembrane domain 4 of
divalent-metal transporter. Biochem J. 372:757–766. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang LH, Wang X, Zheng ZH, et al: Altered
expression and distribution of zinc transporters in APP/PS1
transgenic mouse brain. Neurobiol Aging. 31:74–87. 2010. View Article : Google Scholar
|
|
8
|
Gunshin H, Mackenzie B, Berger UV, et al:
Cloning and characterization of a mammalian proton-coupled
metal-ion transporter. Nature. 388:482–488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erikson KM and Aschner M: Increased
manganese uptake by primary astrocyte cultures with altered iron
status is mediated primarily by divalent metal transporter.
Neurotoxicology. 27:125–130. 2006. View Article : Google Scholar
|
|
10
|
Lee PL, Gelbart T, West C, et al: The
human Nramp2 gene: characterization of the gene structure,
alternative splicing, promoter region and polymorphisms. Blood
Cells Mol Dis. 24:199–215. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackenzie B, Takanaga H, Hubert N, et al:
Functional properties of multiple isoforms of human divalent
metal-ion transporter 1 (DMT1). Biochem J. 403:59–69. 2007.
View Article : Google Scholar :
|
|
12
|
De Domenico I, Nemeth E, Nelson JM, et al:
The hepcidin-binding site on ferroportin is evolutionarily
conserved. Cell Metab. 8:146–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donovan A, Lima CA, Pinkus JL, et al: The
iron exporter ferroportin/Slc40a1 is essential for iron
homeostasis. Cell Metab. 1:191–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan S, Hua Y, Keep RF, et al: Deferoxamine
reduces CSF free iron levels following intracerebral hemorrhage.
Acta Neurochir Suppl. 96:199–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hishikawa T, Ono S, Ogawa T, et al:
Effects of deferoxamine-activated hypoxia-inducible factor-1 on the
brainstem after subarachnoid hemorrhage in rats. Neurosurgery.
62:232–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Inagaki Y and Liu Y: Research
progress on flavonoids isolated from traditional Chinese medicine
in treatment of Alzheimer’s disease. Intractable Rare Dis Res.
2:3–10. 2013.PubMed/NCBI
|
|
17
|
Liu XY, Zi H, Zheng HX, et al: Tissues
distribution of Icariin and its metabolites in kidney of
osteoporosis model rats. Zhongguo Shiyan Fangiixue Zazhi.
20:125–128. 2014.
|
|
18
|
Chen GH and Huang WF: Progress in
pharmacological effects of compositions of Astragalus membranaceus.
Zhongguo Xinyao Zazhi. 17:1482–1485. 2008.
|
|
19
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu Y, Hua Y, Keep RF, et al: Deferoxamine
reduces intracerebral hematoma-induced iron accumulation and
neuronal death in piglets. Stroke. 40:2241–2243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin C, Li S, Zhao W and Feng J: Brain
imaging of mild cognitive impairment and Alzheimer’s disease.
Neural Regen Res. 8:435–444. 2013.PubMed/NCBI
|
|
22
|
Blennow K, de Leon MJ and Zetterberg H:
Alzheimer’s disease. Lancet. 368:387–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imbimbo BP, Lombard J and Pomara N:
Pathophysiology of Alzheimer’s disease. Neuroimaging Clin N Am.
15:727–753. 2005. View Article : Google Scholar
|
|
24
|
Nojima J, Maeda A, Aoki S, et al: Effect
of rice-expressed amyloid β in the Tg2576 Alzheimer’s disease
transgenic mouse model. Vaccine. 29:6252–6258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang J, Wu L, Huang HL, et al: Back
propagation artificial neural network for community Alzheimer’s
disease screening in China. Neural Regen Res. 8:270–276.
2013.PubMed/NCBI
|
|
26
|
Guo C, Wang T, Zheng W, et al: Intranasal
deferoxamine reverses iron-induced memory deficits and inhibits
amyloidogenic APP processing in a transgenic mouse model of
Alzheimer’s disease. Neurobiol Aging. 34(2): 562–75. 2013.
View Article : Google Scholar
|
|
27
|
Overk CR, Perez SE, Ma C, et al: Sex
steroid levels and AD-like pathology in 3xTgAD mice. J
Neuroendocrinol. 25:131–144. 2013. View Article : Google Scholar
|
|
28
|
Marques SC, Lemos R, Ferreiro E, et al:
Epigenetic regulation of BACE1 in Alzheimer’s disease patients and
in transgenic mice. Neuroscience. 220:256–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Shiao C, Hemingway JF, et al:
Suppressed retinal degeneration in aged wild type and APPswe/PS1ΔE9
mice by bone marrow transplantation. PLoS One. 8:e642462013.
View Article : Google Scholar
|
|
30
|
Cheng D, Low JK, Logge W, et al: Novel
behavioural characteristics of female APPSwe/PS1ΔE9 double
transgenic mice. Behav Brain Res. 260:111–118. 2014. View Article : Google Scholar
|
|
31
|
Whitnall M and Richardson DR: Iron: a new
target for pharmacological intervention in neurodegenerative
diseases. Semin Pediatr Neurol. 13:186–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo C, Wang T, Zheng W, et al: Intranasal
deferoxamine reverses iron-induced memory deficits and inhibits
amyloidogenic APP processing in a transgenic mouse model of
Alzheimer’s disease. Neurobiol Aging. 34:562–575. 2013. View Article : Google Scholar
|
|
33
|
Wu J, Bie B, Yang H, et al: Activation of
the CB2 receptor system reverses amyloid-induced memory deficiency.
Neurobiol Aging. 34:791–804. 2013. View Article : Google Scholar
|
|
34
|
Han M, Liu Y, Tan Q, et al: Therapeutic
efficacy of stemazole in a beta-amyloid injection rat model of
Alzheimer’s disease. Eur J Pharmacol. 657:104–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hardy J and Allsop D: Amyloid deposition
as the central event in the aetiology of Alzheimer’s disease.
Trends Pharmacol Sci. 12:383–388. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park MH, Lee JK, Choi S, et al:
Recombinant soluble neprilysin reduces amyloid-beta accumulation
and improves memory impairment in Alzheimer’s disease mice. Brain
Res. 1529:113–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller DL, Papayannopoulos IA, Styles J,
et al: Peptide compositions of the cerebrovascular and senile
plaque core amyloid deposits of Alzheimer’s disease. Arch Biochem
Biophys. 301:41–52. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Delacourte A, Sergeant N, Champain D, et
al: Nonoverlapping but synergetic tau and APP pathologies in
sporadic Alzheimer’s disease. Neurology. 59:398–407. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lue LF, Kuo YM, Roher AE, et al: Soluble
amyloid beta peptide concentration as a predictor of synaptic
change in Alzheimer’s disease. Am J Pathol. 155:853–862. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Jin M, Koeglsperger T, et al:
Soluble Aβ oligomers inhibit long-term potentiation through a
mechanism involving excessive activation of extrasynaptic
NR2B-containing NMDA receptors. J Neurosci. 31:6627–6638. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Masliah E, Mallory M, Alford M, et al:
Altered expression of synaptic proteins occurs early during
progression of Alzheimer’s disease. Neurology. 56:127–129. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sze CI, Troncoso JC, Kawas C, et al: Loss
of the presynaptic vesicle protein synaptophysin in hippocampus
correlates with cognitive decline in Alzheimer disease. J
Neuropathol Exp Neurol. 56:933–944. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jomova K and Valko M: Importance of iron
chelation in free radical-induced oxidative stress and human
disease. Curr Pharm Des. 17:3460–3473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang X, Atwood CS, Moir RD, et al: Trace
metal contamination initiates the apparent auto-aggregation,
amyloidosis, and oligomerization of Alzheimer’s Abeta peptides. J
Biol Inorg Chem. 9:954–960. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu G, Huang W, Moir RD, et al: Metal
exposure and Alzheimer’s pathogenesis. J Struct Biol. 155:45–51.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lovell MA, Robertson JD, Teesdale WJ, et
al: Copper, iron and zinc in Alzheimer’s disease senile plaques. J
Neurol Sci. 158:47–52. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Finefrock AE, Bush AI and Doraiswamy PM:
Current status of metals as therapeutic targets in Alzheimer’s
disease. J Am Geriatr Soc. 51:1143–1148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Colangelo V, Schurr J, Ball MJ, et al:
Gene expression profiling of 12633 genes in Alzheimer hippocampal
CA1: transcription and neurotrophic factor down-regulation and
up-regulation of apoptotic and pro-inflammatory signaling. J
Neurosci Res. 70:462–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang LH, Wang X, Stoltenberg M, et al:
Abundant expression of zinc transporters in the amyloid plaques of
Alzheimer’s disease brain. Brain Res Bull. 77:55–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawabata H, Germain RS, Vuong PT, et al:
Transferrin receptor2-alpha supports cell growth both in
iron-chelated cultured cells and in vivo. J Biol Chem.
275:16618–16625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McKie AT, Marciani P, Rolfs A, et al: A
novel duodenal iron-regulated transporter, IREG1, implicated in the
basolateral transfer of iron to the circulation. Mol Cell.
5:299–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abboud S and Haile DJ: A novel mammalian
iron-regulated protein involved in intracellular iron metabolism. J
Biol Chem. 275:19906–19912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hellman NE and Gitlin JD: Ceruloplasmin
metabolism and function. Annu Rev Nutr. 22:439–458. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Anderson GJ, Frazer DM, McKie AT and Vulpe
CD: The ceruloplasmin homolog hephaestin and the control of
intestinal iron absorption. Blood Cells Mol Dis. 29:367–375. 2002.
View Article : Google Scholar
|
|
55
|
Fleming RE and Sly WS: Hepcidin: a
putative iron-regulatory hormone relevant to hereditary
hemochromatosis and the anemia of chronic disease. Proc Natl Acad
Sci USA. 98:8160–8162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krause A, Neitz S, Mägert HJ, et al:
LEAP-1, a novel highly disulfide-bonded human peptide, exhibits
antimicrobial activity. FEBS Lett. 480:147–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shawki A and Mackenzie B: Interaction of
calcium with the human divalent metal-ion transporter-1. Biochem
Biophys Res Commun. 393:471–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Donovan A, Brownlie A, Zhou Y, et al:
Positional cloning of zebrafish ferroportin1 identifies a conserved
vertebrate iron exporter. Nature. 403:776–781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Anderson GJ and Vulpe CD: Mammalian iron
transport. Cell Mol Life Sci. 66:3241–3261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ke Y, Chang YZ, Duan XL, et al:
Age-dependent and iron-independent expression of two mRNA isoforms
of divalent metal transporter 1 in rat brain. Neurobiol Aging.
26:739–748. 2005. View Article : Google Scholar : PubMed/NCBI
|