Introduction
Leukemia results from abnormal functioning of the
hematopoietic tissues in bone marrow due to variations of
intracellular DNA molecules. As the overproduction of immature
leucocytes interferes with other functions of the bone marrow, the
capability of bone marrow to make other blood cells declines
inevitably. Leukemia cells can spread to the lymph nodes, spleen,
liver, central nervous system and other organs. The incidence and
mortality of leukemia as a malignant clonal disorder of the
hematopoietic stem cells rank first among all pediatric
malignancies (1). The exact causes
of leukemia remain under study, but DNA variations in bone marrow
stem cells generally cause their deterioration, and these may arise
due to exposure to radiation, contact with carcinogens and
variations in the genetic materials in other cells (2). In addition, viruses may cause
leukemia (3,4).
Leukemia drug resistance denotes the insensitivity
or resistance of leukemia cells to chemotherapeutic drugs. When a
patient has received several courses of chemotherapy, but the
percentage of leukemia cells in the bone marrow does not decline
significantly or returns to the pretreatment level soon after a
short-term discontinuation, this condition is considered to be
leukemia drug resistance. Currently, the development of leukemia
cell drug resistance is one of the major causes of leukemia
treatment failure (5,6).
Multidrug resistance (MDR) is the main reason for
chemotherapeutic failure. A number of studies have confirmed that
piperlongumine is able to induce the apoptosis of leukemia cells
and inhibit their proliferation (4,7,8).
Piperlongumine has also been shown to have an inhibitory effect on
tumor proliferation, migration, invasion and angiogenesis by
oxidative stress (7,8). Although piperlongumine is thought to
be able to regulate cell growth, survival, differentiation,
proliferation, migration and other cell processes in addition to
its involvement in multiple signal transduction pathways, it is
still unknown whether piperlongumine is able to reverse drug
resistance in leukemia (9–11).
The present study aimed to investigate the reversal
effect of piperlongumine on drug resistance in a human leukemia
cell line in order to establish an experimental basis for its
clinical applications.
Materials and methods
Cell lines and cell culture
The K562 human leukemia cell line was purchased from
the Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China), and the doxorubicin-resistant K562/A02 cell line
was purchased from the Tianjin Institute of Hematology (Tianjin,
China). The cells were cultured at 37°C with 5% CO2 in
Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum
(Sigma Aldrich, St. Louis, MO, USA). Piperlongumine (Sigma Aldrich)
was dissolved in DMSO to prepare a stock solution, which was
further diluted to the experimental concentrations with serum-free
culture medium and filtered immediately.
MTS assay
A total of 5×103 cells were seeded into
each well of a 96-well plate and the cells were incubated for 24 h
at 37°C with 5% CO2. Then, 0, 2, 5, 10, 20, 50 or 100 μM
piperlongumine was added and the cells were cultured for a further
24 h. Fresh culture medium was then added, followed by 20 μl
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
(MTS; Promega, Madison, WI, USA). Following the incubation of the
cells with MTS for 4 h, the absorbance at 490 nm was determined
using a microplate reader.
The MTS assay was repeated using the same method,
with the exception that 0, 2, 5, 10, 20, 50 or 100 μM doxorubicin
and 2 or 5 μM piperlongumine were added prior to the 24-h culture
instead of the various concentrations of piperlongumine.
Flow cytometric assay
A total of 5×105 cells were seeded into a
6-well plate and cultured for 24 h. Then, 1 μM doxorubicin with 2
or 5 μM piperlongumine was added and the cells were cultured for a
further 24 h. The cells were then collected and incubated with
fluorescein isothiocyanate (FITC)-annexin V and propidium iodide
(PI; BD Franklin Lakes, NJ, USA) for 15 min in the dark. Following
the incubation, the fluorescence intensity of the cells was
measured at 488 nm by flow cytometry (BD FACSCalibur, BD) for the
analysis of apoptosis.
In further experiments, 5×105 cells were
seeded into a 6-well plate and cultured for 24 h. Then, 2 or 5 μM
piperlongumine was added and the cells were cultured for a further
24 h. Following the 24-h incubation, several different analyses
were conducted. i) The cells were collected, fixed and incubated
with PI for 30 min in the dark prior to measurement of the
fluorescence intensity at 488 nm by flow cytometry to evaluate the
cell cycle; ii) the cells were collected, fixed and incubated with
dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime, Shanghai,
China) for 30 min in the dark, and then the fluorescence intensity
was measured at 488 nm by flow cytometry to evaluate the production
of reactive oxygen species (ROS); iii) the cells were collected and
incubated with rhodamine-123 (Rh-123; Beyotime) for 30 min in the
dark, and then the fluorescence intensity was measured at 488 nm by
flow cytometry to determine the intracellular content of Rh-123;
and iv) the cells were collected and incubated with
P-glycoprotein-phycoerythrin (P-gp-PE) antibody (Beyotime) for 30
min in the dark, and then the fluorescence intensity was measured
at 488 nm by flow cytometry to determine the expression of
P-gp.
Realtime polymerase chain reaction (qPCR)
assay
A total of 5×105 cells were seeded into a
6-well plate and cultured for 24 h. Then, 2 or 5 μM piperlongumine
was added and the cells were cultured for another 24 h. A total of
20 μM RNA was extracted from these cells using the TRIzol method
(Beyotime) and the reaction was completed in a ABI 7500 Real-Time
fluorescence quantitative PCR instrument (Applied Biosystems,
Foster City, CA, USA). The PCR products were labeled with
SYBR-Green I (Takara Biotechnology, Dalian, China). The target
genes and reference gene GAPDH in all samples were subjected to
fluorescence qPCR to obtain the respective CT values; the relative
gene expression differences in each sample were analyzed using
2−ΔΔCT method. The primer sequences were retrieved from
the online primer database Primer Bank (http://pga.mgh.harvard.edu/primerbank/), and the
primers were synthesized and purified by Takara Biotechnology
(Dalian) Co., Ltd. (Dalian, China). The PCR process comprised 30
cycles of 94°C for 1 min, 56°C for 50 sec, and 72°C for 1 min. The
genes that were detected and their primer sequences are shown as
Table I.
 | Table IQuantitative PCR primer sequences. |
Table I
Quantitative PCR primer sequences.
| Gene | Primer sequences |
|---|
| p53 | F:
5′-CGGTTTCCGTCTGGGCTTCTT-3′
R: 5′-CCACACGCAAATTTCCTTCCACTC-3′ |
| p27 | F:
5′-GTTAGCGGACGAGTGTCCAG-3′
R: 5′-TGTTCTGTTGGCCCTTTTGTT-3′ |
| MDR1 | F:
5′-GGAGCGGTTCTACGA-3′
R: 5′-ACGATGCCCAGGTGT-3′ |
| MRP1 | F:
5′-CACACTGAATGGCATCACCTTC-3′
R: 5′-CCTTCTCGCCAATCTCTGTCC-3′ |
| Survivin | F:
5′-AGCCCTTTCTCAAGGACCAC-3′
R: 5′-GCACTTTCTTCGCAGTTTCC-3′ |
| GAPDH | F:
5′-GGAGCGAGATCCCTCCAAAAT-3′
R: 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Western blotting assay
A total of 5×105 cells were seeded into a
6-well plate and cultured for 24 h. Then, 2 or 5 μM piperlongumine
was added and the cells were cultured for a further 24 h. The cells
were then lysed and 100 μg total proteins were separated using a
12% SDS-PAGE gel. The proteins were transferred onto a
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA)
and blocked with 5% non-fat milk overnight at 4°C. The membrane was
incubated with primary monoclonal mouse anti-human antibodies
(MDR1, 1:800; MRP1, 1:800; p27, 1:800; survivin, 1:800; p53, 1:800;
p-Akt, 1:400; p-PTEN, 1:800) and β-actin (1:5,000, Sigma) at 4°C
overnight. The membranes were washed with PBST buffer (80 mM
Na2HPO4, 20 mM NAH2PO4,
100 mM NaCl, 0.05% Tween) and incubated for 1 h with horseradish
peroxidase-conjugated anti-rabbit secondary antibody (1:5,000) at
room temperature. Blots were visualized using an
electrochemiluminescence (ECL; Millipore, Billerica, MA, USA)
Western Blotting kit. β-actin acted as the internal control.
Caspase activity
The activities of caspase-3 and -8 were measured
using the Apo-ONE homogeneous caspase-3/7 assay (Beyotime,
Shanghai, China) following the manufacturer’s instructions. In each
assay, 5×103 cells were seeded into a 96-well plate and
cultured for 24 h. Then 2 or 5 μM piperlongumine and the caspase
substrate (Z-DEVD)2-R110 were added, and culturing was
continued for a further 7 h for the detection of caspase-3 activity
or 3 h for the detection of caspase-8 activity. Then, the
fluorescence intensity was measured at 490 nm using a microplate
reader (Multiskan MK3, Hudson, Thermo, NH, USA) to determine the
activity of caspase-3/8.
Assay of the transcriptional activity of
NF-κB and twist
A total of 5×105 cells were seeded into a
6-well plate and cultured for 24 h. A total of 20 μM luciferase
NF-κB and twist reporter plasmids (Apo-ONE Homogeneous caspase
assay, Beyotime) were transfected into the cells using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA) for 6 h. Then 2 or 5 μM piperlongumine was added, and after
culturing for a further 24 h, the cells were collected. The
fluorescence intensity of fluorescein was measured to determine the
transcriptional activity using a dual luciferase reporter gene
assay.
Results
Piperlongumine improves the doxorubicin
sensitivity of the K562/A02 cell line
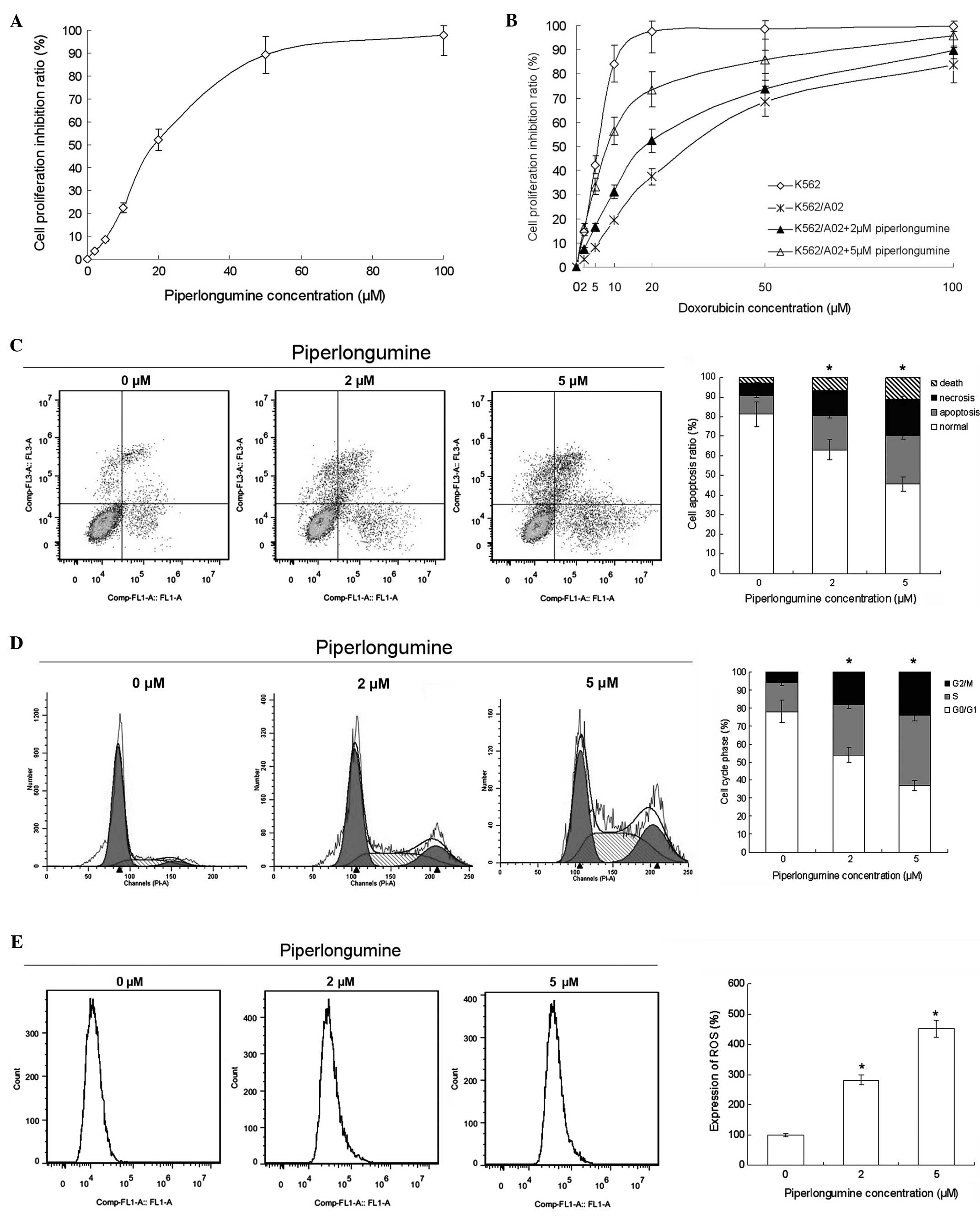
The results of the MTS assay showed that
piperlongumine suppressed K562/A02 cell proliferation with an
IC50 of 18.3 μM. The inhibition rate of 2 μM
piperlongumine was 3.5% (a non-toxic concentration), and the
inhibition rate of 5 μM piperlongumine was 8.6% (a low-toxicity
concentration) (Fig. 1A).
Therefore, the drug-resistance reversal effect and mechanism of
piperlongumine were determined at these non-toxic and low-toxicity
concentrations in order to minimize interference due to toxicity
issues.
With piperlongumine treatment, the sensitivity of
the K562/A02 cells to doxorubicin was improved. The reversal
factor, calculated as the ratio of the IC50 of
doxorubicin in the absence of piperlongumine to that in the
presence of piperlongumine was 2.8 for 2 μM piperlongumine and 6.5
for 5 μM piperlongumine (Fig. 1B).
The flow cytometry results indicated that piperlongumine induced
apoptosis, ROS production and cell cycle arrest in the K562/A02
cells. The cell cycle was arrested at the G2/M phase (Fig. 1C). These results suggest that
piperlongumine reverses the doxorubicin-resistance of K562/A02
cells by inducing apoptosis, ROS production and cell cycle
arrest.
Piperlongumine suppresses the drug efflux
of the K562/A02 cell line
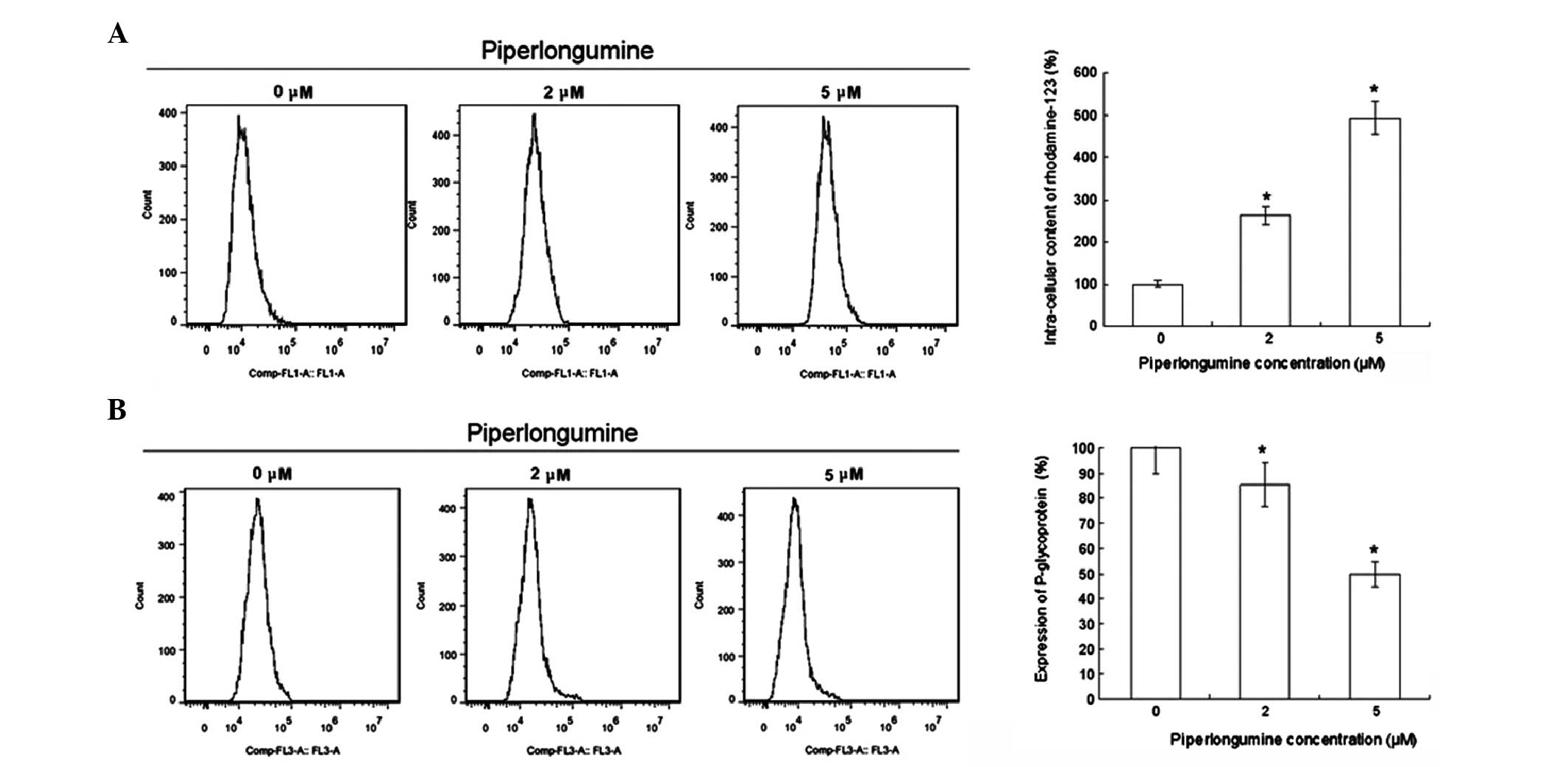
Efflux is an important mechanism associated with the
drug-resistance of tumors (12).
The intracellular concentration of rhodamine-123 was increased and
the cellular surface expression of P-gp was suppressed by
piperlongumine treatment in K562/A02 cells as shown in Fig. 2. These results suggest that
piperlongumine also plays an important role in attenuating the drug
efflux of K562/A02 cells.
Piperlongumine regulates drug
resistance-related genes and signaling pathways
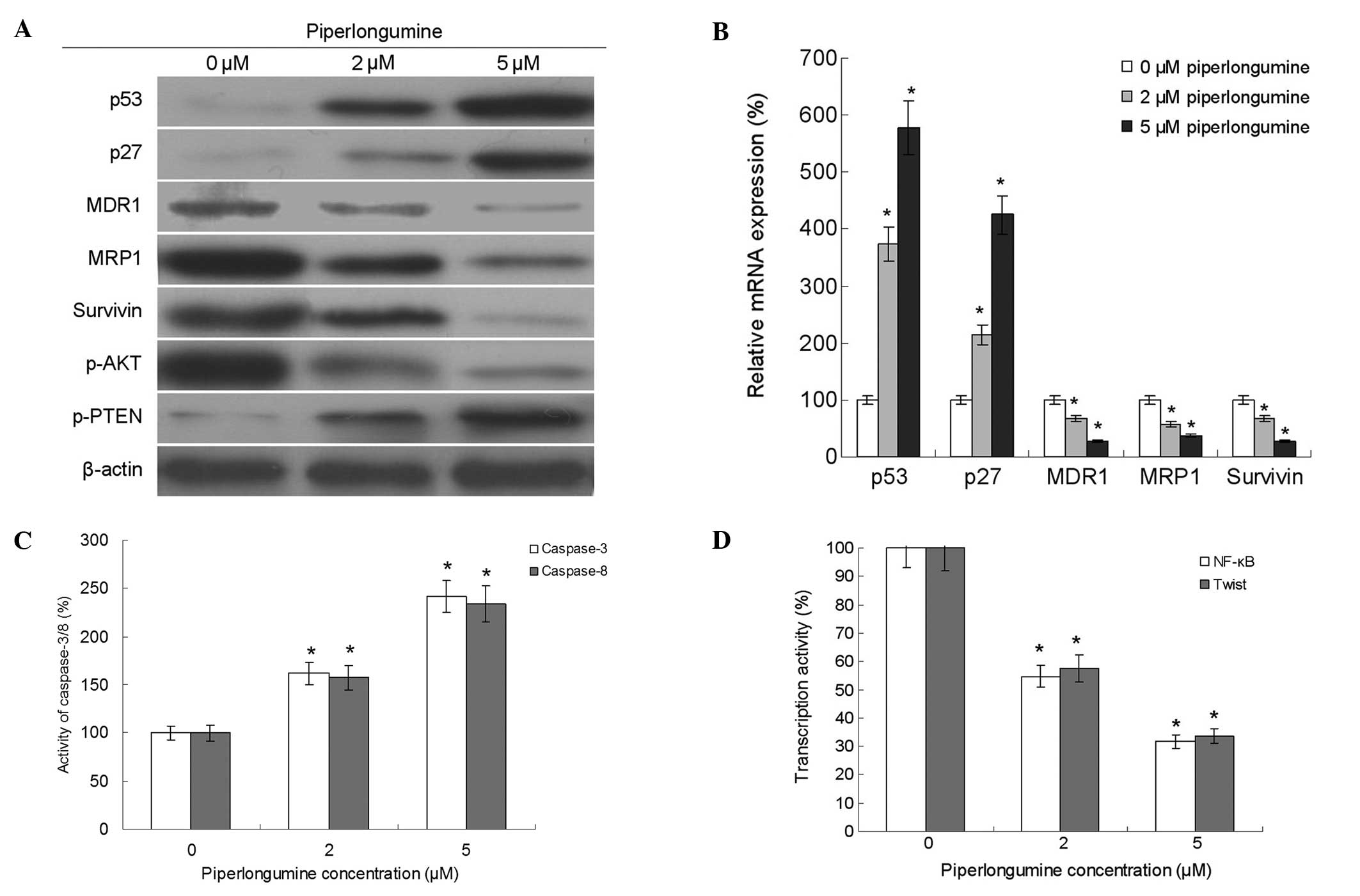
The proliferation and survival of tumor cells may be
increased by the regulation of drug resistance-related genes and
signaling pathways (13). In the
present study, the expression levels of MDR1, MRP1, survivin, p53
and p27 and the activity of caspase-3/8 were detected. The results
showed that piperlongumine decreased the expression of MDR1, MRP1
and survivin, and increased the expression of p53 and p27 and the
activities of caspase-3 and -8. Furthermore, the results also
showed that piperlongumine decreased the phosphorylation of Akt and
the transcriptional activities of NF-κB and twist. In addition, it
increased the phosphorylation of PTEN (Fig. 3).
Discussion
The development of tumor drug resistance is a
multi-step, multi-stage and multi-factorial complex process. There
are two major mechanisms associated with the occurrence of
drug-resistant in leukemia, namely, drug efflux and drug
resistance-related genes (14–19).
Previous studies have shown that piperlongumine is an inhibitor of
human tumors. The results of the present study demonstrate the drug
resistance reversal effects of piperlongumine in K562/A02 human
leukemia cells, and indicate that they are achieved by the
regulation of drug efflux and drug resistance-related genes. The
present study also found that piperlongumine enhanced the
intracellular concentration of rhodamine-123 and reduced the
cellular surface expression of P-gp, suggesting that piperlongumine
may be able to suppress drug efflux. In addition, the results
showed that piperlongumine enhanced ROS production by the tumor
cells, suggesting that ROS may be one of triggers to drug
resistance reversal.
Furthermore, the present study revealed that
piperlongumine induced apoptosis and G2/M phase arrest.
Enhancements in the expression levels of p53 and p27 and reductions
in the expression levels of survivin and the activities of
caspase-3 and -8, suggest that the effects of piperlongumine as an
inhibitor of tumor proliferation and inducer of cell cycle arrest
may be associated with its ability to regulate cell apoptosis and
cell cycle-related genes.
Drug resistance mechanisms also involve the
expression of MDR1 and MRP1 through encoding P-gp and intracellular
transport proteins. They are ATP-dependent drug efflux pumps and
able to actively pump lipophilic drugs out of the cells using the
energy released from ATP hydrolysis due to the extremely low
intracellular drug levels (20,21).
The results of the present study showed that piperlongumine
downregulated the expression of MDR1 and MRP, resulting in the
decreased expression of P-gp and other intracellular transport
proteins.
The results of the present study also showed that
piperlongumine downregulated the phosphorylation of Akt, a kinase
that plays a critical role in the phosphoinositide 3-kinase (PI3K)
signaling pathway and regulates cell survival, differentiation,
proliferation and metabolism by modulating the expression of genes
such as p53 and p27 (22).
Piperlongumine was also demonstrated to inhibit the transcriptional
activities of NF-κB and twist in K562/A02 cells, and there is some
evidence that the transcriptional activities of NF-κB and twist are
associated with tumorigenesis (23–26).
In summary, this study found that piperlongumine is
able to reverse the drug resistance of the K562/A02 human leukemia
cell and that its mechanism of action involved the regulation of
tumor drug resistance-associated gene expression. Thus,
piperlongumine may potentially be useful as a new therapeutic agent
for leukemia.
References
|
1
|
Mardiros A, Brown CE, Budde LE, Wang X and
Forman SJ: Acute myeloid leukemia therapeutics: CARs in the
driver’s seat. Oncoimmunology. 12:e272142013. View Article : Google Scholar
|
|
2
|
Joshi I, Yoshida T, Jena N, Qi X, Zhang J,
Van Etten RA and Georgopoulos K: Loss of Ikaros DNA-binding
function confers integrin-dependent survival on pre-B cells and
progression to acute lymphoblastic leukemia. Nat Immunol.
15:294–304. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rashid A, Lee NG, Jakobiec FA and Freitag
SK: Epstein-Barr virus-positive polymorphous lymphoplasmacytic
infiltrate of the lacrimal glands in a patient with acute
lymphoblastic leukemia. JAMA Ophthalmol. 132:892–894. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuasa M, Ishiwata K, Sugio T, et al:
Herpes simplex virus type 2 fulminant hepatitis after umbilical
cord blood transplantation for acute myeloid leukemia. Rinsho
Ketsueki. 55:682–686. 2014.(In Japanese). PubMed/NCBI
|
|
5
|
Shamroe CL and Comeau JM: Ponatinib: A new
tyrosine kinase inhibitor for the treatment of chronic myeloid
leukemia and Philadelphia chromosome-positive acute lymphoblastic
leukemia. Ann Pharmacother. 11:1540–1546. 2013. View Article : Google Scholar
|
|
6
|
Badura S, Tesanovic T, Pfeifer H, Wystub
S, Nijmeijer BA, Liebermann M, Falkenburg JH, Ruthardt M and
Ottmann OG: Differential effects of selective inhibitors targeting
the PI3K/AKT/mTOR pathway in acute lymphoblastic leukemia. PLoS
One. 11:e800702013. View Article : Google Scholar
|
|
7
|
Han SS, Han S and Kamberos NL:
Piperlongumine inhibits the proliferation and survival of B-cell
acute lymphoblastic leukemia cell lines irrespective of
glucocorticoid resistance. Biochem Biophys Res Commun. 452:669–675.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pei S, Minhajuddin M, Callahan KP, et al:
Targeting aberrant glutathione metabolism to eradicate human acute
myelogenous leukemia cells. J Biol Chem. 288:33542–33558. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makhov P, Golovine K, Teper E, Kutikov A,
Mehrazin R, Corcoran A, Tulin A, Uzzo RG and Kolenko VM:
Piperlongumine promotes autophagy via inhibition of Akt/mTOR
signalling and mediates cancer cell death. Br J Cancer.
110:899–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ginzburg S, Golovine KV, Makhov PB, Uzzo
RG, Kutikov A and Kolenko VM: Piperlongumine inhibits NF-κB
activity and attenuates aggressive growth characteristics of
prostate cancer cells. Prostate. 2:177–186. 2014. View Article : Google Scholar
|
|
11
|
Liu Y, Chang Y, Yang C, et al:
Biodegradable nanoassemblies of piperlongumine display enhanced
anti-angiogenesis and anti-tumor activities. Nanoscale.
6:4325–4337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vasconcelos FC, Nestal de Moraes G,
Moellmann-Coelho A and Maia RC: Phosphorylated Crkl reduction
levels are associated with the lowest P-glycoprotein activity
levels in cells from chronic myeloid leukemia patients. Leuk Res.
12:1711–1718. 2013. View Article : Google Scholar
|
|
13
|
Doroshow JH: Overcoming resistance to
targeted anticancer drugs. N Engl J Med. 19:1852–1853. 2013.
View Article : Google Scholar
|
|
14
|
Ariës IM, Hansen BR, Koch T, van den
Dungen R, Evans WE, Pieters R and den Boer ML: The synergism of
MCL1 and glycolysis on pediatric acute lymphoblastic leukemia cell
survival and prednisolone resistance. Haematologica. 12:1905–1911.
2013. View Article : Google Scholar
|
|
15
|
Daflon-Yunes N, Pinto-Silva FE, Vidal RS,
Novis BF, Berguetti T, Lopes RR, Polycarpo C and Rumjanek VM:
Characterization of a multidrug-resistant chronic myeloid leukemia
cell line presenting multiple resistance mechanisms. Mol Cell
Biochem. 1–2:123–135. 2013. View Article : Google Scholar
|
|
16
|
Wang JY, Yu P, Chen S, Xing H, Chen Y,
Wang M, Tang K, Tian Z, Rao Q and Wang J: Activation of Rac1 GTPase
promotes leukemia cell chemotherapy resistance, quiescence and
niche interaction. Mol Oncol. 5:907–916. 2013. View Article : Google Scholar
|
|
17
|
Karami H, Baradaran B, Esfahani A, Estiar
MA, Naghavi-Behzad M, Sakhinia M and Sakhinia E: siRNA-mediated
silencing of survivin inhibits proliferation and enhances etoposide
chemosensitivity in acute myeloid leukemia cells. Asian Pac J
Cancer Prev. 12:7719–7724. 2013. View Article : Google Scholar
|
|
18
|
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S,
Huang H, Liu N, Liao S, Song W, Zhou P, Wang S, Xu L, Wang X, Dou
QP and Liu J: Gambogic acid induces apoptosis in imatinib-resistant
chronic myeloid leukemia cells via inducing proteasome inhibition
and caspase-dependent Bcr-Abl downregulation. Clin Cancer Res.
1:151–163. 2014. View Article : Google Scholar
|
|
19
|
Chromik J, Safferthal C, Serve H and Fulda
S: Smac mimetic primes apoptosis-resistant acute myeloid leukaemia
cells for cytarabine-induced cell death by triggering necroptosis.
Cancer Lett. 1:101–109. 2014. View Article : Google Scholar
|
|
20
|
Rahgozar S, Moafi A, Abedi M,
Entezar-E-Ghaem M, Moshtaghian J, Ghaedi K, Esmaeili A and
Montazeri F: mRNA expression profile of multidrug-resistant genes
in acute lymphoblastic leukemia of children, a prognostic value for
ABCA3 and ABCA2. Cancer Biol Ther. 1:35–41. 2014. View Article : Google Scholar
|
|
21
|
Ma H, Cheng L, Hao K, Li Y, Song X, Zhou H
and Jia L: Reversal effect of ST6GAL 1 on multidrug resistance in
human leukemia by regulating the PI3K/Akt pathway and the
expression of P-gp and MRP1. PLoS One. 1:e851132014. View Article : Google Scholar
|
|
22
|
Shrivastava S, Kulkarni P, Thummuri D,
Jeengar MK, Naidu VG, Alvala M, Redddy GB and Ramakrishna S:
Piperlongumine, an alkaloid causes inhibition of PI3 K/Akt/mTOR
signaling axis to induce caspase-dependent apoptosis in human
triple-negative breast cancer cells. Apoptosis. 7:1148–1164. 2014.
View Article : Google Scholar
|
|
23
|
Liu Z, Zhang YY, Zhang QW, Zhao SR, Wu CZ,
Cheng X, Jiang CC, Jiang ZW and Liu H: 3-Bromopyruvate induces
apoptosis in breast cancer cells by downregulating Mcl-1 through
the PI3K/Akt signaling pathway. Anticancer Drugs. 4:447–455. 2014.
View Article : Google Scholar
|
|
24
|
Reikvam H, Tamburini J, Skrede S, Holdhus
R, Poulain L, Ersvaer E, Hatfield KJ and Bruserud Ø: Antileukaemic
effect of PI3K-mTOR inhibitors in acute myeloid leukaemia-gene
expression profiles reveal CDC25B expression as determinate of
pharmacological effect. Br J Haematol. 2:200–211. 2014. View Article : Google Scholar
|
|
25
|
Kim MS, Kim GM, Choi YJ, Kim HJ, Kim YJ
and Jin W: TrkC promotes survival and growth of leukemia cells
through Akt-mTOR-dependent up-regulation of PLK-1 and Twist-1. Mol
Cells. 2:177–184. 2013. View Article : Google Scholar
|
|
26
|
Pede V, Rombout A, Vermeire J, Naessens E,
Vanderstraeten H, Philippé J and Verhasselt B: Expression of ZAP70
in chronic lymphocytic leukaemia activates NF-κB signalling. Br J
Haematol. 5:621–630. 2013. View Article : Google Scholar
|