Introduction
Ovarian cancer is a common gynecological malignancy
and remains one of the leading causes of cancer-related mortality
among females (1). Proliferation,
invasion and metastasis are crucial processes in the development of
ovarian cancer, and metastasis occurs prior to diagnosis in the
majority of patients with ovarian cancer. The expression of a
variety of proteins can change during the progression of cancer.
Thus, investigating the functions of these proteins may offer novel
targets for the diagnosis and treatment of ovarian cancer (2).
S100 proteins are a group of low molecular weight
(10–12 kDa) acidic proteins that belong to the largest family of
EF-hand calcium-binding proteins and are only expressed in
vertebrates (3). S100A11, also
known as S100C or calgizzarin, is an important member of the S100
family (4). Overexpression of
S100A11 has been reported in a number of cancers, including
papillary thyroid carcinoma (5),
colon (6), pancreatic (7) and breast cancer (8). A previous study demonstrated that
increased S100A11 expression is correlated with the metastasis of
gastric cancer and poor overall disease prognosis (9). However, decreased expression of
S100A11 has been reported in bladder cancer, and downregulation of
S100A11 has been associated with bladder cancer progression
(10). The role of S100A11 in
ovarian cancer remains unclear. Therefore, the aim of the present
study was to analyze the levels of S100A11 in ovarian cancer cells.
In addition, the effects of S100A11 knockdown on ovarian cancer
cell growth and invasion were investigated in order to determine
the function of S100A11 in ovarian cancer progression.
Materials and methods
Antibodies
An antibody targeting E-cadherin (rabbit polyclonal;
1:1,000; cat. no. sc-21791) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). An antibody targeting Snail
(mouse monoclonal; 1:1,000; cat. no. 3895) was obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA), while antibodies
targeted against S100A11 (mouse monoclonal; 1:1,000; cat. no.
WH0006282M1) β-actin (mouse monoclonal; 1:1,000; cat. no. A3853)
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Cell lines were purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). The human ovarian
surface epithelial cell line, IOSE144, was maintained in MCDB105
medium containing L-glutamine, HEPES (25 mM) and 10% fetal bovine
serum (FBS) (all Sigma-Aldrich). Human ovarian cancer cell lines,
A2780 and SKOV3, were maintained in Dulbecco’s modified Eagle’s
medium containing 10% FBS, while the human ovarian cancer cell
lines, OVCAR3 and HO8910, were maintained in RPMI 1640 medium
containing 10% FBS. Cells were stored at 37°C in a humidified
atmosphere of 5% CO2.
RNA interference of S100A11
An S100A11 short hairpin (sh)RNA vector was designed
and synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China),
with a target sequence of 5′-GGATGGTTATAACTACACT-3′. A scramble
shRNA vector was used as a negative control. HO8910 cells were
transfected with S100A11 or control shRNA using
Lipofectamine® LTX with Plus™ reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The stable cell
clones were isolated using Geneticin® Selective
Antibiotic (G418 Sulfate; Gibco Life Technologies, Beijing,
China).
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer and the protein
concentration was determined using a bicinchoninic acid assay
(Sigma-Aldrich). Subsequently, 50 mg total protein was resolved
using SDS-PAGE and the separated protein was transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked in 5% non-fat milk for 1 h and then
incubated with primary antibodies overnight at 4°C. After washing
three times with phosphate-buffered saline with Tween-20, the
membrane was incubated with peroxidase-conjugated AffiniPure goat
anti-mouse (ZB-2305) and goat anti-rabbit (ZB-2301) immunoglobulin
G secondary antibodies (1:3,000; Zhongshan Jinqiao Biotech, Co.,
Ltd., Beijing, China) for 1 h at room temperature. Bands were
detected with an enhanced chemiluminescence detection kit (Applygen
Technologies, Inc., Beijing, China) and the densitometry of each
band was analyzed with ImageJ software.
Cell growth assay
Cells were seeded into a 24-well plate at
1×104 cells/well and incubated in RPMI 1640 medium
overnight. Cells in the plate were trypsinized (Sigma-Aldrich and
counted using a hemocytometer (Sigma-Aldrich) every day for seven
days. The experiments were repeated three times.
MTT assay
Cell growth rate was determined by an MTT assay.
Briefly, 2×103 cells/well were seeded into a 96-well
plate. Subsequently, 20 μl MTT solution (5 mg/ml; Sigma-Aldrich)
was added to each well and the cells were further incubated at 37°C
in 5% CO2 for 4 h. Next, the RPMI 1640 medium was
removed and dimethyl sulfoxide was added to the wells to dissolve
the colored formazan crystals produced by MTT. Finally, the optical
density (OD) value was measured at 490 nm using a Bio-Rad 2550 EIA
Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Soft agar assay
A soft agar assay was used to examine the
anchorage-independent growth of ovarian cancer cells. Cells at a
density of 0.5×103 cells/ml were suspended in RPMI 1640
medium supplemented with 10% FBS and 0.3% agarose. The cell
suspension was added to a base layer formed by culture medium
containing 0.6% agarose. After 14 days, the colony number of each
well was counted under an inverted microscope (Olympus X71; Olympus
Corporation, Tokyo, Japan) at ×100 magnification.
Invasion assay
A 24-well Transwell® plate was obtained
from Corning, Inc. (Costar®; Corning, NY, USA), and the
cell inserts were coated with Matrigel™ (BD Biosciences, Franklin
Lakes, NJ, USA). Cells (1.0×105) in RPMI 1640 medium
were plated in the upper chambers, while the lower chambers were
filled with RPMI 1640 medium containing 20% FBS as a
chemoattractant. The cells were allowed to invade for 24 h, at 37°C
and 5% CO2. Cells in the upper chambers were removed
with a cotton swab and the filters were stained with eosin. Next,
five random fields were observed and the cell numbers were counted
under an inverted microscope (Olympus X71) at ×100
magnification.
Migration assay
A migration assay was performed with a 24-well
Transwell® plate. Cells were suspended in RPMI 1640
medium and adjusted to a concentration of 5.0×105
cells/ml. A 100-μl cell suspension was plated in the upper
chambers, and RPMI 1640 medium containing 20% FBS was filled in the
lower chambers as a chemoattractant. After 24 h, the non-migrated
cells were removed with a cotton swab and the filters were stained
with eosin. Finally, the number of migrated cells was counted under
an inverted microscope (Olympus X71) at ×100 magnification.
Statistical analysis
Experiments were performed a minimum of three times
and the results are presented as the mean ± standard deviation. The
Student’s t-test was used for statistical analysis, where P<0.05
was considered to indicate a statistically significant
difference.
Results
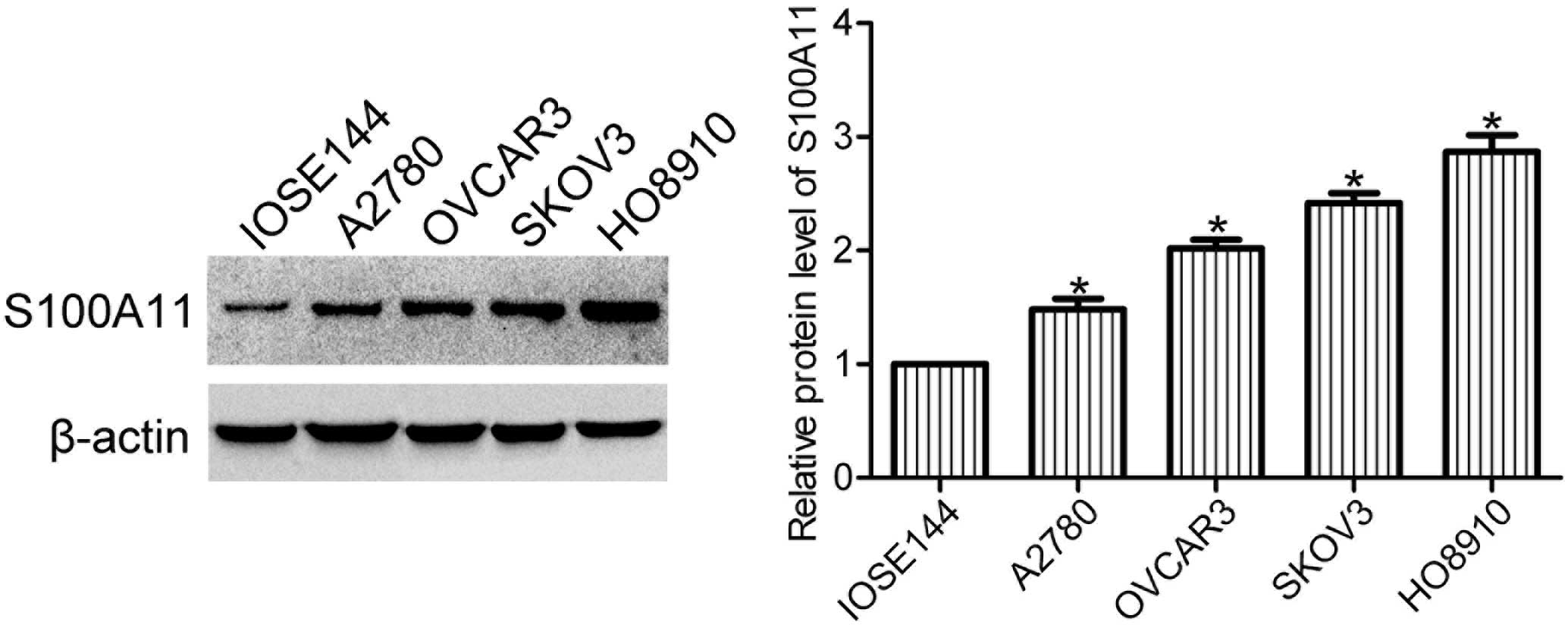
Expression levels of S100A11 are elevated
in ovarian cancer cells
Expression levels of S100A11 in human ovarian
surface epithelial cells (IOSE144) and ovarian cancer cells (A2780,
OVCAR3, SKOV3 and HO8910) were determined using western blot
analysis. The expression levels of S100A11 were markedly increased
in the ovarian cancer cell lines when compared with the ovarian
surface epithelial cell line (Fig.
1), indicating that S100A11 may play a role in the progression
of ovarian cancer.
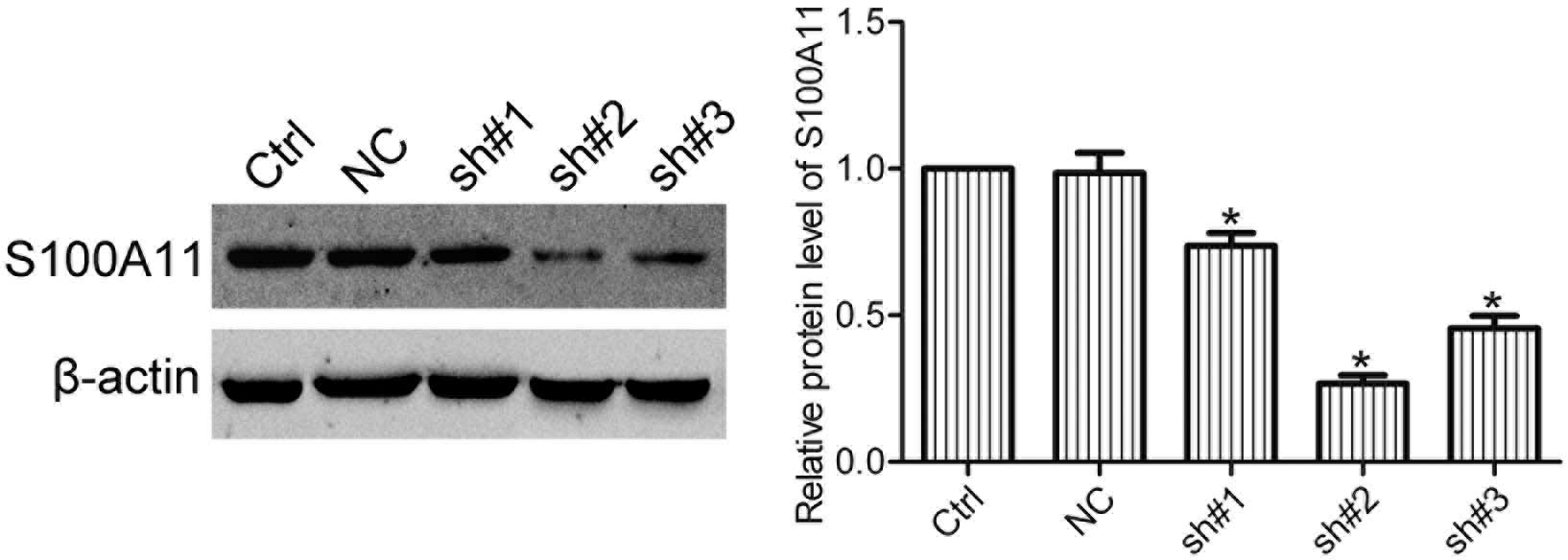
Expression of S100A11 in HO8910 cells is
silenced by shRNA
In order to silence the expression of S100A11 in
ovarian cancer cells, HO8910 cells were transfected with S100A11
shRNA (sh), and three stable cell clones (sh#1, sh#2 and sh#3) were
isolated by G418 selection. Western blot analysis showed that sh#2
cells significantly repressed the expression of S100A11 in HO8910
cells (Fig. 2). Therefore, sh#2
cells were used in the following experiments.
Knockdown of S100A11 inhibits the growth
of ovarian cancer cells
Effects of S100A11 knockdown on the growth of
ovarian cancer cells were examined using a cell growth assay, which
revealed that knockdown of S100A11 inhibited the growth of ovarian
cancer cells (Fig. 3A). In order
to confirm this result, an MTT assay was performed and the results
were found to be consistent with the cell growth assay (Fig. 3B). Furthermore, the effects of
S100A11 knockdown on the anchorage-independent growth of ovarian
cancer cells were examined using a soft agar assay. The results
revealed that knockdown of S100A11 decreased the number of HO8910
cell colonies (Fig. 3C).
Collectively, these results indicated that S100A11 was involved in
the regulation of ovarian cancer cell growth.
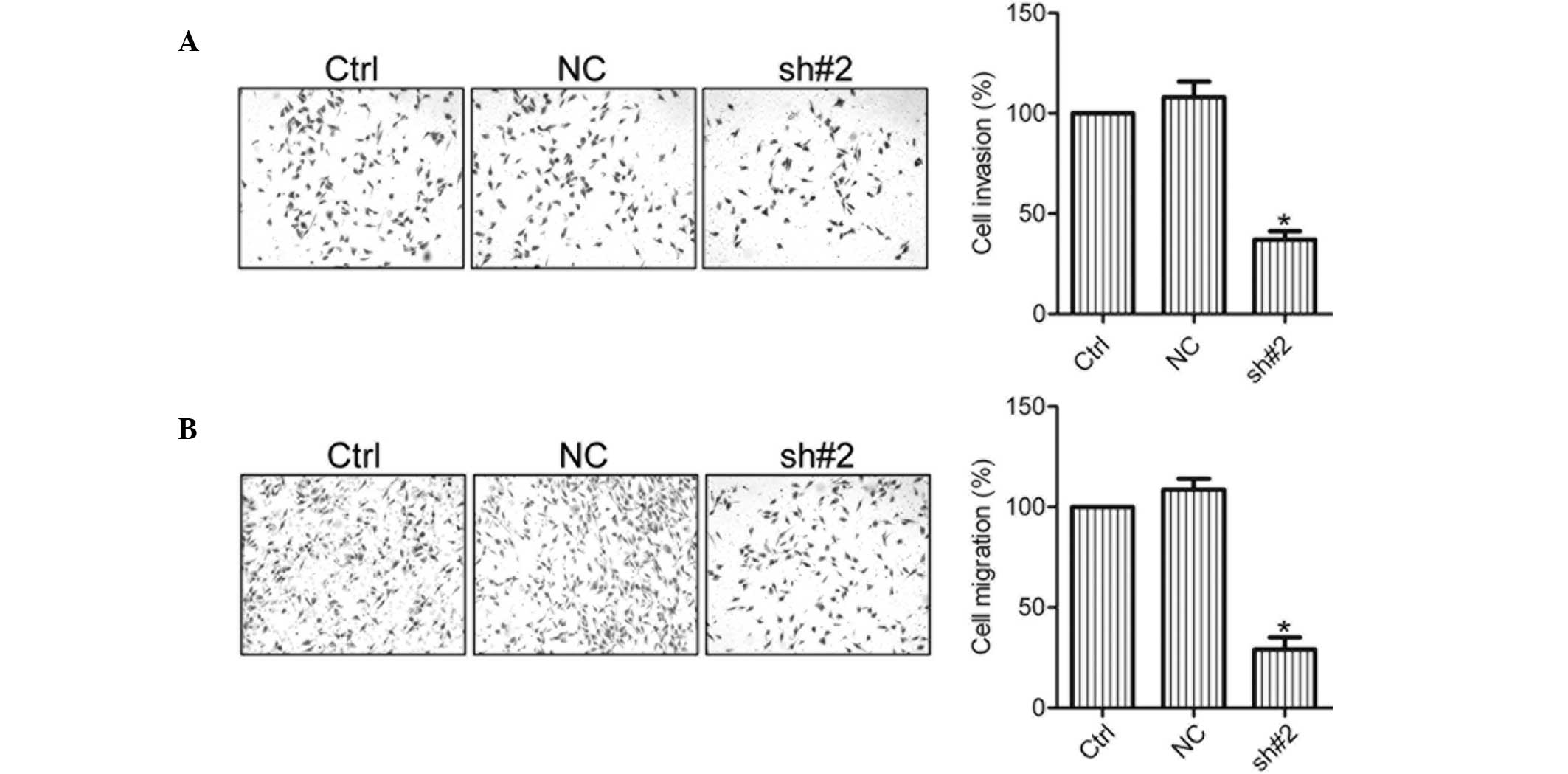
Knockdown of S100A11 suppresses cell
invasion and migration
In order to determine whether S100A11 was associated
with the invasion and migration of ovarian cancer cells, invasion
and migration assays were conducted with mock-transfected control
(Ctrl), negative control (NC) and S100A11 shRNA cells (sh#2). The
results demonstrated that the invasion and migration activity of
HO8910 cells was greatly suppressed in the sh#2 cells when compared
with the Ctrl and NC cells (Fig.
4), indicating that S100A11 was able to promote the invasion
and migration of ovarian cancer cells.
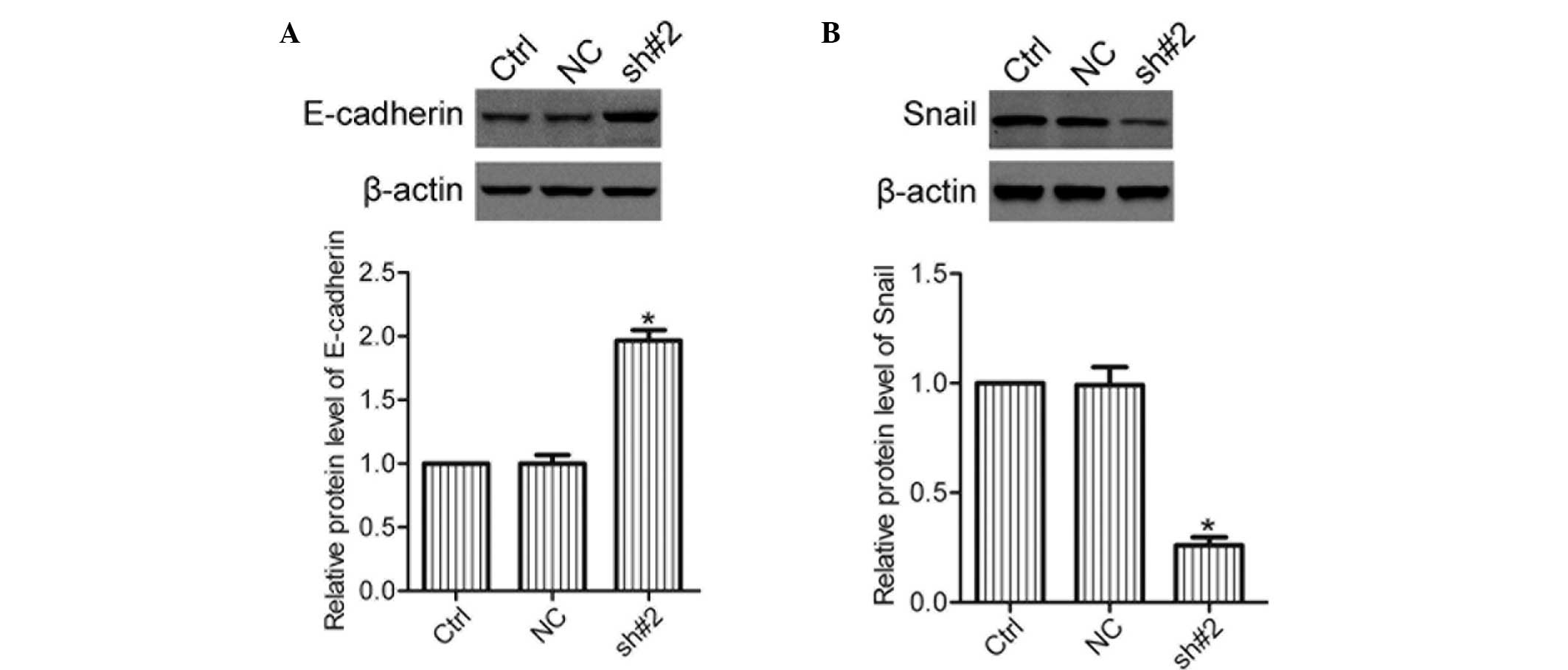
Knockdown of S100A11 decreases the
expression levels of Snail, but increases those of E-cadherin
Snail and E-cadherin are important
epithelial-mesenchymal transition (EMT) markers that are essential
for the invasion and metastasis of cancer (11). The expression levels of E-cadherin
and Snail in the Ctrl, NC and sh#2 cells were analyzed using
western blot analysis. The results revealed that knockdown of
S100A11 caused an increase in E-cadherin and a decrease in Snail
protein expression levels (Fig.
5), suggesting that S100A11 affected the expression of
E-cadherin and Snail in ovarian cancer cells.
Discussion
S100A11 is a member of the S100 protein family, and
is widely expressed in human tissues. Overexpression of S100A11 has
been identified in a variety of human cancer types and elevated
S100A11 expression is closely associated with tumor progression
(12). Increased levels of S100A11
have been reported in ovarian cancer tissues, as compared with
normal ovarian epithelial tissues (13). In the present study, the expression
levels of S100A11 were found to be significantly increased in the
ovarian cancer cell lines. Thus, S100A11 may play an important role
in the progression of ovarian cancer.
S100A11 has a number of contrasting roles in the
regulation of tumor growth. A previous study found that S100A11
promoted the proliferation of lung cancer cells (14). In addition, silencing S100A11
expression has been shown to reduce the anchorage-independent
growth of papillary thyroid carcinoma cells (5). However, S100A11 is also reported to
be a tumor suppressor in hepatocellular carcinoma cells and
epidermal keratinocytes (15,16).
The role of S100A11 in ovarian cancer has not been fully
characterized. The results of the present study demonstrated that
knockdown of S100A11 inhibited the proliferation and
anchorage-independent growth of HO8910 cells, suggesting that
S100A11 contributes to the growth of ovarian cancer cells.
A previous study reported that overexpression of
S100A11 is associated with the metastasis of cancer (17). In addition, experimental studies
have shown that S100A11 is a migration-related protein in laryngeal
squamous cell carcinoma (18), and
is involved in the invasion of hepatocellular carcinoma cells
(19). A recent study showed that
S100A11 is required for the survival of invasive cancer cells
(20). In the present study,
knockdown of S100A11 was found to suppress the invasion and
migration of HO8910 cells, indicating that S100A11 may be involved
in the regulation of ovarian cancer cell invasion and
migration.
EMT plays a key role in the invasion and metastasis
of ovarian cancer (21), and
E-cadherin and Snail are crucial EMT cancer markers (22). E-cadherin is a transmembrane
protein that regulates cell-cell adhesion, while Snail is a
transcriptional repressor of E-cadherin gene expression (23). S100A4 has been reported to enhance
pancreatic cancer cell invasion via the regulation of E-cadherin
expression (24), and
overexpression of S100A6 has been reported to result in the
downregulation of E-cadherin (25). However, whether S100A11 affects the
expression levels of E-cadherin and Snail has not yet been
investigated. The present study demonstrated that knockdown of
S100A11 increased the expression of E-cadherin and decreased the
expression of Snail in ovarian cancer cells. In addition, knockdown
of S100A11 inhibited the invasion and migration of ovarian cancer
cells, indicating that S100A11 may promote ovarian cancer cell
invasion and migration via the regulation of E-cadherin and Snail
expression.
In conclusion, S100A11 is overexpressed in ovarian
cancer cells and the knockdown of S100A11 suppresses the growth,
invasion and migration of ovarian cancer cells. Thus, inhibition of
S100A11 may provide a promising approach to the treatment of
ovarian cancer.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longuespée R, Boyon C, Desmons A, et al:
Ovarian cancer molecular pathology. Cancer Metastasis Rev.
31:713–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schäfer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: functions and
pathology. Trends Biochem Sci. 21:134–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heizmann CW, Fritz G and Schäfer BW: S100
proteins: structure, functions and pathology. Front Biosci.
7:d1356–d1368. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anania MC, Miranda C, Vizioli MG, et al:
S100A11 overexpression contributes to the malignant phenotype of
papillary thyroid carcinoma. J Clin Endocrinol Metab.
98:E1591–E1600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meding S, Balluff B, Elsner M, et al:
Tissue-based proteomics reveals FXYD3, S100A11 and GSTM3 as novel
markers for regional lymph node metastasis in colon cancer. J
Pathol. 228:459–470. 2012.PubMed/NCBI
|
|
7
|
Xiao MB, Jiang F, Ni WK, et al: High
expression of S100A11 in pancreatic adenocarcinoma is an
unfavorable prognostic marker. Med Oncol. 29:1886–1891. 2012.
View Article : Google Scholar
|
|
8
|
Liu XG, Wang XP, Li WF, et al:
Ca2+-binding protein S100A11: a novel diagnostic marker
for breast carcinoma. Oncol Rep. 23:1301–1308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mori M, Shimada H, Gunji Y, et al: S100A11
gene identified by in-house cDNA microarray as an accurate
predictor of lymph node metastases of gastric cancer. Oncol Rep.
11:1287–1293. 2004.PubMed/NCBI
|
|
10
|
Memon AA, Sorensen BS, Meldgaard P, Fokdal
L, Thykjaer T and Nexo E: Down-regulation of S100C is associated
with bladder cancer progression and poor survival. Clin Cancer Res.
11:606–611. 2005.PubMed/NCBI
|
|
11
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKiernan E, McDermott EW, Evoy D, Crown J
and Duffy MJ: The role of S100 genes in breast cancer progression.
Tumour Biol. 32:441–450. 2011. View Article : Google Scholar
|
|
13
|
Wang LN, Tong SW, Hu HD, et al:
Quantitative proteome analysis of ovarian cancer tissues using a
iTRAQ approach. J Cell Biochem. 113:3762–3772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hao J, Wang K, Yue Y, et al: Selective
expression of S100A11 in lung cancer and its role in regulating
proliferation of adenocarcinomas cells. Mol Cell Biochem.
359:323–332. 2012. View Article : Google Scholar
|
|
15
|
Miyazaki M, Sakaguchi M, Akiyama I,
Sakaguchi Y, Nagamori S and Huh NH: Involvement of interferon
regulatory factor 1 and S100C/A11 in growth inhibition by
transforming growth factor beta 1 in human hepatocellular carcinoma
cells. Cancer Res. 64:4155–4161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakaguchi M and Huh NH: S100A11, a dual
growth regulator of epidermal keratinocytes. Amino Acids.
41:797–807. 2011. View Article : Google Scholar
|
|
17
|
Ji YF, Huang H, Jiang F, Ni RZ and Xiao
MB: S100 family signaling network and related proteins in
pancreatic cancer (Review). Int J Mol Med. 33:769–776.
2014.PubMed/NCBI
|
|
18
|
Wang C, Zhang Z, Li L, et al: S100A11 is a
migration-related protein in laryngeal squamous cell carcinoma. Int
J Med Sci. 10:1552–1559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo X, Xie H, Long X, et al: EGFRvIII
mediates hepatocellular carcinoma cell invasion by promoting S100
calcium binding protein A11 expression. PLoS One. 8:e833322013.
View Article : Google Scholar :
|
|
20
|
Jaiswal JK, Lauritzen SP, Scheffer L, et
al: S100A11 is required for efficient plasma membrane repair and
survival of invasive cancer cells. Nat Commun. 5:37952014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallo D, Ferlini C and Scambia G: The
epithelial-mesenchymal transition and the estrogen-signaling in
ovarian cancer. Curr Drug Targets. 11:474–481. 2010. View Article : Google Scholar
|
|
22
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li N, Song MM, Chen XH, Liu LH and Li FS:
S100A4 siRNA inhibits human pancreatic cancer cell invasion in
vitro. Biomed Environ Sci. 25:465–470. 2012.PubMed/NCBI
|
|
25
|
Li Z, Tang M, Ling B, et al: Increased
expression of S100A6 promotes cell proliferation and migration in
human hepatocellular carcinoma. J Mol Med (Berl). 92:291–303. 2014.
View Article : Google Scholar
|