Introduction
Residual tumor cells are a major complication in the
therapy of hematopoietic tumors. The prognosis for patients with
relapse due to the presence of residual tumor cells remains
unsatisfactory (1). Therefore, the
development of therapy for the depletion of these cells is
important in the therapy of hematopoietic tumors.
Immunotherapy to stimulate the immune system of the
patient is a promising method of depleting the residual cells of
hematopoietic tumors. Cytokine-induced killer (CIK) cells are major
histocompatibility complex (MHC)-unrestricted cytotoxic lymphocytes
that can be induced by anti-CD3 monoclonal antibody (mAb),
interleukin (IL)-2, IL-1 and interferon (IFN)-γ, include
CD3+CD56+ cells and have high proliferation
potential. The activity of CIK cells against tumor cells is an
effective and MHC-unrestricted (2,3). CIK
cells have the capacity to migrate toward tumor sites (4,5) and
display antitumor activity in vivo (5). However, the clinical applicability of
CIK cells to deplete residual leukemic cells has not been proven by
various phase I studies performed thus far (6,7). The
most relevant reason may be the limited basal antitumor activity of
CIK cells. CIK cells exhibited a mean lytic activity of only 40%
against the leukemic cells of patients in an in vitro assay
(7). Therefore, it is necessary to
increase the antitumor activity and the clinical applicability of
CIK cells.
Rituximab is an anti-CD20 mAb used in the therapy of
diffuse large B-cell lymphoma (DLBCL). In clinical trials, the use
of rituximab alone or in combination with chemotherapy regimens as
the first-line treatment has been shown to significantly improve
response and survival for DLBCL (8–10).
In the present study,
CD3+CD56+ cells were acquired from the
peripheral blood of healthy donors and cultured in vitro in
the presence of cytokines combined with rituximab to generate CIK
cells. The antitumor activity of CIK cells to the SU-DHL2 and K562
human leukemia cell lines was investigated. A preliminary
investigation to elucidate the mechanism was then performed.
Materials and methods
Human cell lines
One week prior to the experiment, the (SU-DHL2) cell
line and the human chronic myelogenous leukemia cell line K562
(provided by the Cell Bank of the Shanghai Institute of Cell
Biology, Chinese Academy of Science, Shanghai, China) were
maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal calf serum, 50 U/ml penicillin and 50 mg/ml
streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA;
further referred to as ‘complete medium’).
Generation of CIK cells
Peripheral blood CD3+CD56+
cells were isolated by negative selection from 12 healthy donors
from the laboratory and department and collected by venipuncture.
Cells were isolated by negative selection from fresh blood using
magnetic beads (CD3+CD56+ NKT Cell Isolation
kit; Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were
cultured in complete medium at a density of 3×106
cells/ml/well with recombinant human IFN-γ (1×106 U/l),
recombinant human IL-2 (rhIL-2; 5×105 U/l; PeproTech
Inc., Rocky Hill, NJ, USA), mouse anti-human CD3 monoclonal
antibody (50 μg/l; Aibo Trading Co. Ltd, Shenzen, China) and
clinical grade rituximab (5×104 μg/l;
Rituxan®; Roche, Basel, Switzerland) at 37°C with 5%
CO2.
Flow cytometry
Phenotypic analysis of the cells obtained from CIK
cultures after washing twice with phosphate-buffered saline (PBS)
was performed by mAb staining, using peridinin-chlorophyll-protein
complex (PerCP)-anti-CD3, PerCP-anti-CD4, fluorescein
isothiocyanate (FITC)-anti-CD56, FITC-anti-CD25, phycoerythrin
(PE)-anti-perforin, PE-anti-granzyme B (Becton-Dickinson
Biosciences, Franklin Lakes, NJ, USA) and PE-anti-CD314 (Beckman
Coulter, Milan, Italy) on day 14. The cells (1×106) were
incubated with various conjugated mAbs for 30 min at room
temperature, washed twice in PBS and then analyzed using a
FACSCalibur™ flow cytometer (Becton-Dickinson
Biosciences).
Cytotoxicity assays
After 14 days in culture, rituximab was washed out
of the experimental culture using PBS. Cytotoxicity of the CIK
cultures against the SU-DHL2 and K562 cell lines were measured
using the lactate dehydrogenase (LDH; Cytotoxicity Colorimetric
Assay kit; Sigma-Aldrich, St. Louis, MO, USA) assay method.
Effector (CIK) cell/target (SU-DHL2 or K562) cell (E/T) ratios of
10:1, 20:1 and 40:1 were used in the experimental and control
groups. The SU-DHL2 or K562 cells of the two groups were seeded at
a density of 2×105 cells/ml in round-bottomed 96-well
plates (Nunc A/S, Roskilde, Denmark) in RPMI-1640 complete medium
(in a final volume of 200 ml/well) at 37°C, 5% CO2 for
72 h. All the groups were seeded three times concurrently. The
plates were then centrifuged for 1 min at 10,000 × g. Then, 100 μl
supernatant was collected from each well and transferred to a new
flat-bottomed 96-well plate. Subsequent to the effects of the
reaction and stop solutions, the absorbance was measured at the
recommended wavelength using a Multiskan MS ELISA reader (Bio-Tek,
Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After 14 days in culture, rituximab was washed out
of the experimental culture using PBS. Cells in the experimental
and control groups were harvested and used for qPCR analysis. Total
RNA extracted from the cells with TRIzol® reagent
(Invitrogen Life Sciences) was used as the template for all reverse
transcriptase reactions. The cDNA was synthesized with random
priming from 10 μl total RNA with the aid of the Revert Aid™ First
Strand cDNA Synthesis kit (Fermentas, Carlsbad, CA, USA), following
the manufacturer’s instructions. For the PCR, 2 μl cDNA solution
was mixed with 10 μl SYBR® Premix Ex Taq II (Takara Bio
Inc., Shiga, Japan), 0.4 μl each of the forward and reverse primers
and 7.2 μl RNase-free water to provide a total volume of 20 μl. A
fluorescent quantitation PCR cycler (LightCycler®;
Roche) was used for amplification of perforin, granzyme B, INF-γ,
tumor necrosis factor (TNF)-α, TNF-β and Fas ligand (FasL) with the
primer pairs listed in Table I.
The amplification consisted of denaturation at 95°C for 5 sec,
annealing at 62°C for 20 sec and extension at 72°C for 10 sec (43
cycles). The threshold cycle (Ct) was subsequently determined.
Expression levels of perforin, granzyme B, INF-γ, TNF-α, TNF-β and
FasL, normalized to GAPDH and relative to a calibrator, was
expressed as 2−ΔΔCt (fold difference). All procedures
were performed following the manufacturer’s instructions.
 | Table IList of primers used for the
quantitative polymerase chain reaction analysis. |
Table I
List of primers used for the
quantitative polymerase chain reaction analysis.
| Target gene | Primer sequences | Amplification length
(bp) |
|---|
| Perforin | Forward
5′-CAGGTCAACATAGGCATCCACG-3′
Reverse 5′-GAACAGCAGGTCGTTAATGGAG-3′ | 160 |
| Granzyme B | Forward
5′-GAAACGCTACTAACTACAGG-3′
Reverse 5′-CCACTCAGCTAAGAGGT-3′ | 126 |
| INF-γ | Forward
5′-GCAGAGCCAAATTGTCTCCT-3′
Reverse 5′-ATGCTCTTCGACCTCGAAAC-3′ | 290 |
| TNF-α | Forward
5′-CGAGTGACAAGCCTGTAGC-3′
Reverse 5′-CCTTCTCCAGCTGGAAGAC-3′ | 363 |
| TNF-β | Forward
5′-AGGCATGAGGGATCACAG-3′
Reverse 5′-AAAGAGGTTTATTGGGCTTC-3′ | 115 |
| FasL | Forward
5′-TGTTTATGAGCCAGACAAATGG-3′
Reverse 5′-AAGACAGTCCCCCTTGAGGT-3′ | 203 |
Western blot analysis
After 14 days in culture, rituximab was washed out
of the experimental culture using PBS. Cells in the experimental
and control groups were harvested and used for western blot
analysis to detect the constitutively activated signal transduction
pathways in the CIK cells. Total protein was separated by SDS-PAGE
and then electrotransferred to a polyvinylidene fluoride membrane,
using a transfer apparatus set to 100 V for 1 h at 4°C. After
washing, the membranes were probed with the following primary
antibodies: signal transducer and activator of transcription 1
(STAT-1), STAT-3, STAT-5 mouse anti-rat monoclonal antibodies
(1:1,000; Millipore, Billerica, MA, USA), extracellular
signal-regulated kinase (ERK) 1/2 activated mouse anti-rat
monoclonal antibody and p38 mitogen-activated protein kinase (MAPK)
mouse anti-rat polyclonal antibody (1:1,000; Cell signaling
Technology, Inc., Danvers, MA, USA). After the membranes had been
agitated gently for at least 1 h, the antibody solution was poured
off the membrane and the membrane was washed twice for 10 min with
Tris-buffered saline and Tween 20 (TTBS) buffer. The mouse
anti-human monoclonal secondary antibody (Santa Cruz, Dallas, TX,
USA) was added at a dilution of 1:5,000 in 5 ml 0.5% blocking
buffer. The secondary antibody solution was then poured off the
membrane, which was subsequently washed twice. The TTBS buffer was
poured off and the developing reagent added. When the bands were
clearly observed, development was stopped by washing the membrane
with distilled water for 30 min with three changes.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed with SPSS software,
version 12.0 (SPSS, Inc., Chicago, IL, USA). Differences between
repeated experiments of the two groups were investigated using the
unpaired Student’s t-test and F-test. For all statistical tests,
values of P<0.05 were considered as statistically
significant.
Results
Cytotoxic activity of CIK cells
The cytotoxicity of the day-14 CIK cells against the
SU-DHL2 and K562 cell lines was measured using the LDH assay
method. The cytotoxic activity with different E/T ratios was
determined in the two groups against the two cell lines.
Cytotoxicities against the SU-DHL4 or K562 cells at each of the
different E/T ratios in the experimental (rituximab) group were
higher than those of the control group (P<0.05; Fig. 1).
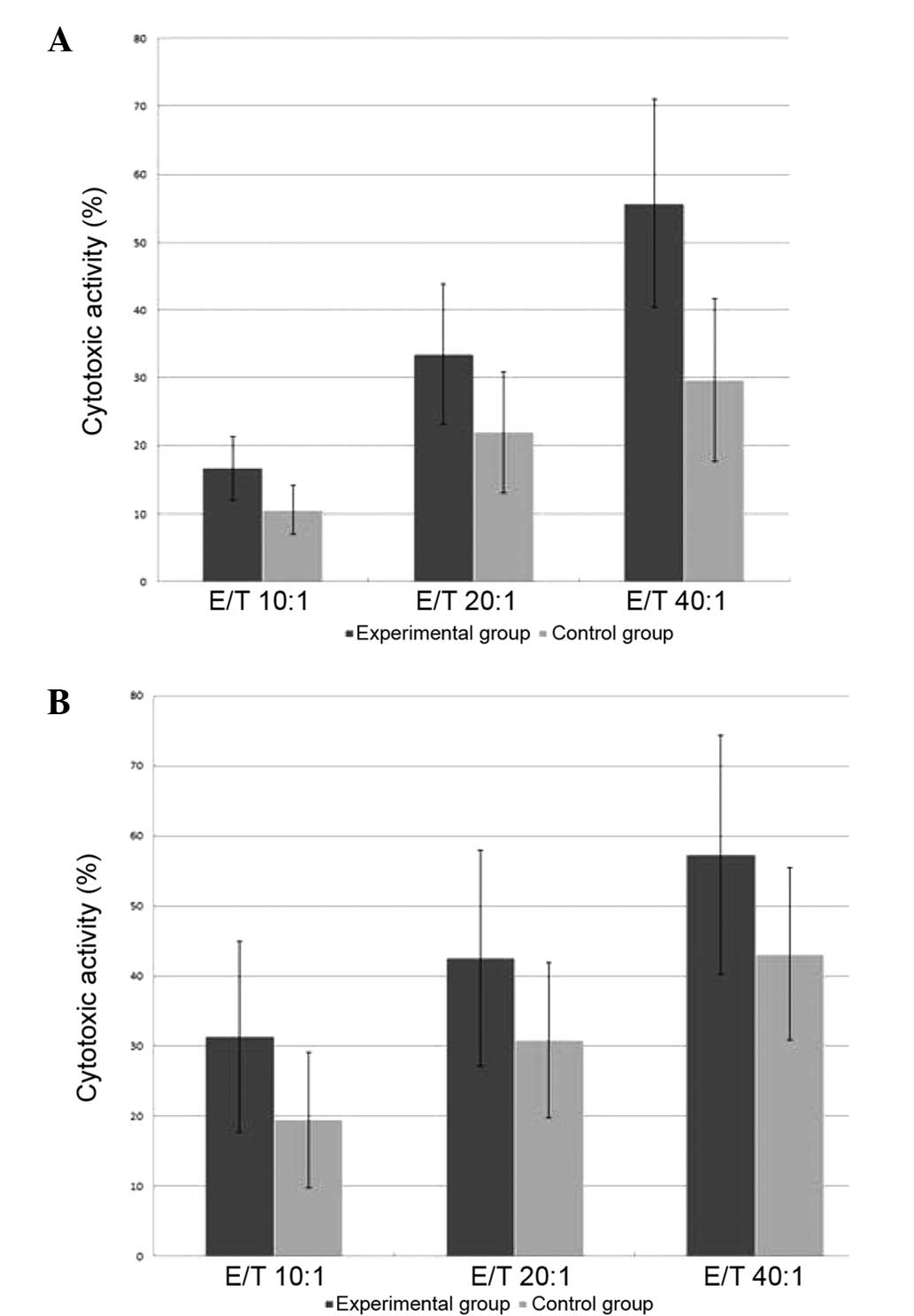 | Figure 1Cytotoxicities of CIK cells against
(A) SU-DHL2 cells at the different ratios of E/T (10:1, 20:1 and
40:1) in the experimental (rituximab) group (16.64±4.75,
33.53±10.36 and 55.73±15.33%, respectively) were higher than those
in the control group (10.54±3.55, 21.96±8.94 and 29.64±12.05%,
respectively; P<0.05). Cytotoxicities of CIK cells against (B)
K562 cells at the different ratios of E/T in the experimental group
(31.32±13.59, 42.58±15.37 and 57.27±17.08%, respectively) were
higher than those in the control group (19.47±9.69, 30.85±11.06 and
43.12±12.31%, respectively; P<0.05). CIK, cytokine-induced
killer; E/T, effector cell/target cell ratio. |
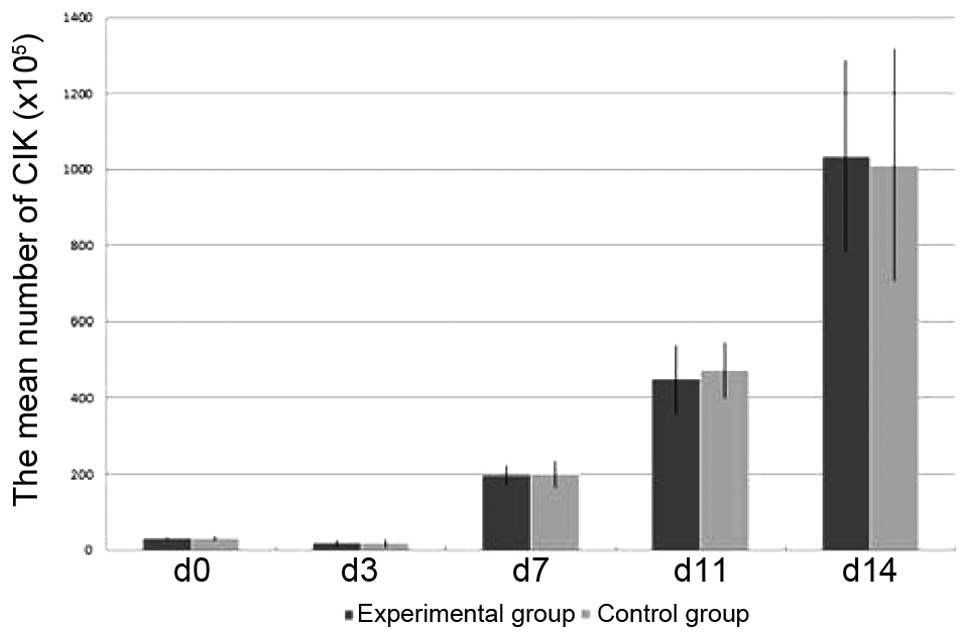
Expansion, phenotype and cytokine
secretion analysis of CIK cells
Starting from (31.1±3.6)x105 cells in the
experimental group and (30.7±5.1)x105 cells in the
control group, the cells of the two groups were counted to
determine the absolute cell number on days 3, 7, 11 and 14 and were
harvested on day 14. After 14 days of expansion, the mean total
number of experimental group cells was
~(1,035.1±251.4)x105, thus representing a mean
33.29-fold expansion. The mean total number of the control group
cells was ~(1,011.8±305.1)x105, thus representing a mean
32.53-fold expansion (Fig. 2). No
significant difference was identified between the mean cell counts
of the two groups (P>0.05).
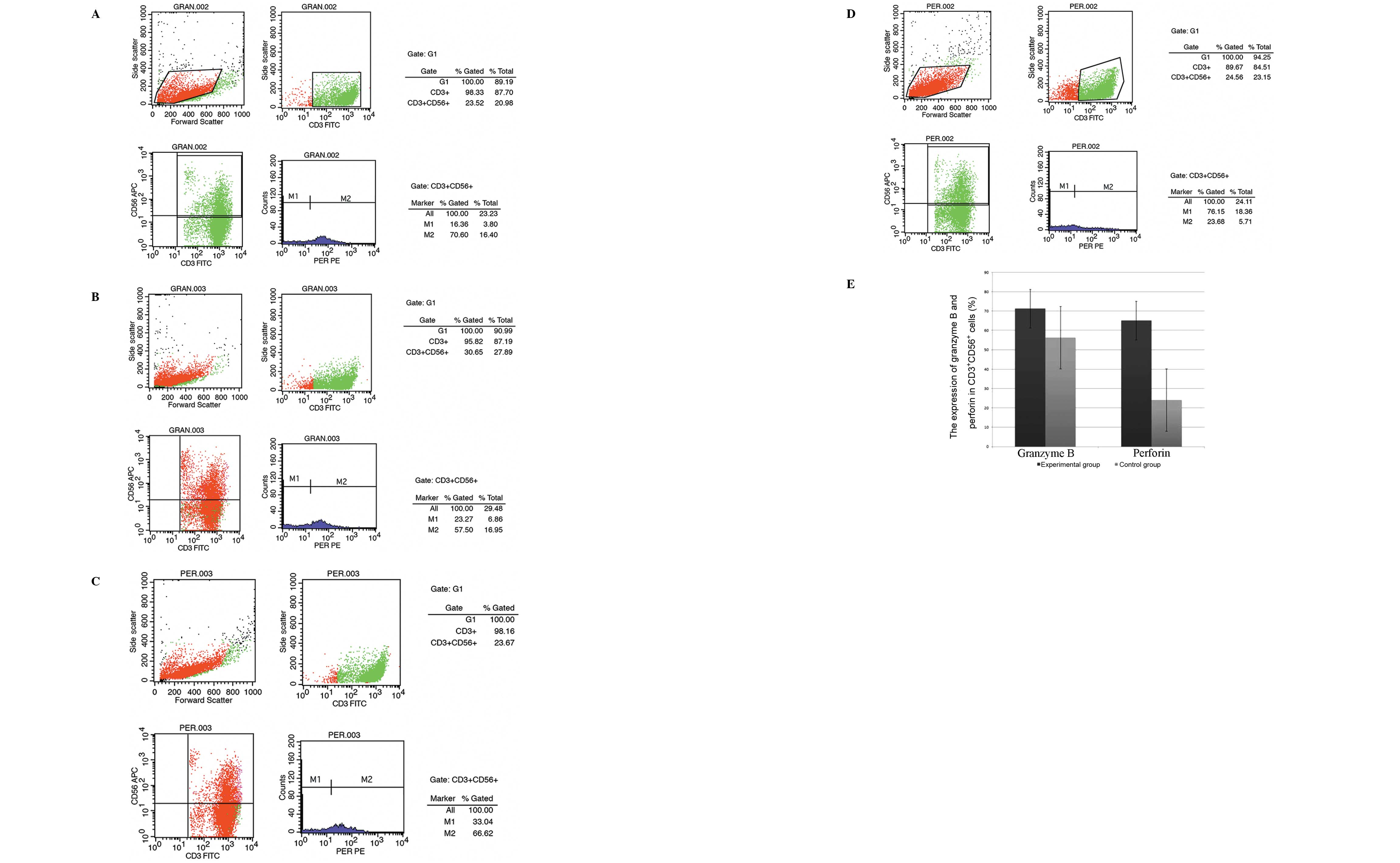
The CD3+CD56+ cells in the two
groups were analyzed on day 14. The final population comprised a
mean of 93.37±17.48% CD3+CD56+ cells in the
experimental group and 94.15±16.96% in the control group. The
expression of granzyme B and perforin in the
CD3+CD56+ cells on day 14 was analyzed. The
final CD3+CD56+ cell population in the
experimental group comprised a mean of 71.25±21.65% granzyme
B-positive cells and 65.08±17.47% perforin-positive cells. However,
in the control group the final CD3+CD56+ cell
population comprised a mean 56.29±16.99% granzyme B-positive cells
and 20.05±6.97% perforin-positive cells. The mean proportions of
granzyme B- and perforin-positive cells among the
CD3+CD56+ cells in the experimental group
were higher than those in the control group at the end of the
expansion period (P<0.05; Fig.
3). The experiment was repeated three times with similar
results.
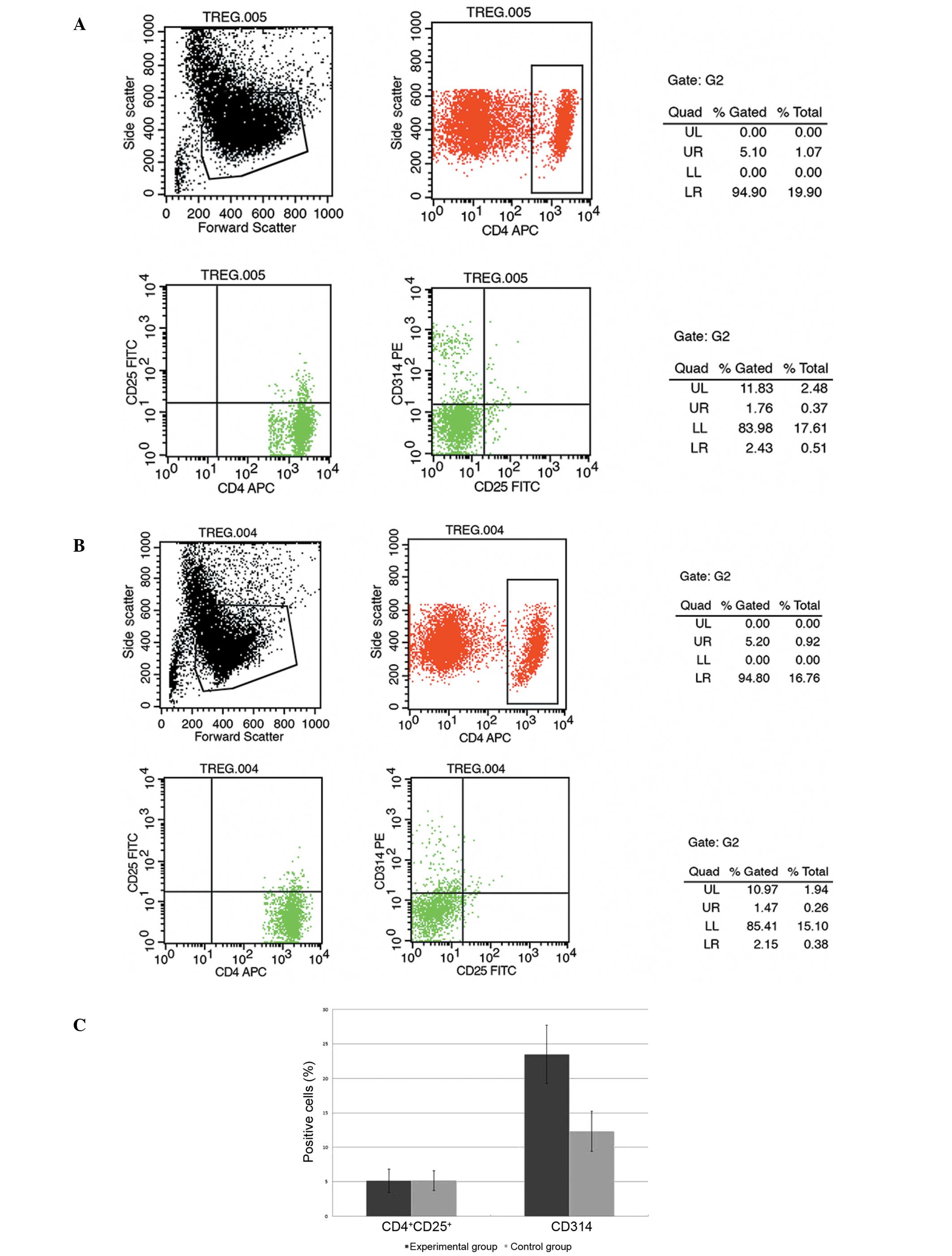
The CD4+CD25+ and
CD314+ cells were analyzed on day 14. The final
population in the experimental group comprised a mean of 5.16±1.68%
CD4+CD25+ cells and 23.49±4.22%
CD314+ cells. However, in the control group the final
population comprised a mean of 5.19±1.43%
CD4+CD25+ cells and 12.35±2.94%
CD314+ cells. The mean proportion of CD314+
cells in the experimental group was higher than that in the control
group at the end of the expansion period (P<0.05; Fig. 4). No difference was identified
between the mean proportions of CD4+CD25+
cells observed in the two groups (P>0.05). The experiment was
repeated three times with similar results.
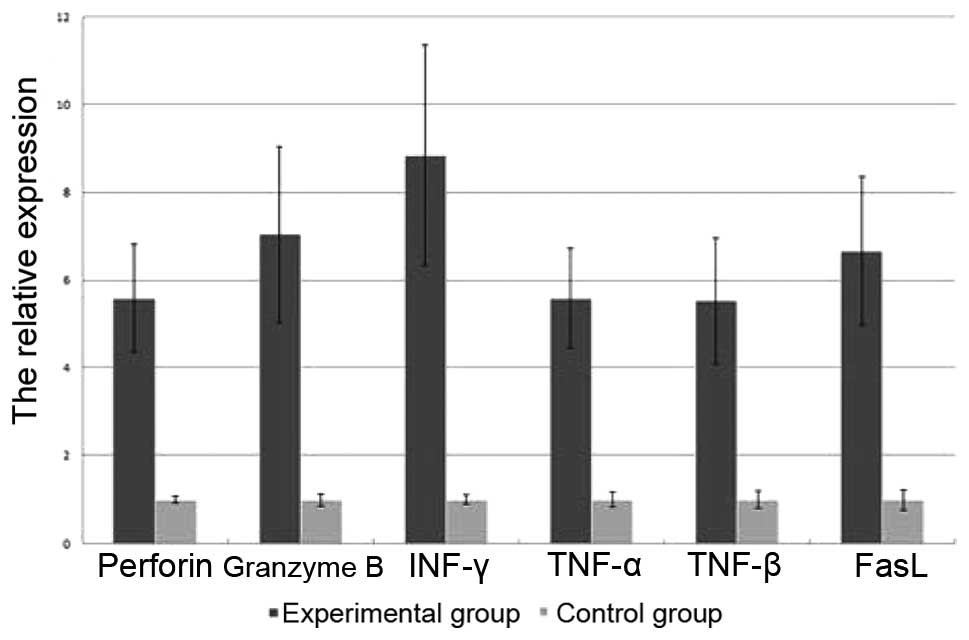
RT-qPCR analysis
The relative expression levels of perforin, granzyme
B, INF-γ, TNF-α, TNF-β and FasL of the two groups were detected by
RT-qPCR analysis on day 14. After 14 days of expansion, the
expression levels of perforin, granzyme B, INF-γ, TNF-α, TNF-β and
FasL in the experimental group were significantly higher than those
in the control group (P<0.05; Fig.
5).
Signaling pathway analysis by western
blotting
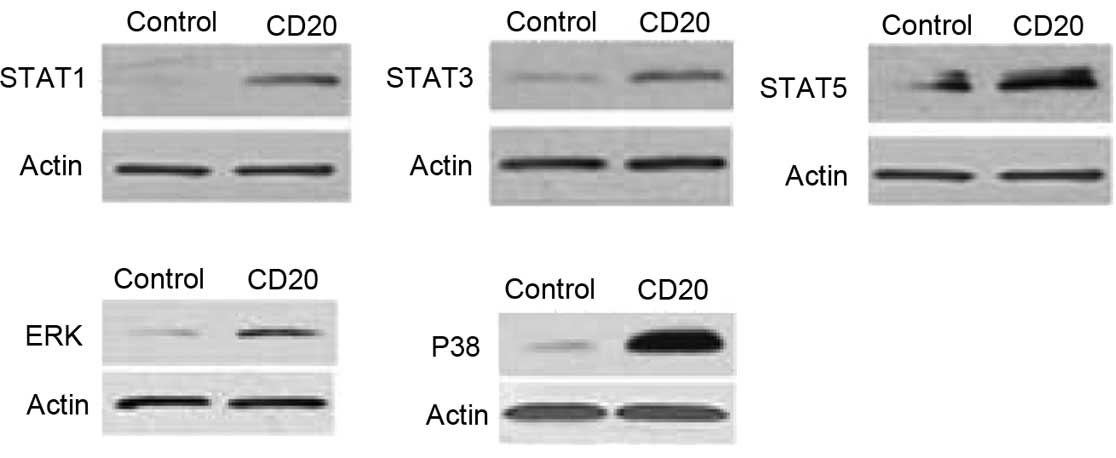
In this study, the effects of rituximab on the
STAT-1, STAT-3, STAT-5, ERK 1/2 and p38 MAPK signaling pathways of
CIK cells associated with proliferation and apoptosis regulation
were analyzed. The results indicate that components of the STAT-1,
STAT-3, STAT-5, ERK 1/2 and p38 MAPK signal transduction pathways
were constitutively phosphorylated in the rituximab group compared
with the control group (Fig.
6).
Discussion
Residual tumor cells represent a major obstacle in
the therapy of malignant hematological disease including acute
leukemia (AL) and lymphoma. The prognosis for patients that relapse
remains unsatisfactory. Therefore, the development of therapy with
regard to the depletion of residual tumor cells is important for
the treatment of such diseases. CD3+CD56+
cells can be generated from healthy donors. CIK cells have
exhibited anticancer activity in vitro and in vivo
(11,12). The cytotoxicity to tumor cells is
non-MHC-restricted, but relies on cell-to-cell contact, and is
perforin-dependent and Fas-independent (13). Additionally, CIK cells express
NKG2D (CD314) and perforin, two molecules that have been
demonstrated to play major roles in CIK-mediated cytotoxicity
(14). Another feature of CIK
cells is the production of effector cytokines including IFN-γ,
TNF-α, IL-2 and IL-12, which are involved in the immunoregulation
in anticancer activity. CIK cells exhibit activity against leukemia
targets while having low or absent activity against normal bone
marrow stem cells and tissues (15). In the present study, CIK cells were
generated from the peripheral blood of healthy donors by exposure
to rituximab (an anti-CD20 mAb) in the culture process. The results
indicated that rituximab increased the cytotoxicity of the CIK
cells against SU-DHL2 and K562 cells. Furthermore, the present
study may provide a novel useful method for the cultivation of CIK
cells and depletion of residual cells for AL and lymphoma
patients.
Rituximab as an anti-CD20 mAb is a main treatment
used in the therapy of a broad variety of B-cell malignancies.
Rituximab alone or as an addition to chemotherapy can enhance the
complete response, long-term remission and cure rate (9). The mechanisms responsible for the
antitumor effects of rituximab are not fully understood. However,
direct signaling, direct induction of apoptosis,
complement-dependent cellular cytotoxicity and antibody-dependent
cellular cytotoxicity (ADCC) are potential mechanisms of action of
rituximab.
In the present study rituximab was added to the CIK
cultures. Although no difference was identified in the expansion in
the experimental and control groups, the cytotoxicity in the
experimental group against the SU-DHL2 and K562 cell lines was
increased. A previous study demonstrated that the addition of
anti-CD20 mAb increased the killing activity of CIK cells toward
B-cell non-Hodgkin’s lymphoma (B-NHL) cell lines (16). This enhancement was mainly due to
ADCC mediated by the 1–10% natural killer cells that contaminated
the CIK cultures. These data suggest that an anti-CD20 mAb could be
used in vivo to enhance CIK therapeutic activity in B-NHL
cell lines.
In the present study, certain other features of the
increased cytotoxicity of CIK cells induced by the anti-CD20 mAb
were demonstrated. It was observed that the proliferation of the
CIK cells was not increased by the addition of anti-CD20 mAb;
however, the relative expression levels of perforin, granzyme B,
INF-γ, TNF-α, TNF-β and FasL detected by RT-qPCR analysis were
significantly enhanced. These effector cytokines are produced by
CIK cells and are involved in the immunoregulation in anticancer
activity. The results revealed that the expression of the CIK cell
phenotype CD3+CD56+ was not improved by the
addition of anti-CD20 mAb. However, the proportions of granzyme B,
perforin and CD314+ cells in the
CD3+CD56+ cells analyzed by flow cytometry
were significantly enhanced. The increase of CIK cell cytotoxicity
is associated with the increase in the expression of the proposed
cytotoxic factors. Therefore, anti-CD20 mAb could play an important
role in the improvement of the cytotoxicity of CIK cells by
enhancing these cytotoxic factors.
The present study demonstrated that in the CIK
cells, the anti-CD20 mAb promoted the STAT and MAPK/ERK signaling
pathways that play central roles in cell growth and apoptosis.
STAT1 is a well-characterized component of the IFN-induced
signaling pathways (17). STAT1 is
directly associated with T-cell apoptosis and may also contribute
to the events that govern the elimination of autoreactive T cells
via negative selection (18).
STAT1, STAT3 and STAT5 are important co-stimulatory molecules that
lead to the enhancement of T-cell proliferation (19). STAT5 is an important component
downstream of cytokines that regulate T-cell biology and a critical
survival factor in T-cell development and survival (20,21).
The present study reveals a critical role for the anti-CD20 mAb in
T-cell development and survival by the upregulation of the
expression of STAT proteins. It was also demonstrated that the
expression of ERK 1/2 and p38 increased due to the effects of the
anti-CD20 mAb. MAPK consists of ERK 1/2, c-Jun N-terminal kinases,
p38 and ERK 5/Big MAPK family members (22). The increased basal and activated
p38 MAPK signaling pathway is critical to death receptor resistance
(23). Due to interaction between
the p38 MAPK pathway and TNF-nuclear factor κB signaling, the role
of p38 in acquired apoptosis resistance is of biological and
therapeutic interest (24). The
apoptosis-resistance effect of the activated MAPK signaling pathway
is a possible mechanism underlying the anti-apoptosis effect of
anti-CD20 mAb on the proliferating CIK cells.
The data suggest that rituximab, an anti-CD20 mAb,
could be used to enhance the antitumor activity of CIK cells
against SU-DHL2 and K562 cell lines. The increase of CIK cell
cytotoxicity derived from the addition of anti-CD20 mAb to the CIK
cell culture was found to be associated with an increase in the
expression of cytotoxic factors. The results also revealed that the
effects of the anti-CD20 mAb were associated with an increase in
the expression of components of the STAT and MAPK/ERK signaling
pathways. Upregulation of the expression of STAT1, STAT3 and STAT5
is important as these co-stimulatory molecules enhance T-cell
proliferation, and the activated MAPK signaling pathway is a
possible mechanism underlying the anti-apoptosis effect on the
proliferating CIK cells. The anti-CD20 mAb was demonstrated to
improve CIK-mediated cytotoxicity to SU-DHL2 or K562 cell lines. In
conclusion, CIK cells cultured with anti-CD20 mAb could be a novel
therapeutic strategy for the depletion of chemotherapy-resistant or
residual cells of AL and B-cell lymphoma.
References
|
1
|
Dölken G: Detection of minimal residual
disease. Adv Cancer Res. 82:133–185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheffold C, Brandt K, Johnston V, et al:
Potential of autologous immunologic effector cells for bone marrow
purging in patients with chronic myeloid leukemia. Bone Marrow
Transplant. 15:33–39. 1995.PubMed/NCBI
|
|
3
|
Nagaraj S, Ziske C and Schmidt-Wolf IG:
Human cytokine-induced killer cells have enhanced in vitro
cytolytic activity via non-viral interleukin-2 gene transfer. Genet
Vaccines Ther. 2:122004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edinger M, Cao YA, Verneris MR, et al:
Revealing lymphoma growth and the efficacy of immune cell therapies
using in vivo bioluminescence imaging. Blood. 101:640–648. 2003.
View Article : Google Scholar
|
|
5
|
Marin V, Dander E, Biagi E, et al:
Characterization of in vitro migratory properties of anti-CD19
chimeric receptor-redirected CIK cells for their potential use in
B-ALL immunotherapy. Exp Hematol. 34:1219–1229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi M, Zhang B, Tang ZR, et al: Autologous
cytokine-induced killer cell therapy in clinical trial phase I is
safe in patients with primary hepatocellular carcinoma. World J
Gastroenterol. 10:1146–1151. 2004.PubMed/NCBI
|
|
7
|
Introna M, Borleri G, Conti E, et al:
Repeated infusions of donor-derived cytokine-induced killer cells
in patients relapsing after allogeneic stem cell transplantation: a
phase I study. Haematologica. 92:952–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feugier P, Van Hoof A, Sebban C, et al:
Long-term results of the R-CHOP study in the treatment of elderly
patients with diffuse large B-cell lymphoma: a study by the Groupe
d’Etude des Lymphomes de I’Adulte. J Clin Oncol. 23:4117–4126.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Habermann TM, Weller EA, Morrison VA, et
al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab
in older patients with diffuse large B-cell lymphoma. J Clin Oncol.
24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pfreundschuh M, Trümper L, Osterborg A, et
al; MabThera International Trial Group. CHOP-like chemotherapy plus
rituximab versus CHOP-like chemotherapy alone in young patients
with good-prognosis diffuse large-B-cell lymphoma: a randomised
controlled trial by the MabThera International Trial (MInT) Group.
Lancet Oncol. 7:379–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leemhuis T, Wells S, Scheffold C, et al: A
phase I trial of autologous cytokine-induced killer cells for the
treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma.
Biol Blood Marrow Transplant. 11:181–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linn YC and Hui KM: Cytokine-induced
killer cells: NK-like T cells with cytotolytic specificity against
leukemia. Leuk Lymphoma. 44:1457–1462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verneris MR, Ito M, Baker J, et al:
Engineering hematopoietic grafts: purified allogeneic hematopoietic
stem cells plus expanded CD8+ NK-T cells in the treatment of
lymphoma. Biol Blood Marrow Transplant. 7:532–542. 2001. View Article : Google Scholar
|
|
14
|
Verneris MR, Karami M, Baker J, et al:
Role of NKG2D signaling in the cytotoxicity of activated and
expanded CD8+ T cells. Blood. 103:3065–3072. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alvarnas JC, Linn YC, Hope EG and Negrin
RS: Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood
progenitor cells of patients undergoing autologous hematopoietic
cell transplantation. Biol Blood Marrow Transplant. 7:216–222.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pievani A, Belussi C, Klein C, et al:
Enhanced killing of human B-cell lymphoma targets by combined use
of cytokine-induced killer cell (CIK) cultures and anti-CD20
antibodies. Blood. 117:510–518. 2011. View Article : Google Scholar
|
|
17
|
David M: Signal transduction by type I
interferons. Biotechniques Suppl. 58–65. 2002.
|
|
18
|
Moro H, Otero DC, Tanabe Y and David M: T
cell-intrinsic and -extrinsic contributions of the IFNAR/STAT1-axis
to thymocyte survival. PLoS One. 6:e249722011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng R, Spolski R, Casas E, et al: The
molecular basis of IL-21-mediated proliferation. Blood.
109:4135–4142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eckelhart E, Warsch W, Zebedin E, et al: A
novel Ncr1-Cre mouse reveals the essential role of STAT5 for
NK-cell survival and development. Blood. 117:1565–1573. 2011.
View Article : Google Scholar
|
|
21
|
Hand TW, Cui W, Jung YW, et al:
Differential effects of STAT5 and PI3K/AKT signaling on effector
and memory CD8 T-cell survival. Proc Natl Acad Sci USA.
107:16601–16606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santen RJ, Song RX, McPherson R, et al:
The role of mitogen-activated protein (MAP) kinase in breast
cancer. J Steroid Biochem Mol Biol. 80:239–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frigo DE, Tang Y, Beckman BS, et al:
Mechanism of AP-1-mediated gene expression by select
organochlorines through the p38 MAPK pathway. Carcinogenesis.
25:249–261. 2004. View Article : Google Scholar
|
|
24
|
Antoon JW, Nitzchke AM, Martin EC, et al:
Inhibition of p38 mitogen-activated protein kinase alters microRNA
expression and reverses epithelial-to-mesenchymal transition. Int J
Oncol. 42:1139–1150. 2013.PubMed/NCBI
|